Abstract
Human susceptibility to environmental carcinogens is highly variable and depends on multiple genetic factors, including polymorphisms in cytochrome P450 genes. Although epidemiological studies have identified individual polymorphisms in cytochrome P450 genes that may alter cancer risk, there is often conflicting data about whether such polymorphisms alter the genotoxicity of environmental carcinogens. This is particularly true of the CYP1A2 polymorphisms that confer differential activation of multiple human carcinogens. To determine whether a single cytochrome P450 polymorphism confers higher levels of carcinogen-associated genotoxicity, we chose an organism that lack enzymes to metabolically activate aflatoxins and expressed individual human P450 genes in budding yeast. We measured the frequencies of recombination, Rad51 foci formation, 7-methoxyresorufin O-demethylase, and the concentrations of carcinogen-associated DNA adducts in DNA repair proficient yeast expressing P450 polymorphisms after exposure to aflatoxin B1 (AFB1). We measured growth of rad4 rad51 cells expressing CYP1A2 polymorphisms while exposed to AFB1. We observed that there was significantly less AFB1-associated genotoxicity in yeast expressing I386F, while yeast expressing CYP1A2 C406Y exhibited intermediate levels of genotoxicity compared to yeast expressing CYP1A2 D348N or wild type. We conclude that differences in carcinogen genotoxicity can be observed in yeast expressing different CYP1A2 alleles. This is the first report that carcinogen-associated P450 polymorphisms can be studied in yeast.
Keywords: aflatoxin B1, budding yeast, cytochrome P450
1. Introduction
An estimated 19% of all cancers are environmentally related [1]. For example, hepatocellular carcinoma (HCC) is correlated with exposure to dietary carcinogens and fungal toxins, such as aflatoxins [2,3,4]. However, there is great individual variability in cancer incidence among exposed individuals. One reason for the variability is that an estimated 75% of environmental carcinogens require metabolic activation [5,6]. These enzymes include cytochrome P450s, which catalyze the hydroxylation of hydrophobic aromatic compounds, rendering them more hydrophilic and soluble for excretion [7]. However, highly reactive intermediates such as epoxides can bind covalently to protein or DNA [8,9], leading to mutations and changes in cell phenotype. P450 enzymes known to activate dietary carcinogens, such as aflatoxin B1 (AFB1), include CYP1A1, CYP1A2, and CYP3A4 [10].
CYP1A2, which is mostly expressed in the liver, contributes 13–15% of the total hepatic CYP activity (for review, see [11,12]). CYP1A2 activates nitroaromatic compounds, heterocylic aryl amines, estrogens and to a lesser extent, polycyclic aromatic hydrocarbons [13,14,15]. While CYP3A4 also metabolically activates mycotoxins, the importance of CYP1A2 is underscored by its ability to activate low concentrations of AFB1 in the liver [16,17]. Although CYP1A2 is non-essential in the mouse [18], the Cyp1a2−/− mice [19] exhibit much longer clearance time for particular drugs, such as caffeine and zoxazolamine, a muscle relaxant. Human CYP1A2, by inference, is likely a non-essential enzyme, which shares similar functions with mouse CYP1A2.
Rapid CYP1A2 activity is a suggested risk factor in colon and bladder cancer (for review see [20]). Interestingly, hepatic expression levels can vary by as much as 60-fold between individuals [21,22]. CYP1A2 enzymatic levels can be affected by cigarette smoking, drug exposure, liver function and inflammation, thus reflecting both environmental and life style factors [22]. Nonetheless, studies involving mono- and dizygotic twins indicate that as much as three-quarters of the variability may result from genetic factors [23]. Such genetic factors include those that act in trans, such as ARNT, AhRR, HNF1α, IL1β, SRC-1, and VDR, as well as single-nucleotide polymorphisms (SNPs) at the CYP1A2 locus [24]. Consequently, it is important to understand which genetic factors affect CYP1A2 activity and its substrate specificity (Klein, 2010 [24]).
Thirty-six CYP1A2 haplotypes have been published [27] and within specific populations, as in Ethiopia, CYP1A2 shows great genetic variability [28]. Allelic variants are found within the 7.8 kb human CYP1A2 gene, a gene that has seven exons, one of which is non-coding [25,26]. Which and how these alleles confer clinical phenotypes are not well understood. CYP1A2*1C, the 5′-upstream variant 3860G > A, has been found to correlate with a decreased risk for HCC [29], but to an increase in recurrence of head and neck cancer [30]. CYP1A2*1F, the intron 1 polymorphism −163C > A located downstream of the untranslated first exon, is associated with pancreatic cancer among heavy smokers [31], with squamous cell lung carcinoma, and with colorectal adenomas [32,33]. Even less is known about the rare polymorphisms that are found in coding sequences, such as those originally found in the French [34] and Japanese populations [35]. Thus, additional methods, besides epidemiological, are required to ascertain the functional significance of CYP1A2 alleles.
