Abstract
Introduction:
We described the clinical and oncological outcomes of patients treated by laparoscopic surgery for non-metastatic pT3 renal cell carcinoma (RCC).
Methods:
We queried a multi-institutional database for patients diagnosed with non-metastatic pathological T3 RCC from 13 Canadian centres treated laparoscopically (radical or partial nephrectomy) between 2008 and 2014. Clinical and pathological outcomes were evaluated. Progression was defined as the development of recurrence or metastatic disease. Log-rank testing and Kaplan-Meier statistical methods assessed for differences and estimated progression-free survival (PFS).
Results:
In total, 176 patients were identified with a median age of 64 years. The median tumour size was 7.0 cm. Pre-clinical stage was cT1 to cT4 in 39%, 28%, 30% and 3%, respectively. The median blood loss was 150 mL (range: 0–6000) and the median operative time was 124 minutes (range: 60–360). Most lesions were clear cell RCC (80%). After a median follow-up of 17.6 months (range: 0.2–75.0), disease progression occurred in 26% (46/176) of patients, consisting of local recurrence in 7% (3/46), and metastatic disease in 93% (43/46). The 3-year PFS was 67%, with a median PFS of 49 months. Of those who progressed, the median time to progression was 10.3 months.
Conclusions:
This study is the largest cohort of pT3 RCC patients treated laparoscopically in the literature and suggests that for properly selected patients, laparoscopic management of locally advanced renal masses yields acceptable short-term oncological outcomes.
Introduction
Surgical management is the primary treatment modality for localized renal cell carcinoma (RCC). Since its introduction in 1991,1 laparoscopic surgery has become widely used to treat renal masses when technically feasible. The reduced morbidity with laparoscopic surgery compared to traditional open surgery is well-established, with less blood loss, decreased analgesic requirements, less disfigurement, shorter hospital admissions, and shorter periods of convalescence.2–4 Additionally, oncological outcomes between laparoscopic and open techniques for T1 and T2 RCC are similar.5–7 Yet, current literature examining the laparoscopic management of advanced RCC remains sparse, but promising.
Pathological T3 (pT3) disease includes renal tumours with venous invasion or peri-nephric tissue involvement. The 5-year disease-free survival for pT3 disease ranges from 30% to 85%.8–13 Among patients who recur, the median time to recurrence ranges from 11 to 22 months.8,9,12–14 If oncological efficacy can be demonstrated, a minimally invasive approach to pT3 disease would be preferred. However, current literature examining outcomes in this patient population are limited to small, single-centre analyses.15–19 In this study using a large, multi-institutional design in a contemporary cohort, we described the oncological outcomes of patients with locally advanced, non-metastatic RCC treated laparoscopically.
Methods
A multi-institutional database, the Canadian Kidney Cancer Information System (CKCis), was developed to compile clinical characteristics and outcomes among patients with RCC from 13 centres in 6 Canadian provinces. This database has been prospectively collected and maintained since January 2011, with retrospective data collection for preceding years. Institutional review board approval was obtained from each contributing site. We included patients who underwent laparoscopic partial (LPN) or radical nephrectomy (LRN) between January 2008 and August 2014 for pathological T3 disease. The choice of procedure was non-randomized and dependent on the preferences of the patient and surgeon. Patients were excluded if they had known metastatic disease prior to surgery, if metastatic disease was found intraoperatively, or if the case was converted to open surgery.
All patients were staged according to the American Joint Committee of Cancer Staging manual, 7th edition.20 Cancer-specific survival and overall survival were not assessed due to the low number of deaths. Progression-free survival (PFS) was calculated based on the time from surgery to the development of metastatic disease, local recurrence, or cancer-related death. Patients were censored at last follow-up or at non-cancer related death. The Kaplan-Meier method was used to estimate survival, and log-rank testing was used to assess for differences in PFS. Cox-proportional hazard ratios and 95% confidence intervals were determined. Statistical analysis was performed using R statistical environment, with statistical significance set at p < 0.05.
Results
Of the 651 patients with pT3 disease, 176 patients met the inclusion criteria with non-metastatic RCC treated laparoscopically. The cohort consisted of predominantly Caucasian males with normal baseline renal function and clinical T1/2 disease (Table 1).
Table 1.
