Introduction
A number of factors must be considered in determining the optimal treatment for patients with renal or ureteral calculi. These factors may be grouped into four broad categories: stone factors (location, size, composition, presence and duration of obstruction); clinical factors (symptom severity, patient’s expectations, associated infection, obesity, coagulopathy, hypertension and solitary kidney); anatomic factors (horseshoe kidney, ureteropelvic junction obstruction and renal ectopia); and technical factors (available equipment, expertise and cost).1 When intervention is indicated, the factors above need to be considered in helping to select the treatment that will achieve maximal stone clearance with minimal morbidity to the patient. In many cases, more than one treatment option will be suitable and the ultimate treatment decision will be based on the patients’ preferences with respect to the balance between invasiveness and morbidity of the procedure versus the likelihood of achieving stone-free status. Access to necessary equipment and technical expertise may also play a key role in the treatment options offered to patients.
The focus of this guideline is the management of ureteral stones. Specifically, the topics covered include: conservative management, medical expulsive therapy, active intervention with either shockwave lithotripsy (SWL) or ureteroscopy (URS), factors affecting SWL treatment success, optimizing success, and special considerations (e.g., pregnancy, urinary diversion). By performing extensive literature reviews for each topic evaluated, we have generated an evidence-based consensus on the management of ureteral stones. The objective of this guideline is to help standardize the treatment of ureteral stones to optimize treatment outcomes.
Methods/guideline development process
Separate reviews of the literature were performed for each of the major topic areas. English language publications from 2000 to 2014 were identified from Medline. For each topic, two authors independently performed the extensive literature review to ensure completeness. The International Consultation on Urologic Disease (ICUD)/WHO modified Oxford Center for Evidence-Based Medicine grading system was used to grade the quality of evidence for each topic assessed. Importantly, all recommendations were based on expert review of the literature and represent the consensus of all authors of these guidelines.
Conservative management
Observation/spontaneous passage
Conservative management to allow spontaneous stone passage is preferred provided that passage is likely in a reasonable time frame, with acceptable patient symptoms and a low risk of complications. Conservative management is not appropriate in the face of infectious symptoms, intolerable patient symptoms, or if conservative management would pose a potential threat to renal function.
Numerous case series have described rates of spontaneous passage based on stone size and location. We have found that 95% of ureteral stones 2 to 4 mm in size will pass spontaneously. This drops to 50% for stones greater than 5 mm.2 Stones greater than 6 mm have a lower rate of spontaneous passage.3 Duration of stone passage may be as long as 40 days.2 A meta-analysis of five series, with a total of 224 patients with stones less than or equal to 5 mm included in the 2007 American Urological Association (AUA)/European Association of Urology (EAU) ureteral stone guidelines, demonstrated a stone passage rate of 68% decreasing to 47% for stones 5 to 10 mm in diameter.4
In determining stone size, the axial diameter (i.e., width) of the stone on unenhanced computed tomography (CT), as opposed to the length, is closely correlated with stone passage rate.5 Furthermore, if coronal reconstruction images are available, they can provide additional information with respect to the maximal stone diameter.6 Also of note, ultra-sound has been shown to overestimate stone size, particularly for stones ≤5 mm, compared with CT scan,7 and so if available, CT-based measurement of stone size should be relied upon for determining a treatment plan.
Recommendation: Spontaneous passage of stones less than 5 mm in size in the distal ureter have a >90% chance of spontaneous passage within 40 days and are appropriate for an attempt at conservative management provided there are no infectious symptoms, intolerable patient symptoms or a threat to renal function. Stones above 5 mm in diameter are less likely to pass spontaneously and patients should be counselled about treatment options (Level of Evidence 4, Grade C).
Medical expulsive therapy
Calcium channel blockers and alpha-receptor antagonists have been studied as adjuncts to improve rates of ureteral stone passage and shorten time to stone passage. The 2007 AUA/EAU ureteral stone guidelines performed a meta-analysis of medical expulsive therapy trials using these agents. Calcium channel blockers did not demonstrate a statistically significant improvement in stone passage whereas significantly more (29%; confidence interval [CI] 20%–37%) patients passed their stones with alpha-blocker therapy than did patients receiving a placebo.4 A Cochrane collaboration meta-analysis demonstrated a higher stone-free rate (RR 1.48, 95% CI 1.33–1.64), a shorter time to stone passage (2.91 days less), with a decreased number of pain episodes, analgesic requirements and hospitalizations for patients with ureteral stones less than 10 mm treated with alpha-receptor antagonists compared to placebo.8 Conversely, a recent large randomized controlled trial failed to show any benefit from the use of tamsulosin or nifedipine to promote stone passage9.
Ultimately, when reviewing all of the available literature there is likely to be a benefit for alpha blockers in treating distal ureteral calculi less than 10 mm. However, clinicians should always weigh the risks and benefits of therapy. Since the risks of alpha-blockers are low, they likely remain an important aspect of medical expulsive therapy.
Recommendation: Medical expulsive therapy with alpha-receptor antagonists potentially shortens the duration and increases the likelihood of spontaneous stone passage. Consideration should be given to offering it to patients with distal ureteral stones less than 10mm in size (Level of Evidence 1a, Grade A).
Comparative outcomes of SWL vs. URS
SWL and URS are the two main modalities presently used to treat ureteral calculi in an attempt to achieve the goal of maximal stone clearance with minimal morbidity to the patient. Below, the stone-free rates and complications of SWL and URS are reviewed with results stratified by stone location and size.
In 2007 the AUA and EAU joined forces to publish the 2007 Guideline for the Management of Ureteral Calculi,4 which represented a synthesis of the best available evidence at the time comparing outcomes for SWL to URS. This joint EAU/AUA Nephrolithiasis Guideline Panel performed a systematic review of the English language literature published since 1997 and comprehensively analyzed outcomes data from the identified studies. The meta-analysis revealed that for stones in the proximal ureter, there was no difference in stone-free rates between SWL and URS. However, for proximal ureteral stones <10 mm, SWL had a higher stone-free rate than URS (90% vs. 80%), whereas for stones >10 mm, URS had superior stone-free rates (79% vs. 68%). As such, the guidelines offer both SWL and URS as equal first-line options for proximal ureteral stones. This contrasts to the previous guidelines from 1997 where SWL was the only first-line option for proximal ureteral stones. In the updated guidelines, the stone-free rate for mid-ureteral stones was not statistically significantly different between URS and SWL, whereas for distal stones, URS yielded better stone-free rates overall and in both size categories (Table 1).
Table 1.
Stone-free rate for SWL and URS in the treatment of ureteral calculi4
| Stone location/size | Stone-free rate* | |
|---|---|---|
| SWL | URS | |
| ureter | 74% | 94% |
| ≤10 mm | 86% | 97% |
| >10 mm | 74% | 93% |
| Mid ureter | 73% | 86% |
| ≤10 mm | 84% | 91% |
| >10 mm | 76% | 78% |
| Proximal ureter | 82% | 81% |
| ≤10 mm | 90% | 80% |
| >10 mm | 68% | 79% |
SWL: shock wave lithotripsy; URS: ureteroscopy;
Stone free rate following first treatment or primary treatment is reported.
Since the publication of AUA/EAU guidelines in 2007, two more recent systematic reviews and meta-analyses have been completed.
First, a Cochrane review, published in 2012, analyzed seven randomized controlled trials (RCTs) comprising 1205 patients treated for ureteric stones.10 The stone-free rates were lower for SWL when compared with URS (RR 0.84, 95% CI 0.73–0.96). Consequently, the need for retreatment, defined as a subsequent intervention for the stone using the same therapeutic technique as the initial treatment, was higher in SWL patients (RR 6.18, 95% CI 3.68–10.38).10 Procedure-related complications were lower for SWL compared to URS patients (RR 0.54, 95% CI 0.33–0.88).10
Second, a meta-analysis by Matlaga and colleauges11 stratified their analysis of SWL versus URS based on stone location in the distal versus proximal ureter. They also specifically compared URS both to SWL with the Dornier HM3 and to other lithotripters. Considering no HM3 lithotripters are in use in Canada, we present only the results of URS versus other lithotripters. For distal ureteral stones, analysis of six studies revealed a 55% greater probability of being stone-free at first follow-up with semi-rigid URS compared with SWL (RR 1.55). However, with time, SWL approached the stone-free rate of semi-rigid URS due to re-treatment of SWL cases. Accordingly, patients treated with semi-rigid URS were less likely to require re-treatment than patients treated with SWL for distal ureteral stones (RR 0.14). A similar number of complications occurred in both the semi-rigid URS and SWL groups (pooled RR 1.28, 95% CI 0.94–1.81). For proximal ureteral stones, there was a greater probability of being stone-free with semi-rigid URS versus SWL (RR 1.15) and a lower risk for retreatment (RR 0.08, 95% CI 0.02–0.32). However in this meta-analysis, no studies directly compared semi-rigid URS to SWL for proximal ureteral stones and as such this is a comparison of outcomes across different studies, which is methodologically undesirable.
Unfortunately, the marked heterogeneity of the existing evidence in terms of study design, stone location, types of ureteroscope, intracorporeal lithotripsy devices, policy variations in stenting after ureteroscopy, and time to follow-up limit the conclusion that can be drawn from both of the aforementioned meta-analyses. Accordingly, it is difficult to provide a definitive recommendation for use in clinical decision-making.
Ultimately, the size and location of stones, the urologist’s expertise and the availability and access to resources and appropriate technologies remain the principal criteria to inform treatment choice for the management of ureteric stones. Large, multicentre, well-designed RCTs and high quality reporting are lacking in the medical literature.
Recommendation: Both SWL and URS are safe and efficacious treatment options for ureteral stones. Based on the available evidence, patients who undergo URS have a higher likelihood of achieving stone-free status, especially for distal stones, at the expense of a greater risk of complications. Patients should be offered both options when suitable and available, and educated on the benefits and risk of each treatment modality (Level of Evidence 2a, Grade B).
Other factors affecting SWL treatment success
Beyond location in the ureter, other stone-related factors, including composition, density of the stone, and skin-to-stone distance on CT, may influence the treating physician and patient’s discussion regarding the choice to proceed with either SWL or URS.
Composition
Most stones, composed of calcium oxalate, will fragment well with SWL treatment. There are certain stone compositions, such as cystine, calcium oxalate monohydrate and brushite, that are more resistant to SWL and may be better served by ureteroscopic management.12 Moreover, in the case of cystine and calcium oxalate monohydrate, the fragments created by SWL may be large which can result in poor clearance. Uric acid stones, while fragile in the face of SWL, present a challenge with respect to localization for SWL treatment. Either the use of ultrasound or pyelography (IVP or retrograde) is required to target the stone. In addition, follow-up cannot be completed in the conventional fashion with plain radiography, and requires the use of either ultrasound or, more often, CT scanning to ensure the patient is successfully treated.
