Abstract
Aim
To investigate the therapeutic and immunoregulatory effects of 1,25-dihydroxyvitamin D (1,25(OH)D3) on 2,4,6-trinitrobenzenesulfonic acid (TNBS) -induced colitis in rats.
Methods
Experimental colitis induced by enema administration of TNBS plus ethanol was treated with 5-aminosalicylic acid (5-ASA) and/or 1,25(OH)D3. Disease activity was measured using the disease activation index (DAI), colon macroscopic damage index (CMDI), histological colonic damage score, and myeloperoxidase (MPO) activity. The expression of toll-like receptor 9 (TLR9) in the colon was determined by reverse transcription-polymerase chain reaction and immunohistochemistry.
Results
Rats with TNBS-induced colitis had significantly elevated DAI, CMDI, histological colonic damage score, and MPO activity (all P < 0.001) compared to rats without colitis. Treatment with 5-ASA or 1,25(OH)D3 ameliorated colitis by lowering CMDI (P = 0.049, P = 0.040, respectively), histological colonic damage score (P = 0.010, P = 0.005, respectively), and MPO activity (P = 0.0003, P = 0.0013, respectively) compared with the TNBS group. Combined treatment with 5-ASA and 1,25(OH)D3 significantly decreased MPO activity (P = 0.003). 1,25(OH)D3 attenuated colitis without causing hypercalcemia or renal insufficiency. TNBS significantly increased the number of TLR9 positive cells compared to control (P < 0.010), while 5-ASA, 1,25(OH)D3, and combined treatment with 5-ASA and 1,25(OH)D3 significantly decreased it compared to TNBS group (all P < 0.010). In TNBS group a moderate correlation was observed between MPO activity and the number of TLR9-positive cells (r = 0.654, P < 0.001).
Conclusion
TLR9 expression correlates with the extent of inflammation in TNBS-induced colitis. 1,25(OH)D3 relieves this inflammation possibly by decreasing TLR9 expression.
Vitamin D is a well-known endocrine regulator of calcium homeostasis and 1,25-dihydroxyvitamin D (1,25(OH)D3) is its biologically active form. Recent studies suggest that vitamin D deficiency is associated with the onset or increased activity of autoimmune diseases, especially Th1-mediated autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, diabetes, multiple sclerosis, and inflammatory bowel disease (IBD) (1-3). Furthermore, in Chinese patients with IBD, vitamin D deficiency was observed to be partially related to higher risk of osteoporosis and increased disease activity (4). Therefore, vitamin D deficiency is considered an environment risk factor of IBD. Some researchers have proposed that vitamin D can modulate intestinal microflora, inhibit the adhesion of intestinal bacteria (5), protect the intestinal mucosal barrier (6,7), and reduce excessive external antigens presentation by antigen presenting cells (APCs) (8,9).
The most important antigen recognition receptors on APCs are toll-like receptors (TLRs). The TLRs signaling pathway is thought to play a key role in both innate immunity and adaptive immunity (10). TLRs recognize diverse ligands, including lipids, lipoproteins, proteins, and nucleic acids derived from microbes. TLR9 recognizes unmethylated cytosine-phosphate-guanine (CpG) DNA motifs in bacteria and DNA viruses (11) and is essential for maturation of dendritic cells and release of pro-inflammatory cytokines. Increase in the expression of TLR9 was related to exposure of colonic epithelial cells to pathogenic bacterial DNA (12). Some reports suggested that the excessive inflammatory response associated with a TLR9 polymorphism correlated with an increased risk of Crohn’s disease (CD) (13,14).