Patient and clinical preoperative characteristics (n = 176)
| Variable | Median (IQR) or n (%) |
|---|---|
| Age | 64 (57–64) |
| Male | 117 (66) |
| Ethnicity | |
| Caucasian | 121 (69) |
| Asian | 6 (3) |
| Aboriginal | 5 (3) |
| Other | 3 (2) |
| Unknown | 41 (23) |
| Preoperative creatinine (μmol/L) | 83 (71–99) |
| Estimated glomerular filtration rate (mL/min) | 78 (61–90) |
| Preoperative hemoglobin (g/L) | 137 (122–145) |
| Laterality | |
| Left kidney | 98 (56) |
| Right kidney | 78 (44) |
| Preoperative clinical stage | |
| T1 | 57 (39) |
| T2 | 42 (28) |
| T3 | 45 (30) |
| T4 | 35(3) |
| Missing | 28 |
IQR: Interquartile range.
The median tumour diameter was 7.0 cm with most patients treated by LRN, for predominantly clear cell RCC. Blood loss was minimal and surgery was completed at a median of about 2 hours. Surgical margins were negative in 158 (90%), positive in 13 (7%), and unknown in 5 patients (3%). For the 13 patients with a positive margin, 8 had a LRN and 5 had a LPN. The positive margin rate stratified by procedure type was 5% (8/155) for LRN and 24% (5/21) for LPN (Table 2).
Table 2.
Operative and pathological characteristics for 176 patients undergoing laparoscopic surgery for pT3 RCC
| Variable | Median (range) or n (%) |
|---|---|
| Tumour size (cm) | 7.0 (1.6–15.0) |
| Surgical approach | |
| Partial nephrectomy | 21 (12) |
| Radical nephrectomy | 155 (88) |
| Blood loss (cc) median | 150 (0–6000) |
| Skin-to-skin OR duration (minutes) | 124 (60–360) |
| Positive surgical margin | |
| Overall | 13 (7) |
| LRN | 8 (5)a |
| LPN | 5 (24)b |
| Histological subtype | |
| Clear cell RCC | 140 (80) |
| Papillary RCC | 13 (7) |
| Chromophobe | 5 (3) |
| RCC unclassified | 9 (5) |
| Sarcomatoid | 2 (1) |
| Other | 7 (34) |
| Furhman grade | |
| 1 | 5 (43) |
| 2 | 53 (31) |
| 3 | 83 (48) |
| 4 | 31 (18) |
| Missing | 4 |
| Pathological stage | |
| pT3a | 150 (85) |
| pT3b | 16 (9) |
| pT3 (unspecified) | 10 (6) |
Compared to total number of LRN (n = 155);
Compared to total number of LPN (n = 21). OR: operation room; LRN: laparoscopic radical nephrectomy; LPN: laparoscopic partial nephrectomy; RCC: renal cell carcinoma.
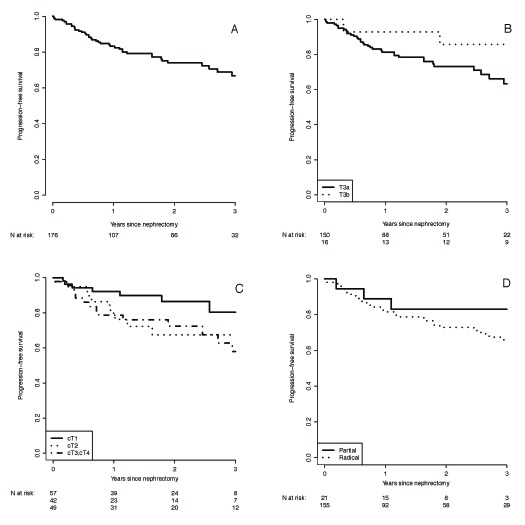
After a median follow-up of 22.6 months (range: 0.2–75.0), 43 (24%) patients developed metastatic disease and 3 (2%) patients had an isolated local recurrence. Lung (70%, 32/46), bone (39%, 18/46), and lymph nodes (30%, 14/46) were the most common sites of metastases. Isolated lymph node recurrences were found in 3 patients. Overall, 6/176 patients (3%) died from kidney cancer, and 1/176 patient (1%) died from other causes. The 3-year estimated PFS was 67%, with a median progression survival of 49 months (Table 3). Of those who progressed, the median time to progression was 10.3 months. Figure 1 shows the Kaplan-Meir PFS estimates for the overall cohort and subgroup comparisons.