In many instances the exact stone composition will not be known prior to treatment, or in the case of recurrent stone formers, it may have changed over time.13 Non-contrast CT scans using dual energy can distinguish some types of stones in vivo. Uric acid stones can be differentiated from calcium stones; however, there is significant overlap in the attenuation of calcium-based stones which makes determining the exact composition difficult. Recent publications on dual energy CT scanning support that different calcium stone compositions can be determined, however this modality is not readily available in clinical practice.14,15
Stone density
As a surrogate for composition, several authors have postulated that the fragmentation of stones with SWL could be predicted based on the measurement of stone density on CT expressed in Hounsfield units (HU). A linear relationship exists between increased stone density and poor stone fragmentation with a threshold of 1000 HU, above which stones are less likely to be successfully fragmented with SWL.16,17 Two prospective studies reinforced these findings with respect to stone densities greater than 1000 HU and 970 HU.18,19
When measuring HU, it is best to maximally magnify the image on the stone, use bone windows, and draw an ellipse within the stone.
Skin-to-stone distance (SSD)
In addition to providing information on stone size and density, CT scans can also allow for measurement of SSD. Several groups have reported reduced SWL success in patients with a greater SSD and high stone density. A large retrospective Canadian series, including renal and ureteral stones, demonstrated on multivariate analysis that a SSD of greater than 11 cm (OR 0.49, CI 0.31–0.78) and density greater than 900 HU (OR 0.49, CI 0.32–0.75) were significant predictors of SWL failure.20 A second large retrospective review of 1282 SWL treatments also demonstrated on multivariate analysis that SSD greater than 10 cm was associated with lower stone-free rates.21
Recommendation: Stone composition, stone density and skin-to-stone distance should be used when possible to counsel patients regarding the success of SWL treatment for patients presenting with ureteral stones. Known cystine, calcium oxalate monohydrate, and brushite stones are likely best treated with URS. Patients with ureteral stones with a density greater than 1000 HU or SSD greater than 10 cm are more likely to fail SWL and may be better served with URS (Level of Evidence 2b, Grade B).
Optimizing treatment outcomes
Shockwave lithotripsy
Despite the advances in ureteroscopes, holmium laser, and endoscopic instrumentation, SWL remains a first-line treatment modality for ureteral calculi. SWL outcomes can be directly influenced by case selection, surgeon technique, and modifiable parameters to enhance safety and maximize successful outcomes. Most of the data for SWL outcomes is derived from patients with renal (rather than ureteric) calculi, but these findings should be generalizable to ureteric stones, particularly for those in the upper ureter, where renal parenchyma is included in the blast path of the shock wave energy.
Coupling
Coupling of the SWL generator head to the patient in an airtight manner, with minimization of gas and air bubbles in the coupling media, is critical to maximizing energy delivery to the stone. Failure to recognize breaks in coupling can lead to failure of stone fragmentation. Changes in lithotripter design have led to a move away from water bath coupling (as was seen with the original HM3 design) to the use of a smaller coupling interface. Coupling can be influenced by the type of SWL machine, type of gel used at the patient-generator interface (a greater volume of lower-viscosity gel being preferable), method of application of that gel (best to apply to shock head first), and patient factors (patient movement during treatment, lifting of the back off the generator leading to “decoupling” and introducing air bubbles into the coupling interface).22–26
Recommendation: SWL operators should ensure proper patient coupling to reduce air bubbles in the SWL blast path, particularly near the centre of the blast path. Patients should receive adequate anesthesia and analgesia to prevent patient movement and “decoupling” during treatment (Level of Evidence 4, Grade C).
Targeting
Proper stone targeting is vital for SWL success. Whether fluoroscopic or ultrasound targeting is superior is an ongoing debate, and varies with urologist expertise, SWL machine type, and stone composition.27 Real-time, in-line imaging is generally considered superior, however in-line (or coaxial) ultrasound imaging is not available with all units. Respiratory excursion hinders targeting by reducing the time that the stone is within the SWL focal zone, even with ideal targeting. Shock wave triggering based on respiration has been abandoned because of increased treatment time, but compression belts reduce renal movement with respiration. Targeting should be confirmed at regular intervals throughout treatment.28 Greater use of fluoroscopy time can lead to improved outcomes.29,30
Recommendation: SWL targeting (whether fluoroscopic or ultrasonic) should occur at regular intervals throughout the treatment. Compression belts may help reduce renal (and ureteric) excursion with treatment (Level of Evidence 4, Grade C).
Dose escalation/pause
SWL energy should be maximized during treatment in order to maximize stone comminution. This is particularly true for mid and distal ureteral stones, where the renal parenchyma is not included in the blast path and thus the risk of renal injury is negligible. However, particularly for upper ureteric stones, SWL energy should be increased gradually, rather than beginning at maximum energy. This allows for better patient accommodation to the sensation of treatment (when treatment is performed under intravenous sedation). This also reduces renal injury by inducing renal vasoconstriction, which is protective in reducing the rate of renal hematomas.31–35 An alternative strategy is to pre-treat the kidney with a series of low energy shocks and then pause treatment for a short period of time before resuming at higher energy levels.31 Of note, if fragmentation is seen at lower energies it is not necessary to increase the energy any further.
Recommendation: Patients with upper ureteric stones should initially receive low-energy shocks, with gradual voltage escalation up to maximum energy (Level of Evidence 1b and 4, Grade C).
Number of treatments
Not all SWL treatments of ureteric stones will be successful and render the ureter stone free. When treatment is unsuccessful, a decision must be made whether to retreat with SWL or to switch to endourologic treatment (retrograde or antegrade URS). This decision-making is often influenced by the degree of fragmentation with the initial SWL session, and by patient factors (patient preferences, impending travel, importance of being rendered stone free quickly, dislike of ureteric stent and prior patient treatment experience). In general, if SWL fails it can be repeated, but the incremental benefit of more than two SWL treatments for the same ureteric stone is small.36,37 Accordingly, after two unsuccessful SWL treatments, consideration should be given to alternative treatment with ureteroscopy. The optimal time interval between SWL treatments is unclear, but can be as short as within two days for mid and distal ureteric stones.
Recommendation: If SWL is unsuccessful, the urologist may elect to treat the stone a second time with SWL in consultation with the patient. More than two SWL treatments to the same ureteric stone have little incremental benefit and URS should be considered (Level of Evidence 4, Grade C).
Treatment rate
A number of randomized trials have indicated that reducing shock wave rate from 120 shocks/min can improve stone fragmentation, particularly for stones larger than 1 cm.38–43 This may also reduce the degree of renal injury, which may be an issue for upper ureteric stones, but is likely less relevant for mid and distal ureteral stones. Slowing treatment rate does increase treatment times. The optimal treatment rate is not clear; however, studies suggest that SWL at 60 to 90 shocks/ min leads to better fragmentation than 120 shocks/min, particularly for larger stones.43–45 Most studies were performed with renal calculi; however, improved outcomes have been demonstrated for upper ureteric stones as well.39
Recommendation: Patients with upper ureteric stones >1 cm, or stones that have failed prior treatment, should be treated with a SWL rate of less than 120 shocks/min (Level of Evidence 1b, Grade A).
Alpha blockers
Given the initial reports showing a benefit with oral alpha blockers in enhancing the spontaneous passage of ureteral stones, several authors have studied the effect of alpha blockers administered as an adjunct to SWL to improve stone-free rates. Their work has been summarized in two meta-analyses with similar findings. The first in 2009 combined the results of four studies, which randomized patients to receive medical expulsive therapy versus placebo or standard of care post treatment. Two of the four studies used tamsulosin, while one used a calcium channel blocker and the other an herbal agent (phyllanthus niruri).46–50 The use of medical expulsive therapy was associated with a 17% increase in SWL success rates with a number needed to treat of six. A more recent meta-analysis focused solely on the use of alpha blockers post-SWL and had similar findings. The authors identified seven trials that met the inclusion criteria and found that the use of alpha-blockers, which was tamsulosin in all seven studies, improved SWL success by 16%.47,51–57 The authors also reported reduced time to fragment passage, reduced pain, and required less analgesia.
Alpha blockers are well-tolerated, inexpensive, and familiar to urologists. The use of alpha blocker therapy as an adjunct to SWL should result in increased fragment passage and a reduction in the need for repeat SWL or more invasive treatments, such as URS. Additional benefits with respect to less pain and reduced need for analgesic use may also be realized.
Recommendation: Alpha blockers, in particular tamsulosin, should be prescribed to patients after SWL for ureteral stones to improve treatment success rates (Level of Evidence 1a, Grade A).
Number of shocks
The optimal number of shocks to administer has not been definitively established. In principle, urologists must balance treatment efficacy with adverse effects (particularly renal damage). For mid to distal ureteric stones, where the renal parenchyma is not affected by SWL energy, treatment can safely be carried out up to 4000 or more shocks.37 However, the incremental benefit of treating ureteric stones beyond 4000 shocks has not been established. For upper ureteral stones the range is from 2000 to 3500 shocks.37 In general, urologists should follow their lithotripter manufacturer’s recommendations for the optimal maximum number of shocks.
Recommendation: An adequate number of shocks should be administered to ensure adequate treatment of ureteric stones. This number varies based on recommendations from the specific SWL machine manufacturers, but generally ranges from 2000 to 4000 shocks for ureteric stones (Level of Evidence 4–5, Grade D).
Stenting
There is good evidence to show that ureteral stenting is not necessary in SWL58 and does not improve the success rate or passage of fragments.59 In fact, having a stent may impede the passage of fragments following SWL. A trial consisting of patients with ureteral stones between 4 to 10 mm undergoing SWL were randomized to a stent or no stent.60 The stone-free rate was much lower in stented patients (68.6%) than non-stented patients (83.7%, p = 0.026). Consequently, stented patients required significantly more adjuvant procedures to render them stone free compared to non-stented patients. On multivariate analysis, the authors noted that the location of the stone, size of stone and presence of a stent were the three factors that significantly affected stone-free rate. Further supporting this, Argyropoulos and Tolley looked at SWL of ureteral stones with a mean size of 8.5 mm in diameter and found that the stone-free rate in stented patients was significantly lower (71%) compared to those who were not stented (93%).61
In addition, based on the available evidence, stents do not appear to decrease the risk of steinstrasse or infection following SWL.62–64
However, consideration should still be given to placing a stent prior to SWL in patients with a solitary kidney.
Recommendation: Ureteral stents do not improve the stone-free rates in SWL and actually impede the passage of fragments resulting in lower stone free rates (Level of Evidence 1a, Grade A). They should be used prior to SWL to treat obstruction, acute kidney injury, intolerable pain, sepsis, and in those with a solitary kidney. If inserted for sepsis, a course of antibiotics should be given prior to SWL and the patient should not be exhibiting signs of sepsis at the time of treatment (Level of Evidence 5, Grade D). Stents do not decrease the risk of steinstrasse or infection following SWL (Level of Evidence 1a, Grade A).