Previous studies have increased our understanding of the crosstalk between vitamin D and TLRs. Dickie et al (15) demonstrated that 1,25(OH)D3 down-regulated intracellular TLR9 expression and TLR9-induced IL-6 production in healthy human monocytes in vitro. It was also shown that by down-regulating the expressions of TLRs and pro-inflammatory cytokines it improved herpes simplex virus-induced Behcet’s disease-like symptoms in mice (16). On the other hand, Edfeldt et al (17) found that TLRs enhanced bioconversion of 25-hydroxyvitamin D3 to its active metabolite, 1,25(OH)D3, by inducting 25-hydroxyvitamin D-1α-hydroxylase. Although previous studies showed that vitamin D could modulate intestinal microflora and TLR9 expression, the effect of vitamin D on IBD and its relationship with TLR9 expression in vivo still remained unclear. Therefore, the aim of this study was to investigate the effect of 1,25(OH)D3 on 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis and TLR9 expression in rats.
Materials and methods
Rats experiments and induction of colitis by TNBS
Male, 8-10 week old Sprague-Dawley rats, weighing approximately 220 g, were obtained from Animal Science Department of Peking University Health Science Center (Beijing, China). All studies were approved by the Ethics Committee of Peking Union Medical College and were in agreement with the Beijing laboratory animal management guidelines.
TNBS (100 mg/kg, Sigma-Aldrich, Shanghai, China) and 50% ethanol were administered to anesthetized rats through a 2 mm polyethylene tube, which was carefully inserted into the rat’s rectum. To ensure uniform distribution of TNBS throughout the entire colon, rats were held in a vertical position for 5 min after the instillation of the TNBS enema.
1,25(OH)D3 (10 μg, Sigma-Aldrich) was dissolved in 4 mL ethanol and diluted with olive oil to obtain the concentration of 0.1 μg/mL. 1,25(OH)D3 and 5-ASA (Dr Falk Pharma GmbH, Freiburg, Germany) was administered by gavage. Thirty rats were randomly divided into five groups. One group was treated with ethanol only and served as control group. One group was treated with TNBS only. Vitamin D treatment rats received 0.2 μg/kg/d 1,25(OH)D3. 5-ASA treatment rats received 0.4 g/kg/d 5-ASA. Combined 1,25(OH)D3 and 5-ASA treatment rats received 0.2 μg/kg/d 1,25(OH)D3 and 0.4g/kg/d 5-ASA. These treatments were initiated one day after the instillation of the TNBS enema and continued for 9 days. On the day 10, the rats were sacrificed and their colons removed. To monitor the adverse reaction of 1,25(OH)D3, serum calcium levels and serum creatinine levels were determined at the end of the experiment.
Assessment of inflammatory activity
Disease Activation Index (DAI). DAI was used to assess the clinical severity of colitis according to Murano et al (18). The calculation was based on the daily body weight, stool consistency, and rectal bleeding. The loss of body weight was scored as follows: 0, no weight loss; 1, weight loss of 0 to 5%; 2, weight loss of 5 to 10%; 3, weight loss of 10 to 20%; and 4, weight loss >20%. Stool consistency was scored as follows: 0, normally formed pellets; 2, pasty and semiformed pellets; and 4, liquid stool. Rectal bleeding was scored as follows: 0, no blood from the rectum and 4, gross bleeding from the rectum. These scores were summed, resulting in a total clinical score ranging from 0 to 12.
Colon Macroscopic Damage Index (CMDI). The assessment of the CMDI was based on the area of inflammation and the presence of ulcers, according to Wallace and Keenan (19). Features were graded as follows: 0, no ulcer, no inflammation; 1, no ulcer, local hyperemia; 2, ulceration without hyperemia; 3, ulceration and inflammation at one site only; 4, two or more sites of ulceration and inflammation; 5, ulceration extending more than 1 cm; 6, ulceration extending more than 2 cm.
Histological colonic damage. For histological examination, a sample of colonic tissue located 4-5 cm above the anal margin was obtained from rats in all treatment groups. The sections were stained with hematoxylin and eosin using routine techniques. Histological colonic damage was scored according to Sykes criteria (20). Features were graded from 0 to 5 in a blinded fashion, as follows: 0, no significant damage; 1, damage confined to the epithelium; 2, focal ulcers, limited to the mucosa; 3, focal transmural inflammation and ulceration; 4, extensive transmural inflammation and ulceration with normal mucosa around; 5, extensive transmural inflammation and ulceration with no normal mucosa.