One promising approach to determine whether CYP variation affects CYP activity and susceptibility to human carcinogens is to express the variant CYP gene in an organism that does not contain any other human CYP gene. Jiang et al. [36] inserted a BAC vector containing human CYP1A2/CYP1A1 locus into a transgenic CYP1A2−/− mouse that lacks the entire mouse CYP1A2/CYP1A1 locus. Several candidate single nucleotide polymorphisms (SNPs) have been introduced into the transgenic, humanized mice; however, results obtained can only account for a small fraction of the CYP1A2 variability [36].
Expressing human CYP genes in Escherichia coli is a rapid approach to determine whether specific CYP variants in coding sequences affect CYP activity. This approach requires modifications of the N terminus to maximize expression and has been successful in characterizing several CYP1A2 polymorphisms, including CYP1A I386F (CYP1A2*4), CYP1A2 C406Y (CYP1A2*5), CYP1A2 D348N, and CYP1A2 R456H [37, 38]). Interestingly, the allele CYP1A2 C406Y actually confers higher kcat/Km (about 2-fold) and a higher mutagenic response after exposure to low doses of 2-amino-3,5-dimethylimadazo[4,5-f]quinoline (MeIQ) in an Escherichia coli mutagenesis assay [37]. These data indicate that although there are some alleles in the amino acid sequences that can confer lower activity, others can confer higher activity [37] or altered substrate specificity [17]. Based on proposed crystal structures by Sansen et al. [39], there is a narrow substrate binding site; CYP1A2 alleles that map near the active site, such as I386F, may confer altered affinities to substrates [17].
The purpose of this study is to functionally characterize CYP1A2 polymorphisms in the budding yeast Saccharomyces cerevisiae. AFB1 activation is solely dependent upon the activity of the introduced CYP1A2 gene, since budding yeast does not contain an analogous gene. Characterization of P450 gene activity is further facilitated in yeast because: 1) the full-length, unmodified P450 genes can be expressed, 2) in vivo genotoxic endpoints can measure carcinogen activation 3) mutants defective DNA repair are hypersensitive to P450-activated carcinogens. Using budding yeast, we had previously detected AFB1-associated DNA adducts and showed that AFB1 exposure is sufficient to stimulate both recombination and mutation in DNA repair proficient strains[40, 41, 42, 43] but rapidly decreases viability in a mutant defective in both nucleotide excision and recombinational repair [43]. In this study we used these genotoxicity endpoints to distinguish phenotypic differences between CYP1A2 alleles. We suggest that this method can be used to detect phenotypic differences between other P450 alleles.
2. Materials and Methods
2.1 Media and chemicals
Standard media were used for the culture of yeast cells. YPD (yeast extract, peptone, dextrose), SC-TRP (synthetic complete lacking tryptophan), SC-URA (synthetic complete lacking uracil) and medium containing 5-fluoro-orotic acid (FOA medium) were described in Burke et al. [44]. Stock solutions of 10 mM AFB1 (Sigma) were dissolved in dimethyl sulfoxide (DMSO).
2.2 Plasmid constructions and site-specific mutagenesis
Standard molecular biology techniques for DNA isolation and bacterial transformation were used to construct vector pRS424-CYP1A2, pRS414-CYP1A2 and pMF-hOR [45]. Human CYP1A2 (CYP1A2*1A, [27]) was subcloned by inserting the SacI CYP1A2 fragment from pCS316 [40] into the multi-copy vector pRS424 and centromere-containing vector pRS414 [46]. pMF::hOR was constructed by inserting the BamH1 fragment containing the hOR (POR*1) gene in pSB229 [40] into pMF [47]. The human hOR gene is thus flanked by DNA sequences both centromere proximal and distal to TRP1.
Site-specific mutagenesis was performed using QuickChange kit (Stratagene) according to the manufacturer’s instructions. We constructed C406Y, D348N, and I386F usingthe primer pairs previously published by Zhou et al. [37]. The entire gene was subsequently sequenced to verify that only the base substitution was introduced.
2.3 Yeast strains
The genotypes of yeast strains used in this study are listed in Table 1. Strains used to measure AFB1-associated recombination or mutagenesis are isogenic to S288c; strains used for detecting Rad51 foci are derived from W303[43]. For measuring AFB1-associated translocation frequencies, diploid strains contained trp1::hOR and recombination substrates, his3-Δ3′ and his3Δ5,′ as described [48]. Plasmids containing CYP1A2 and alleles were introduced into yeast strains by selecting for Trp+ transformants.