Ureteroscopy
Lithotrite (laser vs. electrohydraulic vs. pneumatic)
Common methods of intracorporeal ureteroscopic lithotripsy include pneumatic, eletrohydraulic, and Holmium:YAG (Ho:YAG) laser. Treatment of ureteral stones with Ho:YAG lithotripsy is superior (p < 0.05) to pneumatic lithotripsy when comparing stone-free rate (95–98.6% vs. 80–86%),65–68 operative time (15–20 vs. 25–33 mins),68,69 and need for auxiliary treatment, such as SWL or repeat URS (2%–2.5% vs. 14%–17.5%).65,66 When compared with electrohydraulic lithotripsy, Ho:YAG laser lithotripsy was demonstrated to have superior stone-free rates for stones larger than 15 mm (100% vs. 67%) and faster operative time for stones less than 15 mm (72 vs. 102min).70 Available studies are not sufficiently powered to conclude if a significant difference exists in complication rates such a ureteral perforation, stone migration, or delayed ureteric stricture.
Recommendation: Holmium:YAG laser lithotripsy offers superior stone fragmentation, stone free rates and minimizes the need for auxiliary procedures. It should be considered the method of choice for intracorporeal lithotripsy of ureteral stones (Level of Evidence 2b, Grade B).
Ureteral access sheath
The use of a ureteral access sheath (UAS) has traditionally been advocated at the time of flexible URS for renal stones for several reasons, including: (1) facilitating flexible URS by allowing easy multiple entry and re-entry to the upper urinary tract and renal collecting system; (2) decrease in intrarenal pressure, which could potentially diminish kidney injury;71,72 (3) improved irrigation flow thus optimizing vision;71 and (4) the potential to improve stone-free rates by allowing passive egress or active retrieval of fragments. However, the impact of UAS on stone-free rates is unclear, as the evidence is very limited.73–75
The effect of UAS use on the ureter is also unclear. It has been demonstrated in animal models that the UAS can induce transient ureteral ischemia and promote an acute inflammatory response in the ureter.76 Furthermore, a recent prospective study has questioned the safety of the UAS, demonstrating the potential for ureteral wall injury in 46.5% of patients.77 However, no randomized trials exist comparing the incidence of ureteral stricture with and without a UAS. Retrospective studies show no increased risk of stricture formation.
Ultimately and unfortunately, much of the current data with respect to UAS use are limited by the fact they come from non-randomized studies that are largely retrospective in nature with short follow-up. This limits the recommendations that can be made.
Recommendation: Further studies are needed to confirm safety, define cost-effectiveness, and determine the clinical impact of the reduction of ureteral and intrarenal pressures during sheath deployment before any definitive recommendation on the use of the UAS can be made. Nevertheless, the UAS remains a highly useful tool in the armamentarium of the urologist during flexible URS (Level of Evidence 4, Grade C).
Ureteroscope size
The outer tip diameter of ureteroscopes typically varies between 4.5 and 8.5Fr. for semi-rigid ureteroscopes and 6.75 to 8.7Fr. for flexible ureteroscopes. Recently, digital flexible ureteroscopes have come into more widespread use providing excellent visualization, but some have a larger diameter (8.7Fr. tip with 9.9Fr. shaft), which can make insertion into the non-dilated ureter more difficult. Furthermore, the durability of flexible digital ureteroscopes compared to fibreoptic ureteroscopes remains to be seen.
Semi-rigid ureteroscopes
Semi-rigid (SR) ureteroscopes represent the mainstay for treating most ureteric stones in light of the superior optics, excellent irrigant flow and size of the working channel. Stone-free rates are equivalent between small SR ureteroscopes (4.5–7.5Fr tip) and larger SR ureteroscopes (8.5–10Fr tip).78,79 Larger SR ureteroscopes may require more ureteric dilation and increase minor complications of mucosal abrasion or postoperative hematuria.79
Flexible ureteroscopes
Flexible fibreoptic ureteroscopes range in size from 7.4 to 9.0Fr in diameter and have a progressively higher rate of insertion failure into the undilated ureteral orifice, increasing from 0.9% to 37%, respectively, dependent on size.80 With the introduction of flexible digital ureteroscopes, with typical tip diameters of 8.4 to 8.7Fr broadening to a 9.9Fr shaft, there is an increasing need for ureteral orifice dilation and access sheath use.81 In addition, the greater diameter may result in a higher likelihood of being unable to access and treat stones in the proximal ureter or renal pelvis/calyces. However, based on the available studies comparing larger digital flexible ureteroscopes with fibreoptic flexible ureteroscopes, the larger diameter did not affect stone-free rates and the digital ureteroscope resulted in shorter operative times.82,83
Recommendations: Within the range of commercially available semi-rigid and flexible ureteroscopes, the available evidence suggests stone-free rates and complication rates are similar. When available, use of smaller ureteroscopes may lessen the need for ureteral dilation and slightly reduce minor postoperative complications such as hematuria (Level of Evidence 4, Grade C).
Stenting
Pre-stenting prior to ureteroscopy
Ureteral stents are often placed at the completion of a ureteroscopic case. However, this discussion addresses “prestenting” of a patient prior to a planned URS. Ureteral stents are known to provide drainage, as well as passively dilate the ureter. Accordingly, pre-stenting prior to URS has been shown to ease the insertion of ureteroscopes and UAS. Prestenting did not affect stone-free rates in patients with stones less than 1 cm, but in patients with stones greater than 1 cm, the stone-free rate was significantly better (95.8%) after a single treatment.84 The same study also performed a cost analysis and in those patients with a stone greater than 1 cm, there was a decrease in overall costs to successfully treat the patient from $27 806 (not pre-stented) to $17 706 (pre-stented). Pre-stented patients required less adjuvant procedures to render them stone free, which accounted for the cost savings. Another study found that pre-stented patients had significantly higher stone free rates for ureteral stones 5 mm or greater (99% vs. 90%, p = 0.048).85 There was no difference in stone-free rates for ureteral stones smaller than 5 mm and no difference in complication rates for stones of any size.
Pre-stenting can also be effective in situations where the ureter is narrow and insertion of a UAS or ureteroscope is difficult. In these instances, placing a ureteral stent to help passively dilate the ureter and re-attempting URS at a later date is highly recommended to improve the rate of ureteral access and reduce the rate of complications. Balloon dilation and URS has been shown to be safe and effective in one sitting, but it must be recognized that if this does not work, stenting and performing URS after passive dilation is necessary.86
In a tertiary referral centre examining 119 consecutive patients, the rate of failure to access leading to a ureteral stent with delayed URS was 8%.87 A study of 41 patients with this scenario showed that 71% underwent secondary URS with ease, while 12 patients (29%) had continued resistance.88 Of the 12 patients with continued resistance, nine underwent URS in the secondary setting and two of these patients subsequently developed a ureteral stricture. Overall, in this series of patients who underwent stenting for initial resistance in passing a ureteroscope, 98% had successful subsequent URS. Care should be taken to avoid continuing despite resistance as this can lead to subsequent ureteral stricture, particularly with one-step dilation using a UAS.77 Pre-stenting before the use of a UAS decreased the rate of complication by sevenfold in this particular study.77 Preoperative discussion in consenting patients should include the potential of failed access, placement of a ureteral stent, and delayed URS at another date.
Stenting post ureteroscopy
Stenting post-ureteroscopy is not always necessary. The first description and randomized trial of stent versus stentless URS were both performed in Canada. Hosking and colleagues were the first to describe stentless URS in 93 patients undergoing URS for distal ureteral stones without any further intervention or requirement for subsequent stents or nephrostomy tubes.89 Denstedt and colleagues performed the first prospective randomized trial of stent versus no stent following URS.90 At one week following URS, patients without a stent had significantly less flank pain, abdominal pain, and dysuria compared to stented patients. There were no complications in those who did not have a stent. A subsequent meta-analysis showed an absolute lower risk of complications in those patients who were stented; however, this became insignificant on multivariate-analysis.91 Many other studies have shown no complications and less symptoms in those who did not receive a ureteral stent.92 Stenting after uncomplicated URS did not alter the stone-free rate, complications, urinary tract infection, unplanned medical visits, or fever.
Even when the ureteric orifice has been balloon-dilated to 18Fr, stenting has not been shown to be beneficial. A randomized trial of 144 stented and 142 non-stented patients following rigid URS in which all patients were dilated to 18Fr was undertaken.93 These researchers found no differences in complications or strictures; however, they did find that stented patients had more irritative voiding symptoms (dysuria and urgency).
Another study undertaking SR URS using pneumatic lithotripsy showed that stented patients actually did better than non-stented patients.94 Non-stented patients were more than twice as likely to visit the emergency department following discharge and they also took a longer time to discharge from the hospital on the day of surgery. Narcotic use was also significantly higher in the non-stented group in the first five days after surgery. This study showed evidence that stented patients were actually more comfortable and required less medical attention and narcotics following URS.
If bilateral URS is performed, depending on the situation, consideration should be given to stenting at least one side, to prevent the possibility of bilateral ureteric obstruction postoperatively.
Stenting following use of a UAS
If a UAS is used during URS, there is good data to support placing a ureteral stent in those cases. In a retrospective study of 102 patients, where 51 had no stent following UAS use and the other half were stented, stented patients were less likely to have unscheduled emergency visits and had lower pain scores compared to their non-stented counterparts.95 This was also corroborated by Canadian data where patients who did not have a ureteral stent were more likely to have an emergency room visit in the postoperative period (37% vs. 14%, p = 0.04).96
Duration of stenting
There is no prescribed indwelling time to leave a ureteral stent. The literature is scant regarding this issue. One study retrospectively analyzed 125 patients and found that stents that remained in less than 14 days had less adverse events, such as fever and lumbago, and the authors advocated less than two weeks of stenting following uncomplicated URS.97
Recommendation: Stenting following uncomplicated URS is still a controversial topic and there is evidence to support both sides. There is good evidence that ureteral stents should be left in place following use of a ureteral access sheath with URS. Stenting does not affect stone-free rates or long-term complications such as strictures, but may result in less emergency room visits and narcotic use in the postoperative period. Stenting prior to URS is helpful to improve stone-free rates in stones greater than 1 cm. Stenting prior to URS also facilitates access to the ureter due to passive dilation (Level of Evidence 2a–2b, Grade B).
Special considerations
Pregnancy
No Level 1 Evidence exists regarding the treatment of ureteral stones during pregnancy. Retrospective case series provide some guidance on how to manage this situation. The first diagnostic step in suspected nephrolithiasis during pregnancy should be ultrasound due to the lack of radiation; however, ultra-low dose CT or magnetic resonance imaging (MRI) are good alternatives with very little or no radiation.98,99 A special protocol involving magnetic resonance urography (MRU) involves a half Fourier single-shot turbo spin-echo (HASTE), which is better at imaging ureteral stones than other MRU protocols.100
Most ureteral stones will pass spontaneously and the first option in management is conservative therapy, including hydration and analgesia.101 In a recent study, conservative management was successful in 67% of patients, who had symptomatic obstructing ureteral stones with an average stone size of 8.8 mm.102 Immediate causes for intervention are the same as those in non-pregnant situations (signs of sepsis, renal failure, and unrelenting pain, etc.), but also include induction of premature labour (contractions, fetal distress) in the pregnant patient.103 The most immediate method of intervention is nephrostomy tube or ureteral stent insertion.