Measurement of myeloperoxidase (MPO) activity. The severity of acute colitis was measured by infiltration of neutrophils determined by photometrical assay for the neutrophil-specific MPO enzyme. The MPO activity assay was performed using an MPO kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the method described by Bradley et al (21). The enzyme activity was determined photometrically. The MPO-catalyzed redox reaction of 3,3′-dimethoxybenzidine changed the absorbance at 460 nm. The values were expressed as MPO units per gram of wet tissue.
Reverse transcription-polymerase chain reaction (RT-PCR) of TLR9 mitochondrial RNA (mRNA)
The expression of the TLR9 gene at the mRNA level in samples of rats’ colons was measured by RT-PCR. This method enables detection and semi-quantification of gene expression. RNA was extracted from samples of colonic tissue located 5-6 cm above the anal margin using an RNAprep pure Tissue Kit (Tiangen Biotech, Beijing, China). RNA (2 μg) was used as a template in a cDNA synthesis reaction performed in a Quant cDNA kit (Tiangen Biotech, Beijing, China). Housekeeping gene β-actin was amplified as reference gene for mRNA quantification. The primers for TLR9 were forward 5′- TCAACAAGAACACGCTCAGG-3′ and reverse 5′-GAGAGCTGGGGTGAGACTTG-3′ and β-actin forward 5′ -TCCTGTGGCATCCATGAAACT-3′ and reverse 5′-GAAGCATTTGCGGTGCACGAT-3′. The primers were synthesized by SinoGenoMax (Beijing, China). Amplifications were carried out in an Eppendorf gradient thermal cycler (Eppendorf, Hamburg, Germany). PCR cycling consisted of initial denaturation at 94°C for 3 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and then maintained at 72°C for 5 min. 10 μL of the PCR reaction was run on precast 2.5% agarose Tris/Boric Acid/EDTA gels at 120 V. The level of TLR9 transcripts was calculated by relative quantification (22). The RT-PCR results were expressed as the level of TLR9 transcripts relative to the level of β-actin transcripts in the same samples using Quantity One 4.61 (Bio-Rad, Hercules, CA, USA).
Immunohistochemistry (IHC) of TLR9 protein
IHC was performed using a specific primary mouse monoclonal antibody (Abcam, ab12121, Cambridge, UK) against TLR9 in a final 1:50 dilution. First, deparaffinized sections were heated in citrate buffer (pH 6.0) to induce epitope retrieval. After slow cooling to room temperature, the slides were incubated in 10% hydrogen peroxide and goat serum to block unwanted epitopes. The slides were then washed in phosphate-buffered saline (PBS) twice for 5 min and incubated overnight at 4°C with primary antibody. Following this, they were stained with a chromogen purchased from ZSGB-BIO (PV-9000) (Beijing, China), washed in PBS, and counterstained with hematoxylin. The specimens were processed in the absence of primary antibody as negative controls. Positive staining was defined microscopically by visual identification of brown pigmentation either in the cytoplasm or nucleus. The TLR9 positive cells were counted in 10 high power fields of each lesion by light microscopy at ×400 magnification. The number of TLR9 positive cells in each lesion was determined as the average number in 10 high power fields.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 statistical software (GraphPad Software, Los Angeles, CA, USA). Normality of distribution was tested using Shapiro-Wilk test and the data are expressed as mean ± standard deviation (SD). The differences between groups were tested using one-way analysis of variance and least-significant difference test. Correlation analysis was performed by bivariate correlation. Pearson correlation coefficient (r)>0.95 indicated significant correlation; r ≥0.8 high correlation; 0.5≤r <0.8 moderate correlation; 0.3≤r <0.5 low correlation; r <0.3 no correlation; -1≤r <0 negative correlation. Differences were considered statistically significant at P < 0.050.
