Abstract
With the rise in detection of incidental renal masses on imaging, there has been a commensurate rise in the use of percutaneous biopsies for evaluation of these tumours. Tumour tract seeding had previously been one of the most feared complications of percutaneous biopsy of renal cell carcinoma (RCC). Recently, less emphasis has been placed on this complication, with the assertion that it has only been reported eight times in literature, and thus must be exceedingly rare. However, we report two cases of tumour tract seeding associated with percutaneous biopsy and treatment of RCC over a short time period at a single institution. This report challenges the current extremely low estimates of the frequency of this complication and calls for a more realistic assessment.
Introduction
With the advent of cross-sectional imaging, the incidence of renal tumours has been on the rise, as these tumours are increasingly being detected at earlier stages in asymptomatic patients.1–6 One study estimates that up to 66% of RCCs are detected incidentally.7 Importantly, not all incidentally detected renal masses on imaging are RCC or malignant in nature. In a recent large series of laparoscopic partial nephrectomies for renal masses, 28–34% of the tumours were identified to be benign at final histology.8
The indications for performing percutaneous biopsy of renal tumours were historically limited to diagnosis of lymphoma, metastatic disease, infection, or tumours in patients who have an increased surgical risk.1,5,7,9 With increasing expertise in biopsy performance and the understanding that not all renal masses are malignant and needing invasive measures (such as ablation or nephrectomy), the role of percutaneous biopsy has been on the rise.1–3,5,6 Moreover, recent assessments of safety of renal mass biopsies state that the overall complication rates range from 1.4–4.7%, with major complications reported only in 0.46% of all patients undergoing renal mass biopsies. In particular, the most feared risk of tumour tract seeding has been estimated to be <0.01% with only eight total reported cases in literature to date.2–7,9,10
We report two recent cases of tumour tract seeding associated with percutaneous biopsy and/or treatment (cryoablation) of renal masses at a single institution, suggesting this complication, although rare, may be underreported in literature.
Case reports
Patient A
A 63-year-old woman initially presented in early 2009, after an incidental finding of a 3 cm enhancing left upper pole renal mass found during computed tomography (CT) scan obtained for evaluation of exacerbation of sarcoidosis. The patient was asymptomatic and underwent CT-guided biopsy of the left renal mass by interventional radiology. The procedure was performed with a 19-gauge coaxial needle that was advanced to the superficial margin of the mass, followed by core biopsy specimens that were obtained with a 20-gauge needle (Fig. 1a). Pathology analysis of the biopsy specimens was remarkable for clear cell type of RCC (Fuhrman grade II). The patient subsequently underwent CT-guided cryoablation six weeks after the biopsy. The cryoablation was performed using five probes: four Perc-24 in the mid and caudal aspect of the tumour, and one Perc-17 in the cephalad aspect.
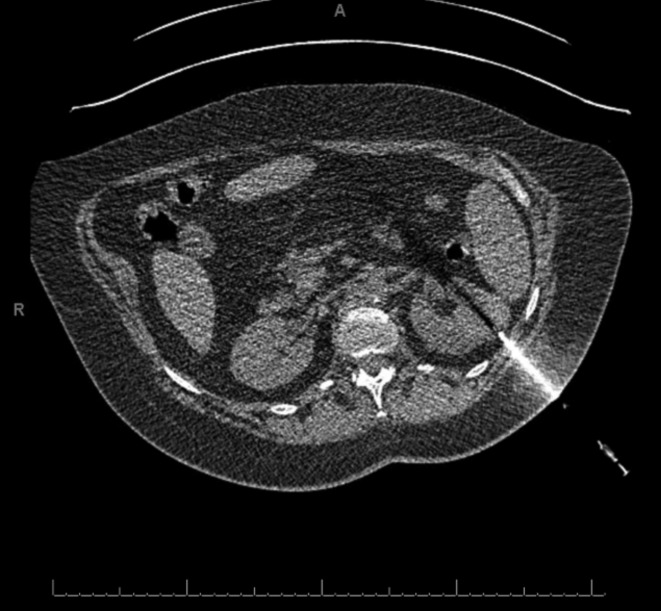
Fig. 1a.
Patient A’s CT scan during renal biopsy showing the enhancing biopsy needle being passed through the left upper pole renal mass. Patient was placed in prone position during the procedure, but the image here has been oriented for ease of comparison with Fig. 1b.