Table 1.
Yeast Strains
| Strain (Synonym) | Genotype | Autonomous plasmid | Reference (Source) |
|---|---|---|---|
| YA101 (MCY726) | MATa ura3-52 his3-Δ200 ade2-101 lys2-801 | M. Carlson | |
| BY4743 | MATa/MATα ura3Δ0/− leu2Δ0/− his3Δ1/− LYS2/lys2Δ0 met15Δ0/MET15 | wiki.yeastgenome.org/index.php/Commonly_used_strains | |
| YB226 | MATa-inc ura3 rad4::KanMX rad51::URA3 | This laboratory | |
| YB318 | MATα ura3-52 his3-Δ200 ade2-n trp1-Δ1 gal3− leu2-3, 112 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs | This Laboratory | |
| YB400 | MATa-inc ura3 rad4::KanMX rad51 | FOA resistant isolate of YB226 | |
| YB403 | MATa-inc ura3 rad4::KanMX rad51 | pCS316 | Ura+ transformant of YB400 |
| YB405 (LSY1957) | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1 ura3 his3 | pRS424-CYP1A2 | L.Symington |
| YB406 | MATa ura3-52 trp1::hOR his3-Δ200 ade2-101 lys2-801 | This Laboratory | |
| YB407 | MATα ura3-52, trp1::hOR his3-Δ200, ade2-101 lys2− 801 | This Laboratory | |
| YB408 | MATa/MATα ura3-52/− his3-Δ200/− ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs | Diploid cross of YB407 and YB318 | |
| YB409 | MATa/MATα ura3-52/− his3-Δ200/− ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs | pRS424 | Trp+ transformant of YB408 |
| YB410 | MATa/MATα ura3-52/− his3-Δ200/− ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3− Δ5′ trp1::his3-Δ3′::HOcs | pRS424 CYP1A2 | Trp+ transformant of YB408 |
| YB411 | MATa/MATα ura3-52/− his3-Δ200/− ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3− Δ5′ trp1::his3-Δ3′::HOcs | pRS424CYP1A2 C406Y | Trp+ transformant of YB408 |
| YB412 | MATa/MATα ura3-52/− his3-Δ200/− ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3− Δ5′ trp1::his3-Δ3′::HOcs | pRS424CYP1A2 D348N | Trp+ transformant of YB408 |
| YB413 | MATa/MATα ura3-52/− his3-Δ200/− ade2-101/ade2-n trp1-Δ1/trp1::hOR leu2-3,112/leu2-801 GAL1::his3-Δ5′ trp1::his3-Δ3′::HOcs | pRS424 CYP1A2 I386F | Trp+ transformant of YB408 |
| YB414 | MATa-inc ura3 rad4::KanMX rad51 | pRS424 | Trp+ transformant of YB400 |
| YB415 | MATa-inc ura3 rad4::KanMX rad51 | pRS424-CYP1A2 | Trp+ transformant of YB400 |
| YB416 | MATa-inc ura3 rad4::KanMX rad51 | pRS424-CYP1A2 C406Y | Trp+ transformant of YB400 |
| YB417 | MATa-inc ura3 rad4::KanMX rad51 | pRS424-CYP1A2 D486N | Trp+ transformant of YB400 |
| YB418 | MATa-inc ura3 rad4::KanMX rad51 | pRS424-CYP1A2 I386F | Trp+ transformant of YB400 |
| YB419 | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1::hOR ura3 his3 | Diploid cross of YB405 and YB407 | |
| YB420 | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1::hOR ura3 his3 | pRS424 | Trp+ transformant of YB419 |
| YB421 | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1::hOR ura3 his3 | pRS424-CYP1A2 | Trp+ transformant of YB419 |
| YB422 | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1 ura3 his3 | pRS424CYP1A2 C406Y | Trp+ transformant of YB419 |
| YB423 | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1 ura3 his3 | pRS424CYP1A2 D348N | Trp+ transformant of YB419 |
| YB424 | MATa YFP-rad51-I345T-URA3-RAD51 ADE2 leu2 trp1 ura3 his3 | pRS424CYP1A2 I386F | Trp+ transformant of YB420 |
For measuring AFB1-associated translocation frequencies, a diploid strain was made by mating the haploid containing trp1::hOR with a haploid (YB318) containing the recombination substrates, his3-Δ3′ and his3Δ5,′ as described [49]. Haploid strains containing trpl::hOR were were made by introducing pMF::hOR (this study) into the Trp+ strain YA101 by selecting for Ura+ transformants. FOA resistant Trp− isolates were obtained that had deleted the pMF sequences but had maintained the human hOR; this was confirmed by PCR using the forward primer AGGAGACAGACGTGGATCTCTCTG and the reverse primer AAGCCAAACACACCCAGGAGACTA.