Failing conservative management, ureteroscopic treatment of stones using laser lithotripsy with either flexible or SR URS is feasible and safe.104 In fact, if ultrasound imaging is non-diagnostic and low-dose CT or MRI is unavailable, URS can also be used for both diagnostic and therapeutic purposes.102 A number of studies have demonstrated that URS is a viable technique to treat stones in pregnancy.102,105,106 Postoperative stenting following URS in this situation is recommended in an attempt to reduce postoperative complications.102 Ideally ureteroscopic treatment should be performed in the second trimester, as teratogenic effects and risks of anaesthesia are higher in the first trimester.103
With regards to intraoperative imaging, if URS or ureteral stent insertion is undertaken, then a lead apron or shield should be put between the x-ray fluoroscopy source and the fetus to shield it from radiation.107 These authors describe inverting the fluoroscopy C-arm so that the energy source is above the supine patient and placing two thyroid collars on the anterior portion of the patient’s abdomen to shield the fetus. This can also be achieved by placing the lead shield or apron underneath the patient if the C-arm energy source comes from below the table; this method also reduces radiation and scatter to operating theatre personnel. Alternatively, URS or ureteral stent insertion can be performed under ultrasound guidance alone, avoiding radiation exposure.
Pregnancy is a contraindication to SWL and although there have been reports of the inadvertent treatment of pregnant patients with SWL, with no adverse sequelae to the fetus,108 it should be avoided. Similarly, percutaneous nephrolithotomy (PCNL), if necessary, should be delayed until after birth as the procedure requires prolonged anaesthesia and radiation exposure.
Recommendation: First-line diagnostic testing for stones in pregnancy is ultrasound, but low-dose CT or MRI can also be used. In some instances, URS can also be diagnostic, as well as therapeutic. Obstructing ureteral stones are typically managed conservatively in the absence of fever, leukocytosis or positive urine culture. In those patients presenting with signs of sepsis, antibiotics and urinary decompression via a nephrostomy tube or ureteral stent are of primary importance. Definitive therapy should be delayed until the infection is treated. URS and laser lithotripsy is safe in pregnancy; however SWL and PCNL are contraindicated in pregnancy (Level of Evidence Level 4, Grade C).
Anti-coagulation
There is little literature regarding surgical management of stone patients with coagulopathies or on anticoagulation therapy. SWL, laparoscopic, percutaneous and open surgeries are contraindicated in these patients.109,110 This is because there is 20- to 40-fold increased risk of peri-renal hematomas and hemorrhagic complications in patients with uncorrected coagulopathies undergoing SWL when compared with patients with a normal bleeding profile.111,112 Therefore, in consultation with a haematologist or a cardiologist, bleeding coagulopathies need to be corrected and anticoagulation therapy appropriately withheld perioperatively.113 In addition, patients with increased risk of thromboembolic disease could be managed by bridging with subcutaneous low molecular weight heparin, while oral anticoagulation is held.114 In a retrospective series of 27 anti-coagulated patients who underwent PCNL with bridging, the stone-free rate was 93% while 7% developed significant bleeding and 4% had thromboembolic complications.115 The only prospective SWL study of patients on anti-platelet agents is that of Zanetti and colleagues.116 In this level 2b study, 23 patients were stratified to being at low-risk or at high-risk of thromboembolic events. Low-risk patients had their antiplatelet agents withheld for eight days prior to SWL, whereas high-risk patients received unfractionated heparin 5000 units thrice daily while anti-platelet agents were held. In both groups anti-platelet agents were re-started within 10 to 14 days of withdrawal and patients were followed with abdominal ultrasound and serial hemoglobin/hematocrit measurements. There were no hematomas or thromboembolic events in either group.116
Recent advances in manufacturing small-calibre ureteroscopes and introduction of Ho:YAG laser energy in lithotripsy have made it possible for patients with coagulopathies to safely undergo URS and laser lithotripsy while anticoagulated.110,117–119 However, this is associated with lower stone-free rates and increased risk of postoperative gross hematuria necessitating admission and bladder irrigation.111,120 Therefore, risks and benefits of withholding anticoagulation or proceeding with URS while anti-coagulated should be discussed with the patient and his/her cardiologist or hematologist.
Recommendations: SWL and PCNL are contraindicated in patients with uncorrected coagulopathies. When possible, coagulopathies should be corrected after consulting with a cardiologist and/or hematologist. However, when risks of withholding anti-coagulants outweigh the benefits, proceeding with URS and laser lithotripsy, while anti-coagulated, is an acceptable option (Level of Evidence 2b, Grade B).
Urinary diversion
Urinary diversions can be classified anatomically into abdominal (such as ileal conduits and catheterizable pouches), urethral (such as orthotopic neobladder) and ureterosigmoidostomy, with ileal conduit representing most (84%) of cases.121,122
Patients with urinary diversions are at high risk of stone formation in light of numerous risk factors, including metabolic abnormalities (i.e., metabolic acidosis, hypocitraturia, hyperoxaluria and hypercalciuria), recurrent infections with urease-splitting organisms (e.g., Proteus), prolonged urinary stasis, prolonged exposure of urine to non-absorbable materials (e.g., staples), anatomical changes following diversion, and reflux of mucous into the upper tract.123
The reported incidence of upper tract calculi in patients with urinary diversion is 1% to 11% depending on the type of diversion, uretero-intestinal anastmosis and the follow-up period. The most common stone types are magnesium ammonium phosphate (struvite) and calcium phosphate stones.124
The established anatomical changes in these patients necessitate accurate preoperative assessment by CT scan to determine whether there are overlying bowel loops, especially if percutaneous access is contemplated.125 Ultrasound-guided access is recommended in these cases to avoid intervening bowel loops.126
Dealing with the stones in these patients represents a challenge to the urologist. Many factors need to be considered when choosing a certain approach. These factors include: stone size, location, patient performance status, availability of advanced SWL machines and flexible ureteroscopes with Ho:YAG laser lithotripsy, and finally, surgeon experience in dealing with these structural changes in the urinary tract.127 In addition, PCNL in these patients is associated with higher rates of postoperative fever or sepsis (8% vs. 0%, p < 0.05) and higher rates of second-look nephroscopy (36% vs. 16%, p < 0.05) compared to those with normal anatomy.126
Minimally invasive modalities, such as SWL, URS and PCNL, that produce the best stone-free rates should be used in these patients since these would avoid open surgical management and are associated with lower morbidity, early convalescence and shorter hospital stay.124 When performing antegrade URS for management of ureteral stones, a ureteral access sheath can be placed in an antegrade fashion to improve irrigation and facilitate access with a flexible ureteroscope.122
Close follow-up in these patients is mandatory because the risk of re-growth and recurrence is as high as 63% at the 5-year follow-up.128 There are many important factors that may prevent or at least prolong the period before recurrence. These include increased fluid intake, timed voiding or frequent clean intermittent catheterization, and frequent irrigation of reservoirs.129 Metabolic workup to identify correctable risk factors, medical management of metabolic consequences of diversion, and long-term antibiotic prophylaxis against recurrent infections are also important to reduce recurrence.125
Also of importance, the urologist should consider the possibility of anastomotic stricture or recurrent malignancy when stones are lodged near the uretero-enteric anastomosis.
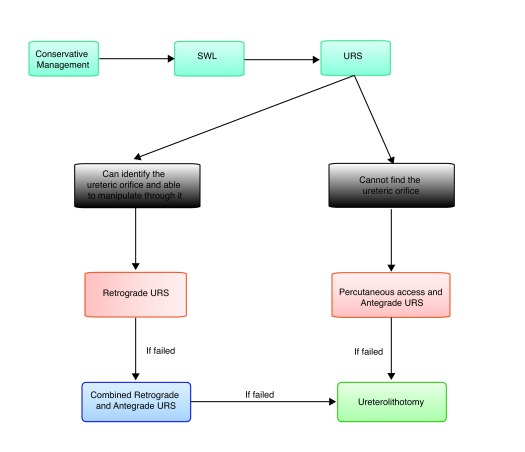
Recommendations: Ureteral calculi in patients with urinary diversions are challenging. While small, non-obstructive, asymptomatic stones could be managed conservatively, SWL could be attempted for obstructive stones (Level of Evidence 4, Grade C). If SWL fails, then retrograde URS with laser lithotripsy could be attempted if the ureteral orifice can be accessed (Fig. 1). However, the most effective modality for clearing large obstructive ureteral stones is percutaneously through antegrade URS (Level of Evidence 4, Grade C). It should be noted, however, that percutaneous renal surgery in patients with urinary diversion is associated with higher rates of postoperative sepsis and higher rates of second-look nephroscopy (Level of Evidence 2b, Grade B). When percutaneous procedures fail, ureterolithotomy is the last option in these patients (Level of Evidence 4, Grade C).
Fig. 1.
Algorithm for approaching ureteral calculi in patients with urinary diversions.
Antegrade URS and ureterolithotomy
In select circumstances, a percutaneous antegrade approach may be necessary instead of a retrograde endoscopic approach. As discussed above, prior urinary diversion represents one of these situations. Antegrade URS can also be considered a treatment option in the following situations: (1) in select cases with a large, impacted proximal ureteral stones; (2) when performed in conjunction with renal stone removal; (3) in select cases following failure of a retrograde ureteroscopic attempt for a large, impacted proximal ureteral stone;130 and (4) when the ureteral stone is in a transplant kidney.131
For large (>15 mm), impacted proximal ureteral stones, the stone-free rate with antegrade URS ranges between 98.5% and 100% with a low risk for complications.130,132–136
Laparoscopic, robotic or open ureterolithotomy may be considered when ureteroscopic and percutaneous procedures have failed or concomitant surgery is required.137
Recommendations: Percutaneous antegrade URS should be considered in the treatment of stones in patients with urinary diversion and select large, impacted proximal ureteral stones, especially when prior retrograde URS has failed (Level of Evidence 4, Grade C). Ureterolithotomy is a salvage option when endoscopic procedures have failed (Level of Evidence 2b, Grade B).
Uric acid stones
Uric acid (UA) urolithiasis is a multi-factorial disease. Persistently low 24-hour urinary pH (≤5.5) is regarded as the most important factor in formation of UA stones. UA stones constitute about 10% of urolithiasis in the general population, but this percentage increases up to 34 % of stones in patients with metabolic syndrome and up to 52.2% of stones in patients with gout.138,139 UA stones are typically radiolucent on plain radiographs and of low attenuation values (<500 HU) on non-contrast CT scans of the abdomen.140 Recently, dual-energy CT scanning has been found to be superior to the conventional “single-energy” non-contrast CT scanning in differentiating non-uric acid stones from UA stones.141 However, dual-energy CT scans are not widely available.