Initial followup with CT scan at four months from the date of biopsy was unremarkable. However, a second followup visit with CT scan at 10 months from the date of biopsy demonstrated a 4 cm enhancing lobulated mass within the left flank musculature at the site of the prior biopsy tract, but without any recurrence at the site of cryoablation (Fig. 1b). The mass was locally excised and was found to be RCC-clear cell type (Fuhrman grade II), thereby confirming tumour tract seeding. Unfortunately, the patient developed metastatic disease a few months later and despite additional treatment, died from her disease two years after her initial diagnosis.
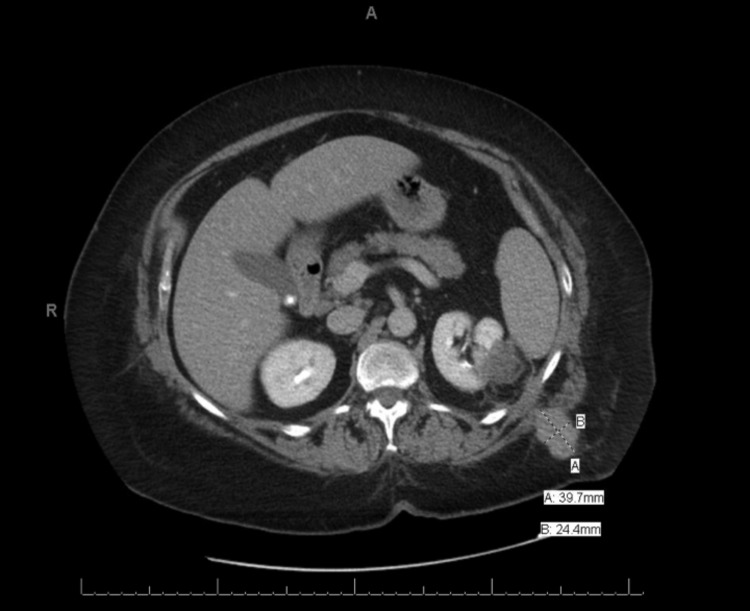
Fig. 1b.
Patient A’s CT scan with contrast at 10 months after percutaneous biopsy and cryoablation of a left T1aN0M0 Grade II renal cancer demonstrating a recurrent tumour mass in the left flank musculature along the course of her needle tracts.
Patient B
A 53-year-old man was found to have an 8 mm exophytic right renal mass on evaluation by CT in early 2010 during an otherwise negative workup for microscopic hematuria. A CT-guided biopsy was performed by interventional radiology with a 19-gauge coaxial needle that was advanced to the superficial margin of the mass, followed by four core biopsy specimens obtained with a 20-gauge needle. Pathology analysis of the biopsy specimens was remarkable for papillary type I RCC.
Two months after the initial biopsy, the patient underwent a robot-assisted partial nephrectomy. Pathological analysis of the final operative specimen confirmed the tumour to be papillary type I RCC. Additionally, several separate small foci of the carcinoma were noted in a linear pattern, adjacent to the tumour in the perinephric fat, consistent with tumour tract seeding from the previous biopsy (Figs. 2a–c). The patient has been followed with biannual cross-sectional imaging since the time of biopsy without evidence of local or distant recurrence at five years.
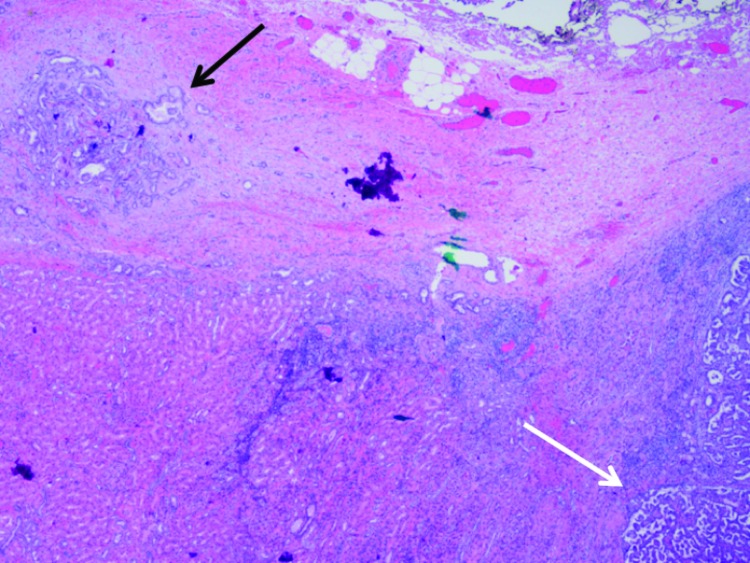
Fig. 2a.