Table 3.
Subgroup analysis of estimated 3-year PFS
| Group | Median time to progression (months) | 3-year estimated PFS | p value |
|---|---|---|---|
| All lap treated pT3 | 49 | 67% | N/A |
| Pathological stage | 0.09 | ||
| pT3a | 46 | 63% | |
| pT3b | N/Aa | 86% | |
| Clinical stage | 0.08 | ||
| cT1 | N/Aa | 80% | |
| cT2 | 42 | 67% | |
| cT3 and cT4 | 45 | 58% | |
| Surgical approach | 0.27 | ||
| LPN | N/Aa | 83% | |
| LRN | 49 | 65% |
PFS: progression-free survival; LRN: laparoscopic radical nephrectomy; LPN: laparoscopic partial nephrectomy; N/A: not available;
Median time to progression not assessed due to a greater than 50% survival rate at the end of the follow-up period.
Fig. 1.
Kaplan-Meier curve for progression free survival for (A) all pT3 renal cell carcinoma treated laparoscopically; (B) those with pT3a and pT3b disease (p = 0.09); (C) stratified according to clinical stage (p = 0.08); and (D) sub-stratified according to surgical approach (p = 0.27).
Discussion
Minimally invasive surgery has gone through many years of advances. With growing experience, more advanced tumours are likely to be managed by this approach; however, there is limited data examining oncological outcomes in the locally advanced RCC population. Using a large, multi-institutional design, our study represents the largest cohort of pT3 RCC treated laparoscopically in the literature. The estimated 3-year PFS was 67%, with a median PFS of 49 months, suggesting that adequate short-term oncological outcomes can be achieved with laparoscopic management of locally advanced RCC.
Some studies have attempted to demonstrate the efficacy and safety of laparoscopic surgery in a similar population;16,17,21–23 however, single-centre analyses, minimal patient numbers or short follow-up periods limit their generalizability. Our multi-institutional design allows us to include many patients in a contemporary time period, not easily accomplished in single-centre studies. Further, including multiple institutions allows us to widen the application of our results by reducing potential patient selection or referral biases inherent in smaller studies. Additionally, single centres may have a certain element of standardization of surgical techniques, which further minimizes generalization of their results.
To date, comparative studies between laparoscopic and open surgery for pT3 disease have shown similar outcomes, but have been limited predominantly to retrospective reviews.17,24 One study used a prospective cohort matched analysis of 25 pairs.23 Although a comparative group is lacking in the present study, our short-term results are concurrent with literature on open surgery.23,24 However, the possibility than open surgery may confer a long-term survival advantage should not be disregarded. In fact, open surgery may be more advantageous as it affords a greater opportunity for retroperitoneal lymph node dissection that offers both diagnostic and therapeutic advantages. In the current study, less than half of the patients had node dissections of which the extent is uncertain. While the role for lymphadenectomy in laparoscopic renal surgery has been debated,25 much of the opposing literature arises from predominantly clinically localized (pT1–2) disease that is likely to have a different biological natural history than pT3 disease. Further, 2% (3/176) patients developed isolated local lymph node recurrences in our cohort and conceivably may have derived benefit from a lymph node dissection.
Nonetheless, our multicentre results corroborate the findings of two recent single-centre series. Guzzo and colleagues evaluated 32 patients undergoing laparoscopic management for non-metastatic pT3b renal cancers, of whom 3 developed recurrences at a mean time of 11 months.16 Stewart and colleagues retrospectively reviewed 77 patients who underwent laparoscopic management of pT3 RCC (36% pT3a and 62% pT3b),19 and after a median follow-up of 17 months, 28.6% of patients developed disease recurrence after a median 13.9 months. Their median predicted PFS was 48 months.
LPN, in select patients, yields similar results to those treated by LRN. In our current study, 12% of patients underwent LPN presumably due to clinical under-staging on preoperative imaging. With microscopic involvement of the venous system, including segmental vessels, it is not uncommon for a partial nephrectomy to be performed with final pathology demonstrating occult adverse pathological features. In fact, 81% of our LPN patients were at clinical stage T1/2, suggesting that the surgeons may have been unaware of the locally advanced nature of the disease. Although, the overall numbers of positive margins were low, the rate of margin positivity was markedly higher in the LPN group, which may reflect clinical under-staging and/or suboptimal patient selection. However, the risk of local recurrence in those with a positive margin remains controversial.26,27 Much of the data on positive margins after partial nephrectomy indicate that these margins do not predict worse outcomes,28 although most of this data were collected in patients with lower risk tumours. It is possible that positive margins are biologically more relevant in pT3 tumours. The difference in positive margins between LRN and LPN in our series was not reflected in the 3-year PFS (Fig. 1, p = 0.27), concurrent with other findings.29 Importantly, one might expect a worse outcome after LRN due to selection for larger tumours, and one could speculate that the absence of this difference could be due to the positive margins.