Results
TNBS induced acute colitis in rats
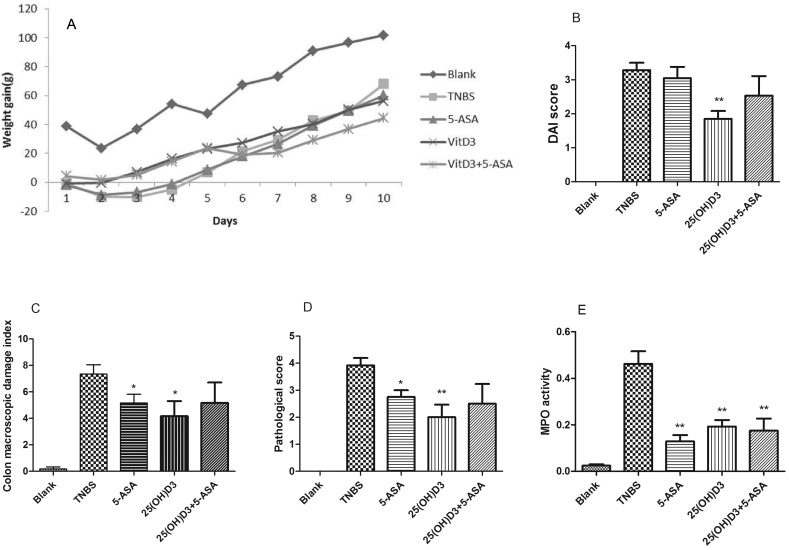
Rats treated with TNBS developed acute colitis, characterized by weight loss, diarrhea, and rectal bleeding, especially in the first three days. Their body weight increased from the day 4 (Figure 1A). On the day 10, the control group had significantly greater weight than other groups (all P < 0.001); while there was no difference between TNBS-treated groups (P = 0.937, P = 0.571, P = 0.941) (Figure 1A). TNBS-treated rats had significantly higher DAI, CMDI, pathological score, and MPO values than control rats (all P < 0.001) (Figure 1B-E). In gross specimens, TNBS colitis is characterized by severe hyperemia and focal mucosal necrosis. In the TNBS colitis groups, transmural inflammation was observed, characterized by infiltration of inflammatory cells in the lamina propria. Some HE sections of TNBS-treated colitis groups showed ulcerations, loss of goblet cells, and diffuse fibrosis. Focal granulomas were also observed (Figure 2B-E).
Figure 1.
Analysis of colitis severity in rats under different treatments. (A) Weight gain. (B) Disease activation index score. (C) Colon macroscopic damage index. (D) Pathological score. (E) Myeloperoxidase activity. TNBS, 2,4,6-trinitrobenzenesulfonic acid; 5-ASA, 5-aminosalicylic acid; VitD3, 1,25-dihydroxyvitamin D. Data are presented as mean ± standard deviation (n = 6). *P < 0.050; **P < 0.010 vs TNBS-treated rats.
Figure 2.
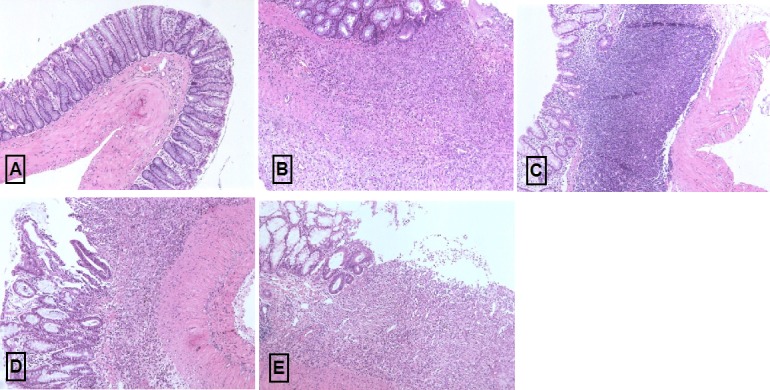
Microscopic features of colons. (A) Control group; (B) TNBS-treated group; (C) TNBS+ 5-ASA treated group; (D) TNBS+ VitD3 treated group; (E) TNBS+5-ASA+ VitD3 treated group. The sections were stained with hematoxylin and eosin. Magnification: 200 × . TNBS, 2,4,6-trinitrobenzenesulfonic acid; 5-ASA, 5-aminosalicylic acid; VitD3, 1,25-dihydroxyvitamin D.