Small satellite lesion in the renal capsule (black arrow), separated from the edge of the main tumour mass (white arrow) (20×).
Fig. 2b.
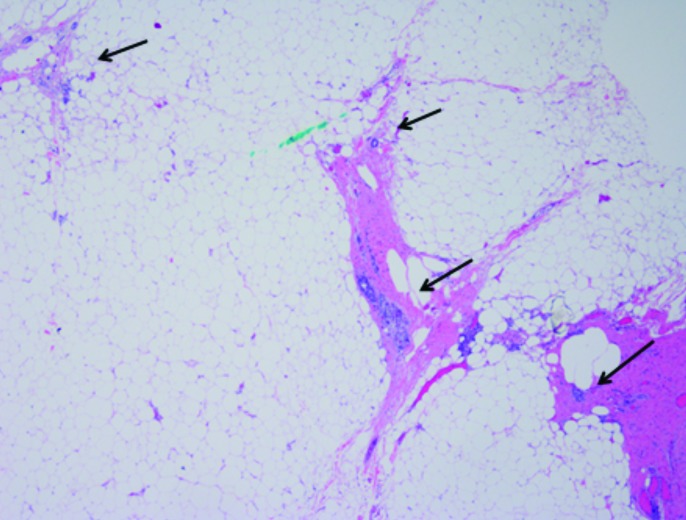
Scanning power of small, isolated, insular foci of tumour (arrows) lying in an interrupted, linear track of fibrous tissue within perinephric adipose tissue, arranged perpendicularly to the renal mass (not pictured) (20×).
Discussion
We have described here two recent cases of RCC needle manipulation: one following biopsy and cryoablation of a clear cell type of RCC, and the other following biopsy of papillary type I of RCC, with convincing evidence of tumour tract seeding. They occurred in the same institution, despite the use of modern biopsy techniques such as coaxial sheaths. Patient A’s tumour was likely aggressive, given that she developed metastatic disease soon after the discovery of tumour tract seeding 10 months after the initial percutaneous biopsy of the renal mass. Patient B’s tumour, with similarly convincing evidence of tract seeding, appears to have a less aggressive phenotype, as the patient has not had any symptoms or signs of disease progression to date.
Patient A’s history of having undergone cryoablation in addition to percutaneous biopsy makes it difficult to conclude with certainty that the complication of tract seeding was due to the biopsy alone. There are four reported cases of tumour tract seeding associated with cryoablation in the literature, suggesting that either procedure could have been the cause in this case.10–13 Nevertheless, these two patients experienced vastly different clinical outcomes despite having experienced the same complication associated with needle manipulation of their RCC.
The institutional volume averages 58.8 percutaneous renal tumour biopsies and cryoablations annually; thus, in last five years, we have had a cumulative incidence of 0.7% (2/286) for tumour tract seeding. While the incidence of tumour tract seeding is still rare at our hospital, it is substantially higher than the previously reported rate of <0.01%, despite the consistent use of a coaxial approach to minimize this risk. We do not believe this is a common problem, but rather that it is underreported in the literature and needs a more realistic assessment. Re-evaluation of the risk of tumour tract seeding on a larger scale is necessary to determine not only the true incidence, but also to understand the prognostic significance of this complication.
Conclusions
The indications for percutaneous biopsy of renal masses has been expanding with increasing detection of incidental masses on cross-sectional imaging and the knowledge that not all renal masses are malignant. The most feared complication of tumour tract seeding is believed to be exceedingly rare and there are only a few reports in literature. This report, from a single institution, which details two separate instances of tumour tract seeding after renal biopsies and a cryoablation, challenges these claims and prompts a reevaluation of our current understanding of the frequency of tumour tract seeding associated with needle manipulation of RCC.
Fig. 2c.