The rad4 rad51 strains, defective in both nucleotide excision and recombinational repair, were used for measuring CYP1A2-mediated AFB1 cytotoxicity[50]. A Ura− derivative (YB400) of the rad4 rad51 strain (YB226) was selected on FOA medium. The UV (60 J/m2) and X-ray sensitivities (4 krads) of the Trp+ or Ura+ transformants of rad4 rad51 containing CYP1A2 alleles were confirmed.
Strains used to detect Rad51 foci were derived from LSY1957, a gift of L. Symington [51]. This strain was crossed with a haploid containing trpl::hOR (YB407) and the meiotic segregant YB419 was obtained that contains both yfp-RAD51 and trpl::hOR.
2.4 Western blot analysis
Expression of CYP1A2 was determined by Western blots. Cells were inoculated in SC-TRP medium. Cells in log growth phase (A600 = 0.5–1) were concentrated and protein extracts were prepared as previously described by Foiani et al. [52] or by Dyavaiah et al [53]; the first protocol yields concentrated protein while the second protocol utilizes a boiling method better suited for the detection of β-tubulin. Proteins were separated on 10% acrylamide/0.266% bis-acrylamide gels and transferred to nitrocellulose membranes. Human CYP1A2 was detected by Western blots using goat anti-CYP1A2 (Abcam), and a secondary bovine anti-goat antibody. For a loading control on Western blots, β-tubulin was detected using a rabbit anti-β-tubulin and a secondary goat anti-rabbit antibody. Signal was detected by chemiluminescence.
2.5 Measuring DNA adducts
To measure the AFB1-associated DNA adducts in yeast, we used liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI/MS) [54]. Three independent log phase cultures of yeast expressing each human CYP1A2 allele were exposed to 50 μM AFB1 for 4 h. Cells were washed twice in 50 mM NaPO4 pH 7.4 and frozen at −80°C. Because standard protocols for isolating yeast DNA involve alkaline buffers, rendering the highly unstable AFB1 N7-Gua DNA adducts labile, we modified the “smash-and-grab” protocol [55] and substituted a neutral buffer containing 10 mM Tris–HCl, 1 mM EDTA, 100 mM NaCl, 2%Triton X-100, 1% SDS, pH 7 for the alkaline lysis buffer. DNA was then isolated and resuspended in H2O from three independent samples of yeast cells. The DNA adducts were identified and measured by high performance liquid chromatography and LC/ESI/MS after acid hydrolysis [54]. Controls included diploid cells that did not contain any CYP1A2 gene.
2.6 Measuring CYP1A2 enzymatic activity
We measured CYP1A2 enzymatic activity using a modified protocol described by Pompon et al [56]. In brief, cells obtained from 100 ml of selective media were pelleted and resuspended in 5 ml Tris EDTA KCl (pH 7.5, TEK) buffer. After 5 min incubation at room temperature, cells were pelleted, resuspended in 1 ml 0.6 M Sorbitol Tris pH 7.5, and glass beads were added. Cells were lysed by agitation. The debris was pelleted at 10,000 x g at 4°C, and the supernatant was diluted in 0.6 M Sorbitol Tris pH 7.5 and made 0.15 M in NaCl and 1% in polyethylene glycol (MW 3350) in a total volume of 5 ml. After incubation on ice for 1 h and centrifugation at 10,000 rpm for 20 min, the precipitate was resuspended in Tris 10% glycerol pH 7.5, and stored at −80°C.
CYP1A2 enzymatic activity was measured in cell lysates by quantifying 7- methoxyresorufin O-demethylase (MROD) activities, using a modified protocol first described for assaying ethoxyresorufin O-deethylase (EROD) activity in yeast [57]. The buffer contained 10 mM Tris pH 7.4, 100 μM methoxyresorufin (Sigma) and 6.7 mM NADPH. The production of resorufin was measured in real-time by fluorescence in a Tecan plate reader, calibrated at 535 nm for excitation and 580 nm for absorption, and standardized using serial dilutions of resorufin. The reaction was started by the addition of NADPH and resorufin was measured at one minute intervals during the one hour incubation at 37 °C; rat liver microsomes (S9) were used as a positive control while the reaction without NADPH served as the negative control. Enzyme activities were measured in duplicate for at least two independent lysates from each strain and expressed in pmole/min/mg protein [58].