Laboratory evaluation should include serum creatinine, potassium, uric acid, and when renal colic subsides, 24-hour urine collection should be obtained and checked for urine volume, urinary pH and uric acid excretion.142
When there are no signs of impending renal failure or sepsis, treatment of UA calculi depends primarily on increased water intake, reduction in the consumption of non-diary animal protein (low purine diet), and urinary alkalinisation using alkalinising agents, such as potassium citrate or sodium bicarbonate leading to stone dissolution.138,142–144 When these agents are given to patients after ESWL or PCNL, they facilitate the dissolution of residual stones <4 mm and prevent stone re-growth and recurrence.145 In addition, the combination of alpha blockers (such as tamsulosin) and potassium citrate have been associated with significantly higher stone-free rates when compared with either therapy alone or with placebo in patients with distal ureteric UA stones (84.4%, 68.8%, 58.7%, and 26.1%, respectively).146
Although UA stones are easy to fragment by SWL, they are difficult to localize due to their radiolucency. Contrast media injection, either through the intravenous route, retrograde ureteropyelography through a ureteral catheter, or antegrade administration through a nephrostomy tube, can be used to localize the radiolucent UA stones. The latest generation of lithotripters come equipped with ultrasonographic targeting, which could be used to localize UA stones. URS and laser lithotripsy are also very effective at treating UA stones in the ureter.
Recommendations: UA stones should be suspected when the stone is radiolucent on plain radiograph, the density is <500 HU on non-contrast CT scan, and it is associated with acidic urine (pH ≤5.5) (Level of Evidence 2b, Grade B). Alkalinization with potassium/sodium citrate or sodium bicarbonate can be used in conjunction with medical expulsive therapy, such as tamsulosin, or endourologic procedures, such as SWL, URS or PCNL, to increase stone-free rates of UA stones (Level of Evidence 1b, Grade A).
Infected obstructing ureteral stones
The basic tenet of treating any infected area or abscess is drainage followed by antibiotics. An obstructing ureteral stone in the setting of infection constitutes a requirement for urgent urologic treatment. Drainage of the obstructed renal unit is paramount and can be performed either by insertion of a ureteral stent or a percutaneous nephrostomy tube. It is generally agreed that definitive treatment of the obstructing stone should not be undertaken until the system has been drained, adequate antibiotics have been administered, and the infection treated. In the only prospective randomized trial, patients presenting with a fever >38°C, leukocytosis, and obstructing stone smaller than 15 mm were randomized to receive a ureteral stent or a nephrostomy tube insertion via interventional radiology.147 There was no difference in time to defervescence, hospital stay, resolution of obstruction, or overall clinical improvement. Others have also found no difference between decompression via nephrostomy or ureteral stent.148–150
The method of decompression should be tailored to each centre and its available resources. Placement of a nephrostomy tube is not on the list of core competencies for all radiologists and therefore may not be available at all centres. One survey found that only 44% of hospitals in the United Kingdom are capable of nephrostomy tube insertion.151 Ureteral stenting should be more widely available, but does require cystoscopy or an operating theatre setting, fluoroscopy or ultrasound, and trained staff. Broad spectrum antibiotics should be started early upon diagnosis; when starting within 1 hour of diagnosis, the survival rate is greater than 80% and each hour delay results in decreased survival (8% per hour).152
Recommendation: Obstructing ureteral stones resulting in urosepsis and infection require emergent drainage. The two methods of decompression, ureteral stenting or nephrostomy tube placement, are equivalent in outcomes and the method chosen will depend on availability of resources at each particular hospital. It is important to start broad-spectrum antibiotics early. Definitive stone treatment should be delayed until decompression and adequate antibiotics have been administered to treat the infection (Level of Evidence Level 2b, Grade B).
Footnotes
Competing interests: Dr. Ordon, Dr. Andonian, Dr. Schuler and Dr. Blew declare no competing financial or personal interests. Dr. Chew is a member of the Advisory Boards for Boston Scientific, Cook Medical, Olympus, Poly-Med, Advatec Inc., and PercSys Inc. He is also participating in clinical trials with Poly-Med Inc., and Advatec Inc. Dr. Pace is a member of the Advisory Board for Boston Scientific.
This paper has been peer-reviewed.
References
- 1.Matlaga BR, Lingeman JE. Surgical management of upper urinary tract calculi. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 10th ed. Philadelphia: PA: Elsevier Saunders; 2012. pp. 1357–410. [DOI] [Google Scholar]
- 2.Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: A guide for patient education. J Urol. 1999;162:688–91. doi: 10.1097/00005392-199909010-00014. [DOI] [PubMed] [Google Scholar]
- 3.Hubner WA, Irby P, Stoller ML. Natural history and current concepts for the treatment of small ureteral calculi. Eur Urol. 1993;24:172. doi: 10.1159/000474289. [DOI] [PubMed] [Google Scholar]
- 4.Preminger GM, Tiselius HG, Assimos DG, et al. 2007 Guideline for the management of ureteral calculi. Eur Urol. 2007;52:1610–31. doi: 10.1016/j.eururo.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002;178:101–3. doi: 10.2214/ajr.178.1.1780101. [DOI] [PubMed] [Google Scholar]
- 6.Berkovitz N, Simanovsky N, Katz R, et al. Coronal reconstruction of unenhanced abdominal CT for correct ureteral stone size classification. Eur Radiol. 2010;20:1047–51. doi: 10.1007/s00330-009-1636-7. [DOI] [PubMed] [Google Scholar]
- 7.Ray AA, Ghiculete D, Pace KT, et al. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76:295–300. doi: 10.1016/j.urology.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;4:CD008509. doi: 10.1002/14651858.cd008509.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Pickard R, Starr K, MacLennan G, et al. Medical expulsive therapy in adults with ureteric colic: A multicentre, randomised, placebo-controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60933-3. [DOI] [PubMed] [Google Scholar]
- 10.Aboumarzouk OM, Kata SG, Keeley FX, et al. Extracorporeal shock wave lithotripsy (ESWL) versus ureteroscopic management for ureteric calculi. Cochrane Database Syst Rev. 2012;5:CD006029. doi: 10.1002/14651858.cd006029.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matlaga BR, Jansen JP, Meckley LM, et al. Treatment of ureteral and renal stones: A systematic review and meta-analysis of randomized, controlled trials. J Urol. 2012;188:130–7. doi: 10.1016/j.juro.2012.02.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saw KC, Lingeman JE. Lesson 20: Management of Calyceal Stones. 1999:154–59. [Google Scholar]
- 13.Lee TT, Elkoushy MA, Andonian S. Are stone analysis results different with repeated sampling? Can Urol Assoc J. 2014;8:E317–22. doi: 10.5489/cuaj.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matlaga BR, Kawamoto S, Fishman E. Dual source computed tomography: A novel technique to determine stone composition. Urology. 2008;72:1164–8. doi: 10.1016/j.urology.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Boll DT, Patil NA, Paulson EK, et al. Renal stone assessment with dual-energy multidetector CT and advanced postprocessing techniques: Improved characterization of renal stone composition--pilot study. Radiology. 2009;250:813. doi: 10.1148/radiol.2503080545. [DOI] [PubMed] [Google Scholar]
- 16.Gupta NP, Ansari MS, Kesarvani P, et al. Role of computed tomography with no contrast medium enhancement in predicting the outcome of extracorporeal shock wave lithotripsy for urinary calculi. BJU Int. 2005;95:1285–8. doi: 10.1111/j.1464-410X.2005.05520.x. [DOI] [PubMed] [Google Scholar]
- 17.Joseph P, Mandal AK, Singh SK, et al. Computerized tomography attenuation value of renal calculus: Can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J Urol. 2002;167:1968–71. doi: 10.1016/S0022-5347(05)65064-1. [DOI] [PubMed] [Google Scholar]
- 18.El-Nahas AR, El-Assmy AM, Mansour O, et al. A prospective multivariate analysis of factors predicting stone disintegration by extracorporeal shock wave lithotripsy: The value of high-resolution noncontrast computed tomography. Eur Urol. 2007;51:1688–94. doi: 10.1016/j.eururo.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 19.Ouzaid I, Al-qahtani S, Dominique S, et al. A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): Evidence from a prospective study. BJU Int. 2012;110:E438–42. doi: 10.1111/j.1464-410X.2012.10964.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiesenthal JD, Ghiculete D, Ray AA, et al. A clinical nomogram to predict the successful shock wave lithotripsy of renal and ureteral calculi. J Urol. 2011;186:556–62. doi: 10.1016/j.juro.2011.03.109. [DOI] [PubMed] [Google Scholar]
- 21.Patel T, Kozakowski K, Hruby G, et al. Skin to stone distance is an independent predictor of stone-free status following shockwave lithotripsy. J Endourol. 2009;23:1383. doi: 10.1089/end.2009.0394. [DOI] [PubMed] [Google Scholar]
- 22.Pishchalnikov YA, Neucks JS, VonDerHaar RJ, et al. Air pockets trapped during routine coupling in dry head lithotripsy can significantly decrease the delivery of shock wave energy. J Urol. 2006;176:2706. doi: 10.1016/j.juro.2006.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain A, Shah TK. Effect of air bubbles in the coupling medium on efficacy of extracorporeal shock wave lithotripsy. Eur Urol. 2007;51:1680. doi: 10.1016/j.eururo.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 24.Neucks JS, Pishchalnikov YA, Zancanaro AJ, et al. Improved acoustic coupling for shock wave lithotripsy. Urol Res. 2008;36:61. doi: 10.1007/s00240-007-0128-y. [DOI] [PubMed] [Google Scholar]