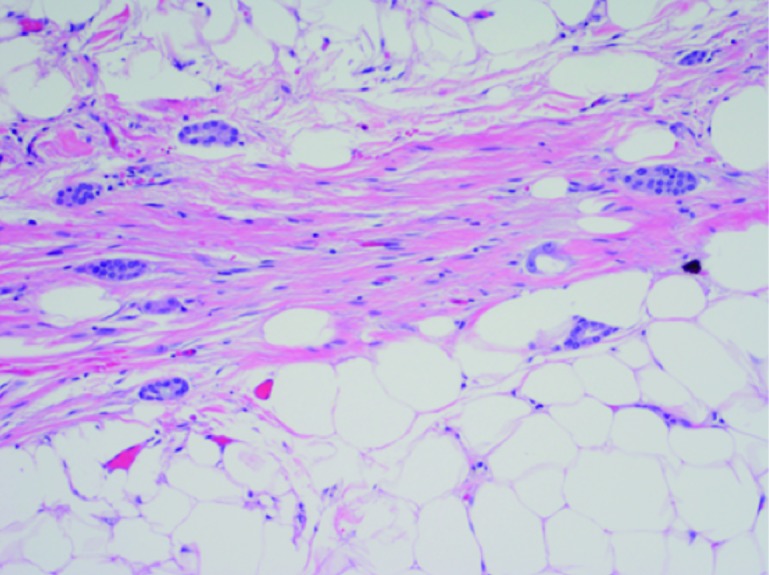
Small tubules of tumour isolated within adipose tissue at high power (200×).
Footnotes
Competing interests: Mr. Viswanathan, Dr. Ingimarsson, and Dr. Schned declare no competing financial or personal interests. Dr. Seigne holds investments in Johnson & Johnson and has participated in clinical trials with Altor BioScience and Viventia Bio.
This paper has been peer-reviewed.
References
- 1.Silverman SG, Gan YU, Mortele KJ, et al. Renal masses in the adult patient: The role of percutaneous biopsy. Radiology. 2006;240:6–22. doi: 10.1148/radiol.2401050061. [DOI] [PubMed] [Google Scholar]
- 2.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60:578–84. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Mally AD, Gayed B, Averch T, et al. The current role of percutaneous biopsy of renal masses. Can J Urol. 2012;19:6243–9. [PubMed] [Google Scholar]
- 4.Mullins JK, Rodriguez R. Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J. 2013;7:E176–9. doi: 10.5489/cuaj.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomaszewski JJ, Uzzo RG, Smaldone MC. Heterogeneity and renal mass biopsy: A review of its role and reliability. Cancer Biol Med. 2014;11:162–72. doi: 10.7497/j.issn.2095-3941.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol. 2014;31(1):20–6. doi: 10.1055/s-0033-1363839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ordon M, Landman J. Renal mass biopsy: “Just do it”. J Urol. 2013;190:1638–40. doi: 10.1016/j.juro.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Volpe A, Mattar K, Finelli A, et al. Contemporary results of percutaneous biopsy of 100 small renal masses: A single-centre experience. J Urol. 2008;180:2333–7. doi: 10.1016/j.juro.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Volpe A, Kachura JR, Geddie WR, et al. Techniques, safety, and accuracy of sampling of renal tumours by fine needle aspiration and core biopsy. J Urol. 2007;178:379–86. doi: 10.1016/j.juro.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 10.Sainani NI, Tatli S, Anthony SG, et al. Successful percutaneous radiologic management of renal cell carcinoma tumour seeding caused by percutaneous biopsy performed before ablation. J Vasc Interv Radiol. 2013;24:1404–8. doi: 10.1016/j.jvir.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 11.Akhavein A, Neuberger MM, Dahm P. Tumour-seeding: A rare complication of ablative therapy for clinically localized renal cell carcinoma. BMJ Case Rep. 2012. 09/30. [DOI] [PMC free article] [PubMed]
- 12.Mayo-Smith WW, Dupuy DE, Parikh PM, et al. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: Techniques and outcomes of 38 treatment sessions in 32 consecutive patients. Am J Roentgenol. 2003;180:1503–8. doi: 10.2214/ajr.180.6.1801503. [DOI] [PubMed] [Google Scholar]
- 13.Krambeck AE, Farrell MA, Charboneau JW, et al. Intraperitoneal drop metastasis after radiofrequency ablation of pararenal tumour recurrences. Urology. 2005;65:797. doi: 10.1016/j.urology.2004.10.017. [DOI] [PubMed] [Google Scholar]