2.7 Measuring AFB1-associated recombination
To measure AFB1-associated genotoxic events, log phase yeast cells (A600 = 0.5–1) were centrifuged and concentrated five-fold in nutrient media (SC-URA or SC-TRP). Cells were exposed to different AFB1concentrations or to the solvent (DMSO) alone. Cells were maintained in nutrient media (SC-URA or SC-TRP) during the carcinogen exposure and then washed twice in H2O. For measuring recombination, cells were directly plated on SC-HIS and an appropriate dilution was inoculated onto YPD to measure viability. Typically, 1–20 colonies were counted on SC-HIS and 100–200 colonies were counted on YPD. Statistical significance was determined using the Student’s t-test.
2.8 Live cell epifluorescence and microscopy analysis
Cells for microscopic analysis were grown to early to mid-log phase overnight in synthetic medium. Strains harboring pRS424-CYP1A2 were grown in SC-TRP. Cells were harvested by centrifugation and concentrated 5-fold in the growth medium. Immobilization of cells was performed by mixing equal volumes of cell suspension and 1.4% low melt agarose plus growth medium solution. Cover slips were sealed with a wax mixture as described by Lisby et al. [33]. Slides were visualized using a Zeiss LSM 510 META confocal microscope.
To determine whether ionizing radiation stimulates the formation of Rad51 foci, cells were washed once in H2O and resuspended in 10 ml H2O and placed in a 81 mm diameter Petri dish. Cells were irradiated at 4 krads (40 Gy) using a Nordion 1.8kCi 137Cs irradiator (6 krad/h). After irradiation cells were concentrated in YPD medium and immobilized on glass slides. Slides were then incubated for at least 90 min at 30°C before being examined by confocal microscopy.
2.9 Growth assays in 96 well plate to measure AFB1 sensitivity
Individual saturated cultures were prepared for each yeast strain. Cell density was adjusted to ~0.8 x 107 cells/ml for all cultures. We maintained the cells in selective medium (synthetic dextrose lacking tryptophan). In each microtiter well, 90 μl medium and 10 μl cell suspension (8 x 104 cells) were aliquoted in triplicate for blank, control and experimental samples. For experimental samples, we added AFB1, dissolved in DMSO, for a final concentration of 100 nM and 10 μM. The microtiter dish was sealed with clear optical tape and placed in a plate reader that is capable of both agitating and incubating the plate at 30° C, as previously described [43]. We measured the OD (A660) at 10 min intervals, for a total period for 24 h, 145 readings. Data at 1h intervals was then plotted.
3. Results
Previous studies have indicated that, when expressed on high-copy number expression vectors, human CYP1A2 enzyme can metabolically activate AFB1 in budding yeast [40, 41, 42]. We used genotoxicity endpoints in budding yeast to detect differences in the metabolic activation conferred by polymorphisms in the coding sequence of CYP1A2. By site-specific mutagenesis, C406Y, D486N and I386F alleles were generated, confirmed by DNA sequencing, and subcloned into the yeast vector pRS424-CYP1A2, and the entire CYP1A2 gene was sequenced to verify the individual base substitutions. The four vectors, each containing an individual P450 allele, were then introduced into yeast strains that also express the human oxidoreductase (hOR). We measured MROD activities and four AFB1-associated genotoxic endpoints, including recombination frequencies, N7-guanine AFB1DNA adducts, Rad51 foci, andgrowth rates. Controls included both cells exposed to solvent alone, and cells containing the empty vector pRS424.