- 25.Bergsdorf T, Chaussy C, Thüroff S. Energy coupling in extracorporeal shock wave lithotripsy—the impact of coupling quality on disintegration efficacy. J Endourol. 2008;22:A161. [Google Scholar]
- 26.Bohris C. Quality of coupling in ESWL significantly affects the disintegration capacity—how to achieve good coupling with ultra-sound gel. In: Chaussy, Haupt G, Jocham D, et al., editors. Therapeutic energy applications in urology II: standards and recent developments. Stuttgart, Germany: Thieme; 2010. pp. 61–4. [Google Scholar]
- 27.Bohris C, Bayer T, Gumpinger R. Ultrasound monitoring of kidney stone extracorporeal shockwave lithotripsy with an external transducer: Does fatty tissue cause image distortions that affect stone comminution? J Endourol. 2010;24:81–8. doi: 10.1089/end.2009.0158. [DOI] [PubMed] [Google Scholar]
- 28.Cleveland RO, Anglade R, Babayan RK. Effect of stone motion on in vitro comminution efficiency of Storz Modulith SLX. J Endourol. 2004;18:629–33. doi: 10.1089/end.2004.18.629. [DOI] [PubMed] [Google Scholar]
- 29.Logarakis NF, Jewett MA, Luymes J, et al. Variation in clinical outcome following shock wave lithotripsy. J Urol. 2000;163:721–5. doi: 10.1016/S0022-5347(05)67791-9. [DOI] [PubMed] [Google Scholar]
- 30.Hartung A, Schwarze W. LithoSpace by AST GmbH. In: Chaussy C, Haupt G, Jocham D, et al., editors. Therapeutic energy applications in urology II: standards and recent developments. Stuttgart, Germany: Thieme; 2010. pp. 53–6. [Google Scholar]
- 31.McAteer JA, Evan AP, Williams JC, Jr, et al. Treatment protocols to reduce renal injury during shock wave lithotripsy. Curr Opin Urol. 2009;19:192–5. doi: 10.1097/MOU.0b013e32831e16e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert EH, Walsh R, Moreno MW, et al. Effect of escalating versus fixed voltage treatment on stone comminution and renal injury during extracorporeal shock wave lithotripsy: A prospective randomized trial. J Urol. 2010;183:580–4. doi: 10.1016/j.juro.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Willis LR, Evan AP, Connors BA, et al. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006;17:663–73. doi: 10.1681/ASN.2005060634. [DOI] [PubMed] [Google Scholar]
- 34.Weizer AZ, Zhong P, Preminger GM. New concepts in shock wave lithotripsy. Urol Clin North Am. 2007;34:375–82. doi: 10.1016/j.ucl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Seemann O, Rassweiler J, Chvapil M, et al. The effect of single shock waves on the vascular system of artificially perfused rabbit kidneys. J Stone Dis. 1993;5:172. [PubMed] [Google Scholar]
- 36.Pace KT, Weir MJ, Tariq N, et al. Low success rate of repeat shock wave lithotripsy for ureteral stones after failed initial treatment. J Urol. 2000;164:1905. doi: 10.1016/S0022-5347(05)66914-5. [DOI] [PubMed] [Google Scholar]
- 37.Rassweiler JJ, Knoll T, Kohrmann KU, et al. Shock wave technology and application: An update. Eur Urol. 2011;59:784–96. doi: 10.1016/j.eururo.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pace KT, Ghiculete D, Harju M, et al. Shock wave lithotripsy at 60 or 120 shocks per minute: A randomized, double-blind trial. J Urol. 2005;174:595–9. doi: 10.1097/01.ju.0000165156.90011.95. [DOI] [PubMed] [Google Scholar]
- 39.Honey RJ, Schuler TD, Ghiculete D, et al. A randomized, double-blind trial to compare shock wave frequencies of 60 and 120 shocks per minute for upper ureteral stones. J Urol. 2009;182:1418–23. doi: 10.1016/j.juro.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Davenport K, Minervini A, Keoghane S, et al. Does rate matter? The results of a randomized controlled trial of 60 versus 120 shocks per minute for shock wave lithotripsy of renal calculi. J Urol. 2006;176:2055–8. doi: 10.1016/j.juro.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Madbouly K, El-Tiraifi AM, Seida M, et al. Slow versus fast shock wave lithotripsy rate for urolithiasis: A prospective randomized study. J Urol. 2005;173:127–30. doi: 10.1097/01.ju.0000147820.36996.86. [DOI] [PubMed] [Google Scholar]
- 42.Yilmaz E, Batislam E, Basar M, et al. Optimal frequency in extracorporeal shock wave lithotripsy: Prospective randomized study. Urology. 2005;66:1160–4. doi: 10.1016/j.urology.2005.06.111. [DOI] [PubMed] [Google Scholar]
- 43.Li K, Lin T, Zhang C, et al. Optimal frequency of shock wave lithotripsy in urolithiasis treatment: A systematic review and meta-analysis of randomized controlled trials. J Urol. 2013;190:1260–7. doi: 10.1016/j.juro.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 44.Kato Y, Yamaguchi S, Hori J, et al. Improvement of stone comminution by slow delivery rate of shock waves in extracorporeal lithotripsy. Int J Urol. 2006;13:1461–5. doi: 10.1111/j.1442-2042.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- 45.Chacko J, Moore M, Sankey N, et al. Does a slower treatment rate impact the efficacy of extracorporeal shock wave lithotripsy for solitary kidney or ureteral stones? J Urol. 2006;175:1370–4. doi: 10.1016/S0022-5347(05)00683-X. [DOI] [PubMed] [Google Scholar]
- 46.Schuler TD, Shahani R, Honey RJ, et al. Medical expulsive therapy as an adjunct to improve shockwave lithotripsy outcomes: A systematic review and meta-analysis. J Endourol. 2009;23:387–93. doi: 10.1089/end.2008.0216. [DOI] [PubMed] [Google Scholar]
- 47.Bhagat SK, Chacko NK, Kekre NS, et al. Is there a role for tamsulosin in shock wave lithotripsy for renal and ureteral calculi? J Urol. 2007;177:2185–8. doi: 10.1016/j.juro.2007.01.160. [DOI] [PubMed] [Google Scholar]
- 48.Gravina GL, Costa AM, Ronchi P, et al. Tamsulosin treatment increases clinical success rate of single extracorporeal shock wave lithotripsy of renal stones. Urology. 2005;66:24–8. doi: 10.1016/j.urology.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Porpiglia F, Destefanis P, Fiori C, et al. Role of adjunctive medical therapy with nifedipine and deflazacort after extracorporeal shock wave lithotripsy of ureteral stones. Urology. 2002;59:835–8. doi: 10.1016/S0090-4295(02)01553-4. [DOI] [PubMed] [Google Scholar]
- 50.Micali S, Sighinolfi MC, Celia A, et al. Can Phyllanthus niruri affect the efficacy of extracorporeal shock wave lithotripsy for renal stones? A randomized, prospective, long-term study. J Urol. 2006;176:1020–2. doi: 10.1016/j.juro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Duijvesz D, Rovers MM, et al. Alpha-nlockers to assist stone clearance after extracorporeal shock wave lithotripsy: A meta-analysis. BJU Int. 2010;106:256–61. doi: 10.1111/j.1464-410X.2009.09014.x. [DOI] [PubMed] [Google Scholar]
- 52.Gravas S, Tzortzis V, Karatzas A, et al. The use of tamsulozin as adjunctive treatment after ESWL in patients with distal ureteral stone: Do we really need it? Results from a randomised study. Urol Res. 2007;35:231–5. doi: 10.1007/s00240-007-0106-4. [DOI] [PubMed] [Google Scholar]
- 53.Kupeli B, Irkilata L, Gurocak S, et al. Does tamsulosin enhance lower ureteral stone clearance with or without shock wave lithotripsy? Urology. 2004;64:1111–5. doi: 10.1016/j.urology.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Micali S, Grande M, Sighinolfi MC, et al. Efficacy of expulsive therapy using nifedipine or tamsulosin, both associated with ketoprofene, after shock wave lithotripsy of ureteral stones. Urol Res. 2007;35:133–7. doi: 10.1007/s00240-007-0085-5. [DOI] [PubMed] [Google Scholar]
- 55.Naja V, Agarwal MM, Mandal AK, et al. Tamsulosin facilitates earlier clearance of stone fragments and reduces pain after shockwave lithotripsy for renal calculi: Results from an open-label randomized study. Urology. 2008;72:1006–11. doi: 10.1016/j.urology.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi M, Naya Y, Kino M, et al. Low dose tamsulosin for stone expulsion after extracorporeal shock wave lithotripsy: Efficacy in Japanese male patients with ureteral stone. Int J Urol. 2008;15:495–8. doi: 10.1111/j.1442-2042.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang HJ, Liu K, Ji ZG, et al. Application of Alpha1-adrenergic antagonist with extracorporeal shock wave lithotripsy for lower ureteral stone [in Chinese] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008;30:506. [PubMed] [Google Scholar]
- 58.Musa AA. Use of double-J stents prior to extracorporeal shock wave lithotripsy is not beneficial: Results of a prospective randomized study. Int Urol Nephrol. 2008;40:19–22. doi: 10.1007/s11255-006-9030-8. [DOI] [PubMed] [Google Scholar]
- 59.Pettenati C, El Fegoun AB, Hupertan V, et al. Double J stent reduces the efficacy of extracorporeal shock wave lithotripsy in the treatment of lumbar ureteral stones. Cent European J Urol. 2013;66:309. doi: 10.5173/ceju.2013.03.art14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sfoungaristos S, Polimeros N, Kavouras A, et al. Stenting or not prior to extracorporeal shockwave lithotripsy for ureteral stones? Results of a prospective randomized study. Int Urol Nephrol. 2012;44:731–7. doi: 10.1007/s11255-011-0062-3. [DOI] [PubMed] [Google Scholar]
- 61.Argyropoulos AN, Tolley DA. Ureteric stents compromise stone clearance after shockwave lithotripsy for ureteric stones: Results of a matched-pair analysis. BJU Int. 2009;103:76–80. doi: 10.1111/j.1464-410X.2008.07886.x. [DOI] [PubMed] [Google Scholar]
- 62.Lucio J, 2nd, Korkes F, Lopes-Neto AC, et al. Steinstrasse predictive factors and outcomes after extracorporeal shockwave lithotripsy. Int Braz J Urol. 2011;37:477. doi: 10.1590/S1677-55382011000400006. [DOI] [PubMed] [Google Scholar]
- 63.Mustafa M, Ali-El-Dein B. Stenting in extracorporeal shockwave lithotripsy may enhance the passage of the fragments. J Pak Med Assoc. 2009;59:141. [PubMed] [Google Scholar]
- 64.Duvdevani M, Lorber G, Gofrit ON, et al. Fever after shockwave lithotripsy--risk factors and indications for prophylactic antimicrobial treatment. J Endourol. 2010;24:277–81. doi: 10.1089/end.2009.0283. [DOI] [PubMed] [Google Scholar]
- 65.Bapat SS, Pai KV, Purnapatre SS, et al. Comparison of holmium laser and pneumatic lithotripsy in managing upper-ureteral stones. J Endourol. 2007;21:1425–8. doi: 10.1089/end.2006.0350. [DOI] [PubMed] [Google Scholar]