5-ASA and 1,25(OH)D3 attenuate TNBS colitis
We then treated TNBS-induced colitis rats with 5-ASA, 1,25(OH)D3 and their combination. 5-ASA-treated rats showed significantly decreased CDMI (P = 0.049), pathological score (P = 0.010), and MPO activity (P < 0.001). 5-ASA treatment also restored normal histological appearance of the samples (Figure 2C). 1,25(OH)D3-treated rats also showed significantly decreased DAI value (P = 0.001), CDMI (P = 0.040), pathological score (P = 0.005), and MPO activity (P = 0.005). 1,25(OH)D3 treatment diminished the intensity of inflammatory infiltration in the lamina propria (Figure 2D).
Rats treated with both 5-ASA and 1,25(OH)D3 showed significantly decreased MPO activity (P = 0.003), but the difference in pathological score and MPO activity was not significant (P = 0.099) (Figure 1D-E). Thus, the combined treatment did not induce a synergistic effect.
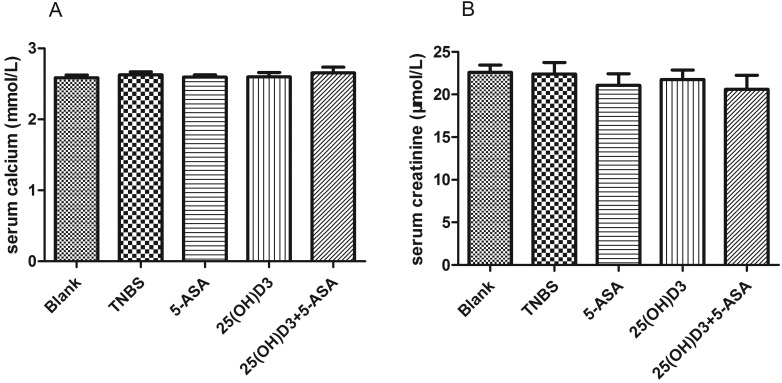
Rat blood serum calcium and creatinine profiles
To monitor the side effects of 1,25(OH)D3, hypercalcemia and renal insufficiency, we measured serum calcium levels and serum creatinine levels. Treatment with 1,25(OH)D3 alone and in combination with 5-ASA did not significantly alter serum calcium and creatinine levels (Figure 3).
Figure 3.
Serum calcium and creatinine profiles. (A) serum calcium levels (mmol/L). (B) serum creatinine levels (μmol/L). TNBS, 2,4,6-trinitrobenzenesulfonic acid; 5-ASA, 5-aminosalicylic acid; VitD3, 1,25-dihydroxyvitamin D. Data are presented as mean ± standard deviations (n = 6).
5-ASA and 1,25(OH)D3 down-regulate TLR9 expression and the number of TLR9 positive cells in TNBS-induced colitis
The mRNA expression of the TLR9 gene was determined using RT-PCR. TLR9 mRNA expression was significantly higher in the TNBS than in the control group (P = 0.008). In 5-ASA-treated rats (P = 0.194) and 1,25(OH)D3-treated rats (P = 0.178) TLR9 gene expression was reduced compared to TNBS group, but neither reduction was statistically significant (Figure 4A).
Figure 4.
Expression of toll-like receptor 9 (TLR9) in colon. (A) Relative expression of TLR9 mRNA level in the colons from each group using β-actin as the internal control gene. (B) The number of TLR9 positive cells in colon from each group. (C) Schematic diagram of TLR9 activation increases myeloperoxidase activity through nuclear factor kB signaling pathway. TLR, toll-like receptor; TNBS, 2,4,6-trinitrobenzenesulfonic acid; 5-ASA, 5-aminosalicylic acid; VitD3, 1,25-dihydroxyvitamin D; NF-kB, nuclear factor kB; TNF, tumor necrosis factor; IL, interleukin; IFN, interferon; MPO, myeloperoxidase; nVDR, nuclear vitamin D receptor. Data are presented as mean ± standard deviation (n = 6). **P < 0.010 vs TNBS-treated rats.