3.1 Recombination can be used as an endpoint to measure differences in metabolic activation of aflatoxin B1
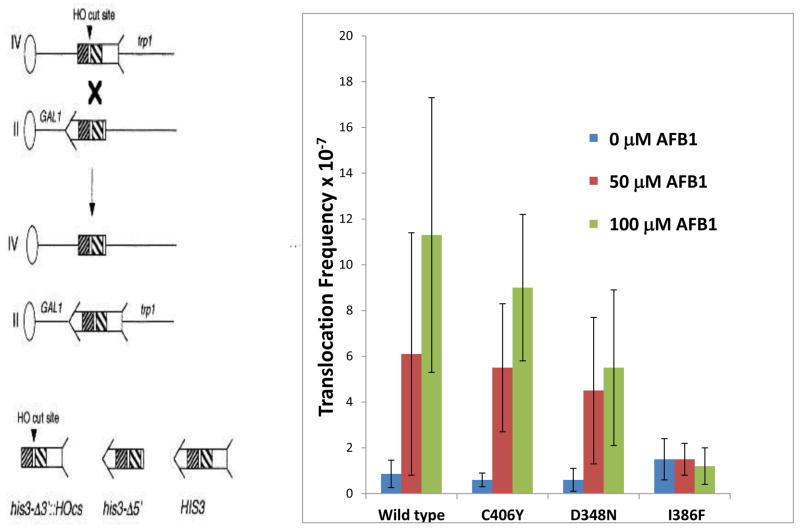
Previously, AFB1 has been shown to be a potent yeast recombinagen, with translocation frequencies increasing in the linear range after exposures between 50 μM and 100 μM AFB1 [40,41,42]. We measured recombination after exposing cells to 0 μM, 50 μM and 100 μM AFB1, using a diploid strain in which hOR had been inserted at the TRP1 locus in yeast. By western blots, we showed that each CYP1A2 allele is expressed in yeast, although wild-type CYP1A2 protein appeared more abundant in Western blots (Figure 1). Expression of the allelic variants is comparable to wild type CYP1A2 (~60–90%) when normalized to β-tubulin protein levels (N =2, supplemental Figure 1). Expression of P450 alleles per se does not increase the frequency of spontaneous translocations and is ~1 x 10−7 (recombinants/viable cell) for all strains. Because 107 cells are inoculated per plate, it is likely that most of the recombinants that appear after exposure to AFB1 result from carcinogen exposure. Overall, levels of AFB1-associated recombination in cells expressing CYP1A2 using the pRS424 vector were slightly lower than in cells expressing pCS316, a high-copy number vector containing CYP1A2 and hOR. Two transformants were analyzed for each strain. Cells expressing CYP1A2 C406Y and CYP1A2 D384N exhibited intermediate levels of translocation frequencies (Figure 2), compared to wild type; however, the frequencies of AFB1-associated recombination were not significantly different from that of wild type (P> 0.05). Cells expressing I386F exhibited no significant increase in recombination frequencies. To confirm that AFB1-associated His+ recombinants contain the expected translocations, the electrophoretic karyotype was determined by contour-clamped homogeneous electric field (CHEF, data not shown), as in previous publications [40,42,47], and the expected translocations were observed (Figure 1). These results indicate that AFB1-associated recombination occurred in diploid cells expressing three different CYP1A2 alleles, with the exception of I386F.
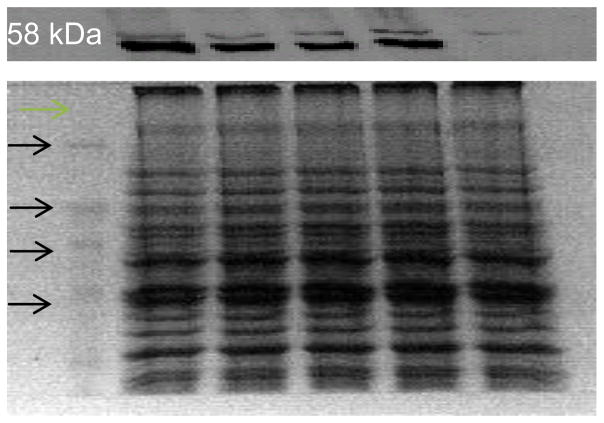
Figure 1.
Expression of human CYP1A2 in diploid yeast strains as detected by Western blots. Lanes correspond to molecular weight markers, and extracts from strains expressing CYP1A2 (YB410), CYP1A2 C406Y (YB411), CYP1A2 D348N (YB412), CYP1A2 I386F (YB413) and empty vector pRS424 (YB409), as marked. Each lane contains 10 μg of protein, as determined by Bradford assay. Equal amounts of protein were loaded as indicated by a parallel Coomassie-stained gel (bottom panel). The molecular weight markers are indicated, proceeding from 120 kDa (top), 80 kDa, 60 kDa, to 40 kDa (bottom).
Figure 2.
Frequencies of AFB1-associated recombination in diploid cells expressing CYP1A2 (YB410), CYP1A2 I386F (YB413), CYP1A2 C406Y (YB411), and CYP1A2 D348N (YB412). Figure on the left is a model of the recombination assay. The oval represents a centromere and the line represents the chromosome; the left arm of the chromosome is not shown for simplicity. The his3 fragment is shown with arrow and feathers. The shaded areas represent shared homology. An “X” denotes where a cross-over event would occur. The product of the recombination event is shown below where CEN2 is linked to the long arm of chromosome IV and CEN4 is linked to the long arm of chromosome II. Figure on the right shows the recombination frequencies after cells were exposed to 0, 50 μM, and 100 μM AFB1. The CYP1A2 allele is indicated on the x axis.