Clinical under-staging is an important phenomenon to discuss. Larger tumours in particular are associated with an elevated risk of being identified as pT3 on final pathological analysis.27 While surgical planning is highly dependent on preoperative imaging, it should be noted that segmental renal vein involvement and fat invasion are often difficult to identify with current cross-sectional imaging techniques. In fact, two-thirds of our cohort had clinical stage T1/2 disease. While clinical stage was not a significant predictor of progression, patients with cT1 tumours trended towards improved outcomes with an 80% 3-year PFS compared to 67% and 58% in those with cT2 and cT3/4, respectively (p = 0.08). This may be related to the prognostic value of tumour size. In fact, when accounting for size in regression modelling, this trend was no longer appreciated (p = 0.36).
We acknowledge that a number of tumour characteristics present risk factors for pathological upstaging from cT1 to pT3 disease, including high tumour complexity, increasing tumour diameter and hilar location.30 While we have not compared the rates of margin positivity between LPN and open partial nephrectomy, as this would be limited by selection bias, it is worth considering whether the tumours in this series may have been better managed with open partial nephrectomy if the indication was truly mandatory. It must also be acknowledged that this is a very select group of patients, with most being treated for presumed clinical T1/2 disease. We are not suggesting that T3 disease be managed by partial nephrectomy. However, should pT3 disease be identified on final pathological analysis, acceptable short-term oncological outcomes can be achieved.
While operating time and blood loss only represent a portion of the morbidity associated with this procedure, in our experience, reduced operative times and comparable blood loss to other studies were seen, despite similar tumour sizes.16,21 In this regard, laparoscopic surgery appears technically safe and feasible in this population.
There are a number of limitations. First, the multi-institutional design, while advantageous to reduce biases found within single-centre studies, is also subject to heterogeneity in data collection and follow-up. Second, we did not capture data on specific postoperative complications, although others have shown similar complication rates between laparoscopically treated pT1, pT2 and pT3 disease.11,21 Third, we acknowledge that pT3 RCC includes a heterogeneous population, including those with both venous and fat invasion, whose differential prognoses are debatable. Fourth, there is an inherent selection bias in those selected for laparoscopic surgery, which we are unable to adjust. Fifth, the median follow-up in our study was 22.6 months and the median PFS was 49 months, so that further cases of local recurrence or metastatic disease will be anticipated with longer follow-up. Lastly, cancer-specific survival and overall survival were not assessed due to the low number of deaths, precluding any meaningful conclusions in this regard.
Conclusion
This multi-centered study constitutes the largest cohort of patients to have undergone laparoscopic renal surgery for pT3 RCC. For properly selected patients, our findings provide preliminary evidence that laparoscopic management of locally advanced renal masses yields acceptable oncological outcomes, and may be considered in the treatment paradigm on locally advanced disease. Under-staging in patients undergoing laparoscopic partial nephrectomy remains a concern.
Acknowledgments
The authors acknowledge the comments received from presentations at the Canadian Urological Association annual meeting, the American Urological Association annual meeting and for the financial support from The Kidney Cancer Research Network of Canada.