We then assessed the number of TLR9-positive cells by IHC. In the control group, only a few inflammatory TLR9-positive cells were present in the epithelium, lamina, and submucosa. In TNBS-treated group, the number of TLR9-positive cells was significantly higher (all P < 0.010) and they were mostly distributed in the lamina propria. Some goblet cells and epithelial cells were TLR9 positive (Figure 5A-E). 5-ASA treatment, 1,25(OH)D3 treatment, and combined treatment with 5-ASA and 1,25(OH)D3 significantly reduced the number of TLR9-positive inflammatory cells (P < 0.001, P = 0.008, P < 0.001, respectively) (Figure 4B). In TNBS-induced colitis group, a moderate correlation was detected between MPO activity and the number of TLR9-positive cells (r = 0.654, P < 0.001) and a low non-significant correlation between MPO activity and TLR9 gene expression (r = 0.394, P = 0.057).
Figure 5.
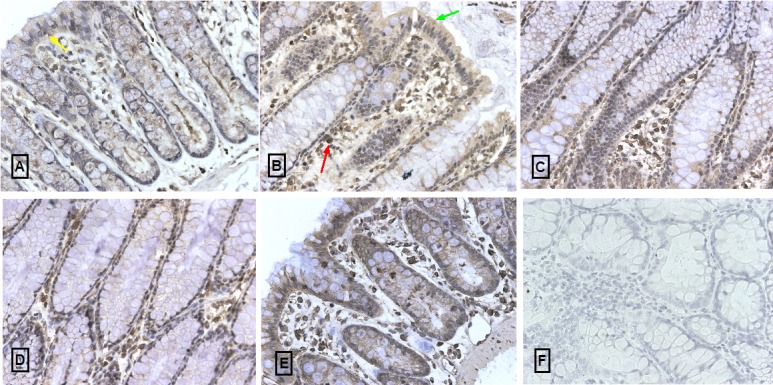
Immunohistochemical staining using an anti-TLR9 primary antibody. (A) Control group. (B) TNBS group. Cells that expressed TLR9 were mostly unevenly distributed in the lamina propria (red arrow). Some goblet cells and epithelium cells were TLR9-positive (green arrow). (C) TNBS+5-ASA group. (D) TNBS+ VitD3 group. (E) TNBS+5-ASA+ VitD3 group. (F) Negative control. Magnification: 400 × . TLR, toll-like receptor; TNBS, 2,4,6-trinitrobenzenesulfonic acid; 5-ASA, 5-aminosalicylic acid; VitD3, 1,25-dihydroxyvitamin D.
Discussion
Consistent with the results of previous research (23,24), our study showed that 1,25(OH)D3 reduced inflammation in TNBS-induced colitis by suppressing infiltration of neutrophil granulocytes. However, the combined application of 5-ASA and 1,25(OH)D3 was no more effective than treatment with 1,25(OH)D3 alone, suggesting no synergistic effects. Vitamin D usage is considered as an adjuvant therapy in IBD, based on previous murine experiments and human studies (25,26). It is possible that the therapeutic effect of vitamin D is not substantial. Also the lack of synergistic effects in our study might reflect an insufficient statistical power of our study. Though numerous studies have reported anti-inflammatory effect of 1,25(OH)D3 in vitro, only one placebo-controlled trial evaluated its effect in CD patients (27). Jorgensen et al (27) reported that treatment with vitamin D markedly reduced the rate of clinical relapses from 29% to 13%, but the reduction was not significant. It is possible that 1,25(OH)D3 is beneficial to IBD patients, but further large-scale, multi-center clinical trials are needed to investigate this issue.