3.2 Correlations between AFB1-associated DNA adduct levels and AFB1-associated recombination
We measured N7-Guanine AFB1 DNA adduct levels after 50 μM AFB1 exposure in the same diploid strains used to measure recombination (Table 2). DNA was extracted from cells, using a protocol to maintain the stability of the N7-adduct at neutral pH. Diploid strains that do not express CYP1A2 exhibit no detectable levels of AFB1 DNA adducts by LC/ESI/MS, consistent with previous studies using 3H- AFB1 to detect DNA adducts [59]. The levels of N7-Guanine AFB1 DNA adducts in diploid cells expressing pRS424-CYP1A2 were the same as those observed in haploid cells containing pCS316 plasmid.. We expected that, compared to wild type, low levels of AFB1-associated DNA adducts would correspond to low levels of AFB1-associated recombination. Expression of I386F and C406Y alleles yielded lower levels of recombination and adduct levels; however, only adduct levels from cells expressing I586F were significantly different than wild type (P ≤ 0.05). D384N, on the other hand, exhibited about equivalent levels of recombination and DNA adducts. We conclude, therefore, that significantly low levels of DNA adducts correspond to low levels of AFB1-associated recombination.
Table 2.
Concentrations of AFB1 7N-Gua Adducts in DNA.
| CYP450a | AFB1 7N-Guanine/mg DNAb | Ratioc |
|---|---|---|
| CYP1A2 | 5.0 ± 3.9 pmole | 1 |
| C406Y | 2.2 ± 0.05 pmole | 0.4 |
| I386F | 0.3 ± 0.16 pmole | <0.1 |
| D348N | 8.3 ± 6.9 pmole | 1.7 |
| None | ≤0.05 pmole | <0.01 |
Indicates CYP1A2 allele expressed in yeast
N > 2, generated after exposure to 50 μM AFB1
Adducts CYP1A2 allele/Adducts WT, numbers in bold indicate significant differences with wild type.
We also correlated CYP1A2 enzymatic activities with levels of recombination by obtaining microsomal fractions from yeast strains containing CYP1A2 alleles and measuring MROD activities. Guo et al [58] previously observed that diploid strains containing pCS316 express between 3 and 12 pmole/min/mg MROD activity. We observed that MROD enzymatic activities varied between 1–4 pmole/min/mg (2.4 ± 1, N =3) pmole/min/mg in the diploid BY4743 that contained pCS316. In diploid strains in which hOR was expressed from a chromosomal copy while CYP1A2 was expressed from a multi-copy plasmid, we found that cells expressed eight - fold lower activity (0.3 pmole/min/mg), compared to cells that contain pCS316. In diploid strain containing CYP1A2 C406Y, we observed lower activity (0.2 pmole/min/mg) compared to the strain containing CYP1A2 wild type; however, the difference was not statistically different (P = 0.3). Diploid strains containing CYP1A2 D348N or CYP1A2 I386F expressed barely detectable activities (<0.1 pmole/min/mg), while no activity was observed in diploids that did not contain any CYP1A2. Thus, similar to DNA adduct levels, strains expressing CYP1A2 I386F express low levels of enzymatic activities that correlate with low levels of DNA damage-associated recombination.
3.3 Rad51 foci can be used as an endpoint to detect AFB1 activation in cells expressing CYP1A2 alleles
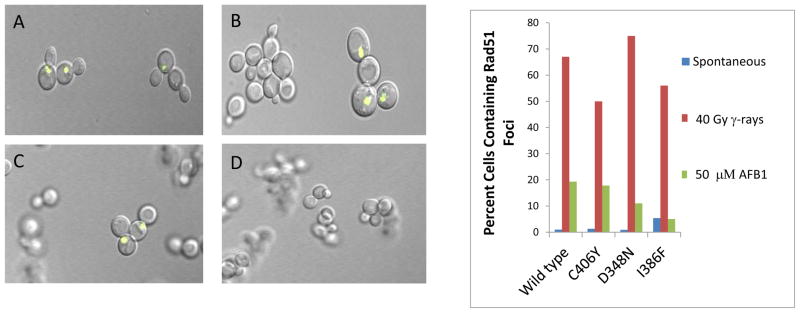
Previous experiments have indicated that Rad51 foci appear in yeast (LSY1957) cells expressing both CYP1A2 and yellow fluorescent protein (ypf)-tagged Rad51 after exposure to AFB1 [43]. To facilitate CYP1A2activity, we inserted the human oxidoreductase gene at the chromosomal TRP1 locus (trpl::hOR), rendering the strain Trp−. We introduced CYP1A2 alleles contained on pRS424 into this strain (YB419) by selecting for Trp+ transformants. We observed similar numbers of AFB1-DNA adducts in transformants of LSY1957 containing pRS424 CYP1A2 as in the YB163 strain containing pCS316 after exposing them to 50 μM AFB1 [42]. To determine that Trp+ transformants were equally competent to form DNA damage-associated Rad51 foci, we exposed them to 4 krads (40 Gy) of radiation and consistently observed that ≥ 50% of cells contain foci (Figure 3). For each strain expressing the CYP1A2 allele, fewer than 5% of the cells contain spontaneous Rad51 foci, a finding consistent with previous results [43].