Footnotes
Competing interests: Dr. Nayak, Dr. Patel, Dr. Bjazevic, Dr. Liu, and Dr. Saarela declare no competing financial or personal interests. Dr. Kapoor is a member of the Speakers bureau for, and has received grants and honoraria from, Pfizer Oncology, GSK Oncology, Novartis Oncology and Amgen. He has also participated in clinical trials within the past 2 years with NCIC, Pfizer, GSK, Novartis and Amgen. Dr. Rendon is a member of the Advisory Board and the Speakers bureau for Amgen, Astellas, Ferring and Janssen. Dr. Rendon has received honoraria from Amgen, Astellas, Ferring and Janssen for participation in Advisory Boards and their Speaker’s bureau. Dr. Kawakami hs received grants from Pentopharm and Baxter for travel grants. Dr. Tanguay is a member of the advisory board for Pfizer and has received grants from Pfizer, Novartis, and GSK. Dr. Breau has received a grant from Pfizer. Dr. Black is a member of the advisory boards for Amgen, Janssen, Ferring, Astellas, and AbbVie. He is also currently participating in a clinical trial with Ferring, Astellas, GSK, and Janssen. Dr. Drachenberg has attended Advisory Boards for Astellas and Janssen and has been a speaker for Amgen and Actavis (formerly Watson). He has also been an investigator in clinical trials run by Cancer Care Manitoba (CCMB).
This paper has been peer-reviewed.
References
- 1.Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: Initial case report. J Urol. 1991;146:278–82. doi: 10.1016/s0022-5347(17)37770-4. [DOI] [PubMed] [Google Scholar]
- 2.Dunn MD, Portis AJ, Shalhav AL, et al. Laparoscopic versus open radical nephrectomy: A 9-year experience. J Urol. 2000;164:1153–9. doi: 10.1016/S0022-5347(05)67131-5. [DOI] [PubMed] [Google Scholar]
- 3.Shuford MD, McDougall EM, Chang SS, et al. Complications of contemporary radical nephrectomy: Comparison of open vs. laparoscopic approach. Urol Oncol. 2004;22:121–6. doi: 10.1016/S1078-1439(03)00137-6. [DOI] [PubMed] [Google Scholar]
- 4.Hemal AK, Kumar A, Kumar R, et al. Laparoscopic versus open radical nephrectomy for large renal tumors: A long-term prospective comparison. J Urol. 2007;177:862–6. doi: 10.1016/j.juro.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 5.Ono Y, Kinukawa T, Hattori R, et al. The long-term outcome of laparoscopic radical nephrectomy for small renal cell carcinoma. J Urol. 2001;165:1867–70. doi: 10.1016/S0022-5347(05)66230-1. [DOI] [PubMed] [Google Scholar]
- 6.Gill IS, Meraney AM, Schweizer DK, et al. Laparoscopic radical nephrectomy in 100 patients: A single center experience from the United States. Cancer. 2001;92:1843–55. doi: 10.1002/1097-0142(20011001)92:7<1843::AID-CNCR1701>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Luo JH, Zhou FJ, Xie D, et al. Analysis of long-term survival in patients with localized renal cell carcinoma: Laparoscopic versus open radical nephrectomy. World J Urol. 2010;28:289–93. doi: 10.1007/s00345-009-0487-9. [DOI] [PubMed] [Google Scholar]
- 8.Levy DA, Slaton JW, Swanson DA, et al. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol. 1998;159:1163–7. doi: 10.1016/S0022-5347(01)63541-9. [DOI] [PubMed] [Google Scholar]
- 9.Hafez KS, Novick AC, Campbell SC. Patterns of tumor recurrence and guidelines for followup after nephron sparing surgery for sporadic renal cell carcinoma. J Urol. 1997;157:2067–70. doi: 10.1016/S0022-5347(01)64675-5. [DOI] [PubMed] [Google Scholar]
- 10.Ljungberg B, Alamdari FI, Rasmuson T, et al. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–11. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 11.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–5. doi: 10.1016/S0022-5347(05)67896-2. [DOI] [PubMed] [Google Scholar]
- 12.Gofrit ON, Shapiro A, Kovalski N, et al. Renal cell carcinoma: Evaluation of the 1997 TNM system and recommendations for follow-up after surgery. Eur Urol. 2001;39:669–74. doi: 10.1159/000052525. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson AJ, Chetner MP, Rourke K, et al. Guidelines for the surveillance of localized renal cell carcinoma based on patterns of relapse after nephrectomy. J Urol. 2004;172:58–62. doi: 10.1097/01.ju.0000132126.85812.7d. [DOI] [PubMed] [Google Scholar]