TLRs are usually expressed in inflammatory cells, such as macrophages and dendritic cells, and play a central role in the innate immune system. TLR9 binds to unmethylated CpG DNA in intestinal bacteria and activates an intestinal immune response through the NF-κB signaling pathway. We demonstrated that a small amount of TLR9 was expressed in the colon of control rats, which is consistent with previous studies (28,29). The limited expression of TLR9 in control rat colons could reduce intestinal commensal bacteria antigen recognition, and help control the specific immune response. In TNBS-induced colitis, a significant increase in TLR9 expression was observed. Consistent with the results of previous studies in IBD patient specimens (30,31) and other animal experiments (32), our data showed that TLR9 was not only expressed in inflammatory cells in rats with colitis but also in epithelial cells and goblet cells. Lee et al (33) found that apical TLR9 stimulation conferred tolerance to subsequent TLR challenge. Due to TLR9-assoiated activation, antigen-presenting cells produce IFN-α, resulting in activated Th1 cells, which produce pro-inflammatory cytokines, such as interleukin 6 and TNF-α. These pro-inflammatory cytokines activate neutrophil granulocytes, increasing the activity of MPO (34). The positive correlation between TLR9 expression and MPO activity detected in our experiment is consistent with a previous study (35). Our data, along with these observations, suggested that TLR9 plays a crucial role in maintaining intestinal homeostasis.
Studies by Sadeghi et al and Dickie et al (36,37) both reported that 1,25(OH)D3 suppressed the expression of TLR proteins and mRNA in human monocytes in a time- and dose-dependent fashion in vitro and reduced the recognition of bacterial specific antigens through monocytes. Another recent study showed that it decreased TLR-induced cytokine production and enhanced cytokine levels in peripheral mononuclear cells and monocyte-derived dendritic cells from CD patients (38). Our data showed that treating TNBS-induced colitis with 1,25(OH)D3 could reduce TLR9 expression in the rat colon. Moreover, we demonstrated that the severity of colitis was correlated with TLR9 expression. Thus, we hypothesize that 1,25(OH)D3 could relieve inflammation in TNBS-induced colitis by reducing TLR9 expression.
One of the study limitations is the small sample size, however the heterogeneity among rats was very low. We also did not use various concentration gradients of 1,25(OH)D3. According to the United States Institute of Medicine, the recommended dietary allowance of vitamin D in adult people is 15 μg/d (39). Different doses of supplemental vitamin D are based on the 1,25(OH)D concentration. Patients suffering from osteoporosis are usually treated with calcitriol at a dose of 1 μg/d. Thus, we used 0.2 μg/kg/d 1,25(OH)D3 to treat TNBS induced colitis, which is equivalent to 1 μg/d in a human subject. Finally, we only used TNBS, instead of dextran sulfate sodium, to induce IBD in rats. Our choice was based on previous studies, which showed that the TNBS-induced acute colitis is a Th1-mediated colitis, which resembles CD.
As vitamin D supplementation is readily available at a low cost, it is potentially a very attractive therapeutic option. The vitamin D analog, 22-ene-25-oxa-vitamin D (ZK156979) could both preserve immunosuppressive activity in vitro and ameliorate inflammation in TNBS-induced colitis without causing hypercalcemia (40,41), which offers a new therapeutic option for the treatment of human IBD. In conclusion, our study showed that 1,25(OH)D3 relieved TNBS-induced colitis in rats by down-regulating TLR-9, which might be associated with inflammation. Further studies are needed to clarify the exact molecular mechanisms involved in this process.
Acknowledgments
Funding received from Janssen Research Council China; Grant number: 2010 and Chinese National Scientific Research Special-purpose Project in Public Health Profession; Grant number: 201002020.
Ethical approval received from the Ethics Committee of Peking Union Medical College.