- 66.Binbay M, Tepeler A, Singh A, et al. Evaluation of pneumatic versus holmium:YAG laser lithotripsy for impacted ureteral stones. Int Urol Nephrol. 2011;43:989–95. doi: 10.1007/s11255-011-9951-8. [DOI] [PubMed] [Google Scholar]
- 67.Maghsoudi R, Amjadi M, Norizadeh D, et al. Treatment of ureteral stones: A prospective randomized controlled trial on comparison of Ho:YAG laser and pneumatic lithotripsy. Indian J Urol. 2008;24:352–4. doi: 10.4103/0970-1591.39549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demir A, Karadag MA, Cecen K, et al. Pneumatic versus laser ureteroscopic lithotripsy: A comparison of initial outcomes and cost. Int Urol Nephrol. 2014;46:2087–93. doi: 10.1007/s11255-014-0787-x. [DOI] [PubMed] [Google Scholar]
- 69.Atar M, Bodakci MN, Sancaktutar AA, et al. Comparison of pneumatic and laser lithotripsy in the treatment of pediatric ureteral stones. J Pediatr Urol. 2013;9:308–12. doi: 10.1016/j.jpurol.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Teichman JM, Rao RD, Rogenes VJ, et al. Ureteroscopic management of ureteral calculi: Electrohydraulic versus holmium:YAG lithotripsy. J Urol. 1997;158:1357–61. doi: 10.1016/S0022-5347(01)64214-9. [DOI] [PubMed] [Google Scholar]
- 71.Rehman J, Monga M, Landman J, et al. Characterization of intrapelvic pressure during ureteropyeloscopy with ureteral access sheaths. Urology. 2003;61:713–8. doi: 10.1016/S0090-4295(02)02440-8. [DOI] [PubMed] [Google Scholar]
- 72.Auge BK, Pietrow PK, Lallas CD, et al. Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol. 2004;18:33–6. doi: 10.1089/089277904322836631. [DOI] [PubMed] [Google Scholar]
- 73.Kourambas J, Byrne RR, Preminger GM. Does a ureteral access sheath facilitate ureteroscopy? J Urol. 2001;165:789–93. doi: 10.1016/S0022-5347(05)66527-5. [DOI] [PubMed] [Google Scholar]
- 74.L’Esperance JO, Ekeruo WO, Scales CD, Jr, et al. Effect of ureteral access sheath on stone-free rates in patients undergoing ureteroscopic management of renal calculi. Urology. 2005;66:252–6. doi: 10.1016/j.urology.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Berquet G, Prunel P, Verhoest G, et al. The use of a ureteral access sheath does not improve stone-free rate after ureteroscopy for upper urinary tract stones. World J Urol. 2014;32:229–32. doi: 10.1007/s00345-013-1181-5. [DOI] [PubMed] [Google Scholar]
- 76.Lallas CD, Auge BK, Raj GV, et al. Laser Doppler flowmetric determination of ureteral blood flow after ureteral access sheath placement. J Endourol. 2002;16:583–90. doi: 10.1089/089277902320913288. [DOI] [PubMed] [Google Scholar]
- 77.Traxer O, Thomas A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J Urol. 2013;189:580–4. doi: 10.1016/j.juro.2012.08.197. [DOI] [PubMed] [Google Scholar]
- 78.Yaycioglu O, Guvel S, Kilinc F, et al. Results with 7.5F versus 10F rigid ureteroscopes in treatment of ureteral calculi. Urology. 2004;64:643–6. doi: 10.1016/j.urology.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 79.Atis G, Arikan O, Gurbuz C, et al. Comparison of different ureteroscope sizes in treating ureteral calculi in adult patients. Urology. 2013;82:1231–5. doi: 10.1016/j.urology.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 80.Hudson RG, Conlin MJ, Bagley DH. Ureteric access with flexible ureteroscopes: Effect of the size of the ureteroscope. BJU Int. 2005;95:1043–4. doi: 10.1111/j.1464-410X.2005.05462.x. [DOI] [PubMed] [Google Scholar]
- 81.Bach C, Nesar S, Kumar P, et al. The new digital flexible ureteroscopes: ‘size does matter’--increased ureteric access sheath use! Urol Int. 2012;89:408–11. doi: 10.1159/000341429. [DOI] [PubMed] [Google Scholar]
- 82.Somani BK, Al-Qahtani SM, de Medina SD, et al. Outcomes of flexible ureterorenoscopy and laser fragmentation for renal stones: Comparison between digital and conventional ureteroscope. Urology. 2013;82:1017–9. doi: 10.1016/j.urology.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 83.Binbay M, Yuruk E, Akman T, et al. Is there a difference in outcomes between digital and fiberoptic flexible ureterorenoscopy procedures? J Endourol. 1929;24:1929–34. doi: 10.1089/end.2010.0211. [DOI] [PubMed] [Google Scholar]
- 84.Chu L, Farris CA, Corcoran AT, et al. Preoperative stent placement decreases cost of ureteroscopy. Urology. 2011;78:309–13. doi: 10.1016/j.urology.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 85.Netsch C, Knipper S, Bach T, et al. Impact of preoperative ureteral stenting on stone-free rates of ureteroscopy for nephroureterolithiasis: A matched-paired analysis of 286 patients. Urology. 2012;80:1214–20. doi: 10.1016/j.urology.2012.06.064. [DOI] [PubMed] [Google Scholar]
- 86.Bourdoumis A, Tanabalan C, Goyal A, et al. The difficult ureter: stent and come back or balloon dilate and proceed with ureteroscopy? What does the evidence say? Urology. 2014;83:1–3. doi: 10.1016/j.urology.2013.08.073. [DOI] [PubMed] [Google Scholar]
- 87.Cetti RJ, Biers S, Keoghane SR. The difficult ureter: What is the incidence of pre-stenting? Ann R Coll Surg Engl. 2011;93:31–3. doi: 10.1308/003588411X12851639106990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ambani SN, Faerber GJ, Roberts WW, et al. Ureteral stents for impassable ureteroscopy. J Endourol. 2013;27:549–53. doi: 10.1089/end.2012.0414. [DOI] [PubMed] [Google Scholar]
- 89.Hosking DH, McColm SE, Smith WE. Is stenting following ureteroscopy for removal of distal ureteral calculi necessary? J Urol. 1999;161:48–50. doi: 10.1016/S0022-5347(01)62058-5. [DOI] [PubMed] [Google Scholar]
- 90.Denstedt JD, Wollin TA, Sofer M, et al. A prospective randomized controlled trial comparing nonstented versus stented ureteroscopic lithotripsy. J Urol. 2001;165:1419–22. doi: 10.1016/S0022-5347(05)66320-3. [DOI] [PubMed] [Google Scholar]
- 91.Makarov DV, Trock BJ, Allaf ME, et al. The effect of ureteral stent placement on post-ureteroscopy complications: A meta-analysis. Urology. 2008;71:796–800. doi: 10.1016/j.urology.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 92.Pengfei S, Yutao L, Jie Y, et al. The results of ureteral stenting after ureteroscopic lithotripsy for ureteral calculi: A systematic review and meta-analysis. J Urol. 2011;186:1904–9. doi: 10.1016/j.juro.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 93.Baseskioglu B, Sofikerim M, Demirtas A, et al. Is ureteral stenting really necessary after ureteroscopic lithotripsy with balloon dilatation of ureteral orifice? A multi-institutional randomized controlled study. World J Urol. 2011;29:731–6. doi: 10.1007/s00345-011-0697-9. [DOI] [PubMed] [Google Scholar]
- 94.Cevik I, Dillioglugil O, Akdas A, et al. Is stent placement necessary after uncomplicated ureteroscopy for removal of impacted ureteral stones? J Endourol. 2010;24:1263–7. doi: 10.1089/end.2009.0153. [DOI] [PubMed] [Google Scholar]
- 95.Torricelli FC, De S, Hinck B, et al. Flexible ureteroscopy with a ureteral access sheath: When to stent? Urology. 2014;83:278–81. doi: 10.1016/j.urology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Rapoport D, Perks AE, Teichman JM. Ureteral access sheath use and stenting in ureteroscopy: Effect on unplanned emergency room visits and cost. J Endourol. 2007;21:993–8. doi: 10.1089/end.2006.0236. [DOI] [PubMed] [Google Scholar]
- 97.Shigemura K, Yasufuku T, Yamanaka K, et al. How long should double J stent be kept in after ureteroscopic lithotripsy? Urol Res. 2012;40:373–6. doi: 10.1007/s00240-011-0426-2. [DOI] [PubMed] [Google Scholar]
- 98.White WM, Johnson EB, Zite NB, et al. Predictive value of current imaging modalities for the detection of urolithiasis during pregnancy: A multicenter, longitudinal study. J Urol. 2013;189:931–4. doi: 10.1016/j.juro.2012.09.076. [DOI] [PubMed] [Google Scholar]
- 99.Masselli G, Derme M, Laghi F, et al. Imaging of stone disease in pregnancy. Abdom Imaging. 2013;38:1409–14. doi: 10.1007/s00261-013-0019-3. [DOI] [PubMed] [Google Scholar]
- 100.Mullins JK, Semins MJ, Hyams ES, et al. Half Fourier single-shot turbo spin-echo magnetic resonance urography for the evaluation of suspected renal colic in pregnancy. Urology. 2012;79:1252–5. doi: 10.1016/j.urology.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 101.Semins MJ, Matlaga BR. Management of stone disease in pregnancy. Curr Opin Urol. 2010;20:174–7. doi: 10.1097/MOU.0b013e3283353a4b. [DOI] [PubMed] [Google Scholar]
- 102.Isen K, Hatipoglu NK, Dedeoglu S, et al. Experience with the diagnosis and management of symptomatic ureteric stones during pregnancy. Urology. 2012;79:508–12. doi: 10.1016/j.urology.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 103.Semins MJ, Matlaga BR. Kidney stones during pregnancy. Nat Rev Urol. 2014;11:163–8. doi: 10.1038/nrurol.2014.17. [DOI] [PubMed] [Google Scholar]
- 104.Akpinar H, Tufek I, Alici B, et al. Ureteroscopy and holmium laser lithotripsy in pregnancy: Stents must be used postoperatively. J Endourol. 2006;20:107–10. doi: 10.1089/end.2006.20.107. [DOI] [PubMed] [Google Scholar]
- 105.Watterson JD, Girvan AR, Beiko DT, et al. Ureteroscopy and holmium:YAG laser lithotripsy: An emerging definitive management strategy for symptomatic ureteral calculi in pregnancy. Urology. 2002;60:383–7. doi: 10.1016/S0090-4295(02)01751-X. [DOI] [PubMed] [Google Scholar]
- 106.Bozkurt Y, Penbegul N, Soylemez H, et al. The efficacy and safety of ureteroscopy for ureteral calculi in pregnancy: Our experience in 32 patients. Urol Res. 2012;40:531. doi: 10.1007/s00240-011-0454-y. [DOI] [PubMed] [Google Scholar]
- 107.Cocuzza M, Colombo JR, Jr, Lopes RI, et al. Use of inverted fluoroscope’s C-arm during endoscopic treatment of urinary tract obstruction in pregnancy: A practicable solution to cut radiation. Urology. 2010;75:1505–8. doi: 10.1016/j.urology.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 108.Frankenschmidt A, Sommerkamp H. Shock wave lithotripsy during pregnancy: A successful clinical experiment. J Urol. 1998;159:501–2. doi: 10.1016/S0022-5347(01)63962-4. [DOI] [PubMed] [Google Scholar]