- 14.Lam JS, Shvarts O, Leppert JT, et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466–72. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 15.Martin GL, Castle EP, Martin AD, et al. Outcomes of laparoscopic radical nephrectomy in the setting of vena caval and renal vein thrombus: Seven-year experience. J Endourol. 2008;22:1681–5. doi: 10.1089/end.2008.0035. [DOI] [PubMed] [Google Scholar]
- 16.Guzzo TJ, Schaeffer EM, McNeil BK, et al. Laparoscopic radical nephrectomy for patients with pathologic T3b renal-cell carcinoma: The Johns Hopkins experience. J Endourol. 2009;23:63–7. doi: 10.1089/end.2008.0451. [DOI] [PubMed] [Google Scholar]
- 17.Bensalah K, Salomon L, Lang H, et al. Survival of patients with nonmetastatic pT3 renal tumours: A matched comparison of laparoscopic vs open radical nephrectomy. BJU Int. 2009;104:1714–7. doi: 10.1111/j.1464-410X.2009.08662.x. [DOI] [PubMed] [Google Scholar]
- 18.Bird VG, Shields JM, Aziz M, et al. Laparoscopic radical nephrectomy for patients with T2 and T3 renal-cell carcinoma: Evaluation of perioperative outcomes. J Endourol. 2009;23:1527–33. doi: 10.1089/end.2009.0399. [DOI] [PubMed] [Google Scholar]
- 19.Stewart GD, Ang WJ, Laird A, et al. The operative safety and oncological outcomes of laparoscopic nephrectomy for T3 renal cell cancer. BJU Int. 2012;110:884–90. doi: 10.1111/j.1464-410X.2011.10850.x. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. Springer; New York: 2010. pp. 479–89. [Google Scholar]
- 21.Laird A, Stewart GD, Zhong J, et al. A generation of laparoscopic nephrectomy: Stage-specific surgical and oncologic outcomes for laparoscopic nephrectomy in a single center. J Endourol. 2013;27:1008–14. doi: 10.1089/end.2012.0562. [DOI] [PubMed] [Google Scholar]
- 22.Hammond L, Powell TM, Schwartz BF. Pure laparoscopic radical nephrectomy for stage T(3b) renal-cell carcinoma: More than 2-year follow-up. J Endourol. 2007;21:408–10. doi: 10.1089/end.2006.0014. [DOI] [PubMed] [Google Scholar]
- 23.Laird A, Choy KC, Delaney H, et al. Matched pair analysis of laparoscopic versus open radical nephrectomy for the treatment of T3 renal cell carcinoma. World J Urol. 2015;33:25–32. doi: 10.1007/s00345-014-1280-y. [DOI] [PubMed] [Google Scholar]
- 24.Ganpule AP, Sharma R, Thimmegowda M, et al. Laparoscopic radical nephrectomy versus open radical nephrectomy in T1-T3 renal tumors: An outcome analysis. Indian J Urol. 2008;24:39–43. doi: 10.4103/0970-1591.38602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman TN, Sharma S, Zhang S, et al. Laparoscopic lymph node dissection in clinically node-negative patients undergoing laparoscopic nephrectomy for renal carcinoma. Urology. 2008;71:287–91. doi: 10.1016/j.urology.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 26.Borghesi M, Brunocilla E, Schiavina R, et al. Positive surgical margins after nephron-sparing surgery for renal cell carcinoma: Incidence, clinical impact, and management. Clin Genitourin Cancer. 2013;11:5–9. doi: 10.1016/j.clgc.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Breda A, Stepanian SV, Liao J, et al. Positive margins in laparoscopic partial nephrectomy in 855 cases: A multi-institutional survey from the United States and Europe. J Urol. 2007;178:47–50. doi: 10.1016/j.juro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Ani I, Finelli A, Alibhai SM, et al. Prevalence and impact on survival of positive surgical margins in partial nephrectomy for renal cell carcinoma: A population-based study. BJU Int. 2013;111:E300–5. doi: 10.1111/j.1464-410X.2012.11675.x. [DOI] [PubMed] [Google Scholar]
- 29.Weight CJ, Lythgoe C, Unnikrishnan R, et al. Partial nephrectomy does not compromise survival in patients with pathologic upstaging to pT2/pT3 or high-grade renal tumors compared with radical nephrectomy. Urology. 2011;77:1142–6. doi: 10.1016/j.urology.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 30.Gorin MA, Ball MW, Pierorazio PM, et al. Outcomes and predictors of clinical T1 to pathological T3a tumor up-staging after robotic partial nephrectomy: A multi-institutional analysis. J Urol. 2013;190:1907–11. doi: 10.1016/j.juro.2013.06.014. [DOI] [PubMed] [Google Scholar]