Declaration of authorship ZD performed the experiments and data collection, and wrote the article. BT performed the experiments. HY took part in research design. OW took part in research design. JQ provided the idea. HL conceived the project and provided the idea.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–7. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 3.Bours PH, Wielders JP, Vermeijden JR, van de Wiel A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int. 2011;22:2857–67. doi: 10.1007/s00198-010-1484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan B, Li P, Lv H, Li Y, Wang O, Xing XP, et al. Vitamin D levels and bone metabolism in Chinese adult patients with inflammatory bowel disease. J Dig Dis. 2014;15:116–23. doi: 10.1111/1751-2980.12118. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YG, Wu S, Sun J. Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers. 2013;1:e23118. doi: 10.4161/tisb.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 8.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 9.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 10.Parker LC, Prince LR, Sabroe I. Translational Mini-Review Series on Toll-like Receptors: Networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–9. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bank S, Skytt AP, Burisch J, Pedersen N, Roug S, Galsgaard J, et al. Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS ONE. 2014;9:e98815. doi: 10.1371/journal.pone.0098815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torok HP, Glas J, Tonenchi L, Bruennler G, Folwaczny M, Folwaczny C. Crohn's disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004;127:365–6. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 15.Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford) 2010;49:1466–71. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 16.Choi B, Lee ES, Sohn S. Vitamin D3 ameliorates herpes simplex virus-induced Behcet's disease-like inflammation in a mouse model through down-regulation of Toll-like receptors. Clin Exp Rheumatol. 2011;29(4) Suppl 67:S13–9. [PubMed] [Google Scholar]
- 17.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010;107:22593–8. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–8. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990;258:G527–34. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- 20.Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, et al. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther. 1999;13:1535–42. doi: 10.1046/j.1365-2036.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 21.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett JMS, Stirling D. A short history of the polymerase chain reaction. PCR protocols. methods in molecular biology. 226 (2nd ed.). Totowa, NJ: Humana Press; 2003. [DOI] [PubMed] [Google Scholar]
- 23.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–86. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–32. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 26.Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, et al. Comparison of the effects of 1,25 Dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis. 2009;15:1656–62. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, et al. Clinical trial: vitamin D3 treatment in Crohn's disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 28.Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 29.Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–9. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Mraz M, et al. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol. 2008;151:34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267–74. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgener IA, Konig A, Allenspach K, Sauter SN, Boisclair J, Doherr MG, et al. Upregulation of toll-like receptors in chronic enteropathies in dogs. J Vet Intern Med. 2008;22:553–60. doi: 10.1111/j.1939-1676.2008.0093.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Rachmilewitz D, Raz E.Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci. 2006;1072:351-5 [DOI] [PubMed] [Google Scholar]
- 34.Fűri I, Sipos F, Germann TM, Kalmár A, Tulassay Z, Molnár B, et al. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: clinico-pathogenic aspects. World J Gastroenterol. 2013;19:4119–26. doi: 10.3748/wjg.v19.i26.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H, Jiang JX, Zhu PF, Wang ZG, Zhang DJ. The relationship of the expression of principal pattern recognition receptor with the pulmonary injury in abdominal infection-induced sepsis. Zhonghua Wai Ke Za Zhi. 2006;44:613–7. [PubMed] [Google Scholar]
- 36.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–70. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 37.Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford) 2010;49:1466–71. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 38.Dionne S, Calderon MR, White JH, Memari B, Elimrani I, Adelson B, et al. Differential effect of vitamin D on NOD2- and TLR-induced cytokines in Crohn's disease. Mucosal Immunol. 2014;7:1405–15. doi: 10.1038/mi.2014.30. [DOI] [PubMed] [Google Scholar]
- 39.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel C, Radeke HH, Sartory NA, Zahn N, Zuegel U, Steinmeyer A, et al. The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. J Pharmacol Exp Ther. 2006;319:622–31. doi: 10.1124/jpet.106.107599. [DOI] [PubMed] [Google Scholar]
- 41.Daniel C, Schlauch T, Zugel U, Steinmeyer A, Radeke HH, Steinhilber D, et al. 22-ene-25-oxa-vitamin D: a new vitamin D analogue with profound immunosuppressive capacities. Eur J Clin Invest. 2005;35:343–9. doi: 10.1111/j.1365-2362.2005.01492.x. [DOI] [PubMed] [Google Scholar]