- 109.Alsaikhan B, Andonian S. Shock wave lithotripsy in patients requiring anticoagulation or antiplatelet agents. Can Urol Assoc J. 2011;5:53. doi: 10.5489/cuaj.09140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aboumarzouk OM, Somani BK, Monga M. Flexible ureteroscopy and holmium:YAG laser lithotripsy for stone disease in patients with bleeding diathesis: A systematic review of the literature. Int Braz J Urol. 2012;38:298. doi: 10.1590/s1677-55382012000300002. [DOI] [PubMed] [Google Scholar]
- 111.Klingler HC, Kramer G, Lodde M, et al. Stone treatment and coagulopathy. Eur Urol. 2003;43:75. doi: 10.1016/s0302-2838(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 112.Razvi H, Fuller A, Nott L, et al. Risk factors for perinephric hematoma formation after shockwave lithotripsy: A matched case-control analysis. J Endourol. 2012;26:1478–82. doi: 10.1089/end.2012.0261. [DOI] [PubMed] [Google Scholar]
- 113.Tsuboi T, Fujita T, Maru N, et al. Transurethral ureterolithotripsy and extracorporeal shock wave lithotripsy in patients with idiopathic thrombocytopenic purpura. Hinyokika Kiyo. 2008;54:17. [PubMed] [Google Scholar]
- 114.Kaatz S, Paje D. Update in bridging anticoagulation. J Thromb Thrombolysis. 2011;31:259–64. doi: 10.1007/s11239-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 115.Kefer JC, Turna B, Stein RJ, et al. Safety and efficacy of percutaneous nephrostolithotomy in patients on anticoagulant therapy. J Urol. 2009;181:144–8. doi: 10.1016/j.juro.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 116.Zanetti G, Kartalas-Goumas I, Montanari E, et al. Extracorporeal shockwave lithotripsy in patients treated with antithrombotic agents. J Endourol. 2001;15:237–41. doi: 10.1089/089277901750161656. [DOI] [PubMed] [Google Scholar]
- 117.Kuo RL, Aslan P, Fitzgerald KB, et al. Use of ureteroscopy and holmium:YAG laser in patients with bleeding diatheses. Urology. 1998;52:609–13. doi: 10.1016/S0090-4295(98)00276-3. [DOI] [PubMed] [Google Scholar]
- 118.Watterson JD, Girvan AR, Cook AJ, et al. Safety and efficacy of holmium:YAG laser lithotripsy in patients with bleeding diatheses. J Urol. 2002;168:442–5. doi: 10.1016/S0022-5347(05)64654-X. [DOI] [PubMed] [Google Scholar]
- 119.Turna B, Stein RJ, Smaldone MC, et al. Safety and efficacy of flexible ureterorenoscopy and holmium:YAG lithotripsy for intrarenal stones in anticoagulated cases. J Urol. 2008;179:1415–9. doi: 10.1016/j.juro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 120.Elkoushy MA, Violette PD, Andonian S. Ureteroscopy in patients with coagulopathies is associated with lower stone-free rate and increased risk of clinically significant hematuria. Int Braz J Urol. 2012;38:195. doi: 10.1590/S1677-55382012000200007. [DOI] [PubMed] [Google Scholar]
- 121.Mottet N, Castagnola C, Rischmann P, et al. Quality of life after cystectomy: French national survey conducted by the French Association of Urology (AFU), the French Federation of Stoma Patients (FSF) and the French Association of Enterostomy Patients (AFET) in patients with ileal conduit urinary diversion or orthotopic neobladder [in French] Prog Urol. 2008;18:292. doi: 10.1016/j.purol.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 122.Stuurman RE, Al-Qahtani SM, Cornu JN, et al. Antegrade percutaneous flexible endoscopic approach for the management of urinary diversion-associated complications. J Endourol. 2013;27:1330–4. doi: 10.1089/end.2012.0371. [DOI] [PubMed] [Google Scholar]
- 123.Dhar NB, Hernandez AV, Reinhardt K, et al. Prevalence of nephrolithiasis in patients with ileal bladder substitutes. Urology. 2008;71:128–30. doi: 10.1016/j.urology.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 124.El-Nahas AR, Shokeir AA. Endourological treatment of nonmalignant upper urinary tract complications after urinary diversion. Urology. 2010;76:1302–8. doi: 10.1016/j.urology.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 125.Okhunov Z, Duty B, Smith AD, et al. Management of urolithiasis in patients after urinary diversions. BJU Int. 2011;108:330–6. doi: 10.1111/j.1464-410X.2011.10194.x. [DOI] [PubMed] [Google Scholar]
- 126.Fernandez A, Foell K, Nott L, et al. Percutaneous nephrolithotripsy in patients with urinary diversions: A case-control comparison of perioperative outcomes. J Endourol. 2011;25:1615–8. doi: 10.1089/end.2011.0045. [DOI] [PubMed] [Google Scholar]
- 127.L’Esperance JO, Sung J, Marguet C, et al. The surgical management of stones in patients with urinary diversions. Curr Opin Urol. 2004;14:129–34. doi: 10.1097/00042307-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 128.Cohen TD, Streem SB, Lammert G. Long-term incidence and risks for recurrent stones following contemporary management of upper tract calculi in patients with a urinary diversion. J Urol. 1996;155:62–5. doi: 10.1016/S0022-5347(01)66540-6. [DOI] [PubMed] [Google Scholar]
- 129.Hensle TW, Bingham J, Lam J, et al. Preventing reservoir calculi after augmentation cystoplasty and continent urinary diversion: the influence of an irrigation protocol. BJU Int. 2004;93:585–7. doi: 10.1111/j.1464-410X.2003.04664.x. [DOI] [PubMed] [Google Scholar]
- 130.Kumar V, Ahlawat R, Banjeree GK, et al. Percutaneous ureterolitholapaxy: The best bet to clear large bulk impacted upper ureteral calculi. Archivos espanoles de urologia. 1996;49:86. [PubMed] [Google Scholar]
- 131.Rhee BK, Bretan PN, Jr, Stoller ML. Urolithiasis in renal and combined pancreas/renal transplant recipients. J Urol. 1999;161:1458–62. doi: 10.1016/S0022-5347(05)68926-4. [DOI] [PubMed] [Google Scholar]
- 132.Maheshwari PN, Oswal AT, Andankar M, et al. Is antegrade ureteroscopy better than retrograde ureteroscopy for impacted large upper ureteral calculi? J Endourol. 1999;13:441. doi: 10.1089/end.1999.13.441. [DOI] [PubMed] [Google Scholar]
- 133.Goel R, Aron M, Kesarwani PK, et al. Percutaneous antegrade removal of impacted upper-ureteral calculi: still the treatment of choice in developing countries. J Endourol. 2005;19:54. doi: 10.1089/end.2005.19.54. [DOI] [PubMed] [Google Scholar]
- 134.Karami H, Arbab AH, Hosseini SJ, et al. Impacted upper-ureteral calculi >1 cm: Blind access and totally tubeless percutaneous antegrade removal or retrograde approach? J Endourol. 2006;20:616. doi: 10.1089/end.2006.20.616. [DOI] [PubMed] [Google Scholar]
- 135.Topaloglu H, Karakoyunlu N, Sari S, et al. A comparison of antegrade percutaneous and laparoscopic approaches in the treatment of proximal ureteral stones. Biomed Res Int. 2014 doi: 10.1155/2014/691946. 691946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu H, Ye X, Xiao X, et al. Retrograde, antegrade, and laparoscopic approaches to the management of large upper ureteral stones after shockwave lithotripsy failure: A four-year retrospective study. J Endourol. 2014;28:100–3. doi: 10.1089/end.2013.0391. [DOI] [PubMed] [Google Scholar]
- 137.Lopes Neto AC, Korkes F, Silva JL, 2nd, et al. Prospective randomized study of treatment of large proximal ureteral stones: Extracorporeal shock wave lithotripsy versus ureterolithotripsy versus laparoscopy. J Urol. 2012;187:164–8. doi: 10.1016/j.juro.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 138.Maalouf NM. Metabolic syndrome and the genesis of uric acid stones. J Ren Nutr. 2011;21:128–31. doi: 10.1053/j.jrn.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marchini GS, Sarkissian C, Tian D, et al. Gout, stone composition and urinary stone risk: A matched case comparative study. J Urol. 2013;189:1334. doi: 10.1016/j.juro.2012.09.102. [DOI] [PubMed] [Google Scholar]
- 140.Ciudin ALP, Diaconu MG. Predicting urinary stone composition: A radiological study made by urologists. Eur Urol. 2013;12:e436. doi: 10.1016/S1569-9056(13)60920-3. [DOI] [Google Scholar]
- 141.Wisenbaugh ES, Paden RG, Silva AC, et al. Dual-energy vs conventional computed tomography in determining stone composition. Urology. 2014;83:1243–7. doi: 10.1016/j.urology.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 142.Paterson R, Fernandez A, Razvi H, et al. Evaluation and medical management of the kidney stone patient. Can Urol Assoc J. 2010;4:375. doi: 10.5489/cuaj.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pearle MS, Goldfarb DS, Assimos DG, et al. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Turk C, Knoll T, Petrik A, et al. EAU Guidelines on urolithiasis. http://uroweb.org/wp-content/uploads/20_Urolithiasis_LR-March-13-2012.pdf. Accessed November 11, 2015.
- 145.Lojanapiwat B, Tanthanuch M, Pripathanont C, et al. Alkaline citrate reduces stone recurrence and regrowth after shockwave lithotripsy and percutaneous nephrolithotomy. Int Braz J Urol. 2011;37:611. doi: 10.1590/s1677-55382011000500007. [DOI] [PubMed] [Google Scholar]
- 146.El-Gamal O, El-Bendary M, Ragab M, et al. Role of combined use of potassium citrate and tamsulosin in the management of uric acid distal ureteral calculi. Urol Res. 2012;40:219. doi: 10.1007/s00240-011-0406-6. [DOI] [PubMed] [Google Scholar]
- 147.Pearle MS, Pierce HL, Miller GL, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160:1260. [PubMed] [Google Scholar]
- 148.Christoph F, Weikert S, Muller M, et al. How septic is urosepsis? Clinical course of infected hydronephrosis and therapeutic strategies. World J Urol. 2005;23:243. doi: 10.1007/s00345-005-0002-x. [DOI] [PubMed] [Google Scholar]
- 149.Ramsey S, Robertson A, Ablett MJ, et al. Evidence-based drainage of infected hydronephrosis secondary to ureteric calculi. J Endourol. 2010;24:185. doi: 10.1089/end.2009.0361. [DOI] [PubMed] [Google Scholar]
- 150.Yoshimura K, Utsunomiya N, Ichioka K, et al. Emergency drainage for urosepsis associated with upper urinary tract calculi. J Urol. 2005;173:458. doi: 10.1097/01.ju.0000150512.40102.bb. [DOI] [PubMed] [Google Scholar]
- 151.Lynch MF, Anson KM, Patel U. Current opinion amongst radiologists and urologists in the UK on percutaneous nephrostomy and ureteric stent insertion for acute renal unobstruction: Results of a postal survey. BJU Int. 2006;98:1143. doi: 10.1111/j.1464-410X.2006.06513.x. [DOI] [PubMed] [Google Scholar]
- 152.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]