Figure 3.
yfp-Rad51 foci in AFB1-exposed cells expressing CYP1A2 (YB421), CYP1A2 C406Y (YB422), CYP1A2 D348N (YB423) or CYP1A2 I386F (YB424). Rad51 foci were identified by the appearance of yellow fluorescent foci in the confocal microscope. Left panel shows Rad51 foci in A) irradiated cells containing pRS424-CYP1A2, B) irradiated cells containing CYP1A2 I386F, C) AFB1-treated cells containing CYP1A2, D) A AFB1-treated cells containing CYP1A2-I386F. The right panel gives the percentage of CYP1A2-expressing cells containing foci after either no exposure, exposure to AFB1 or 10 Gy irradiation. The percentage of cells containing foci is indicated on the y axis and the CYP1A2-expressing strain is identified on the X axis.
We then measured AFB1-associated Rad51 foci in yeast strains expressing different P450 polymorphisms. As a negative control, we exposed (YB420) cells containing the pRS424 (empty) vector to 50 μM AFB1 and observed that 2.3% (3/127) contained Rad51 foci, indicating that P450-associate AFB1 activation was required for the occurrence of AFB1-associated Rad51 foci. For cells expressing pRS424-CYP1A2 wild type, we observed that approximately 20% of the cells (19/92, N=3) contain Rad51 foci after 50 μM AFB1 exposure. Lower percentages of cells expressing either CYP1A2 C406Y (17/95, N =2) or D384N (21/195, N =2) also contained Rad51 foci; however, the number of Rad51 foci (11/175, N =2) in CYP1A2-I386F expressing cells after AFB1 exposure was similar to those that appear without AFB1 exposure. As in previous experiments, most of the γ-ray associated Rad51 cells appear in large-budded G2 arrested cells, while the AFB1 -associated Rad51 foci also appear in small-budded cells. These data indicate that AFB1-associated Rad51 foci appear in cells expressing CYP1A2 alleles in which AFB1-associated recombination can also be detected.
3.4 Low AFB1 concentrations are cytotoxic in a rad4 rad51 double haploid mutant expressing different P450 alleles
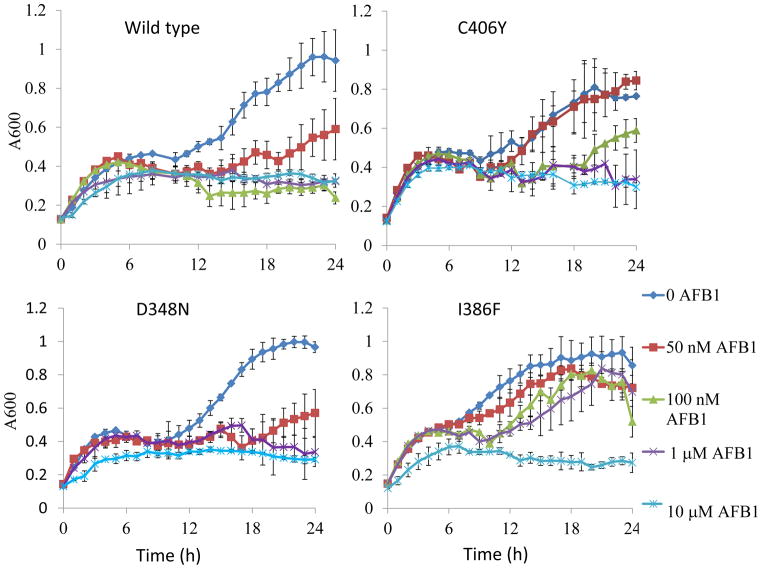
Mutants defective in both nucleotide excision and recombinational repair are extremely sensitive to AFB1 [43,538. We measured growth of rad4 rad51 double mutants individually expressing each of the four different CYP1A2 alleles throughout a 24 hr exposure period to low AFB1 concentrations (Figure 4). Controls indicated that cells exposed to the solvent DMSO or containing the empty vector and exposed to 10 μM AFB1 are as viable as rad4 rad51 cells inoculated in only YPD medium. Although cells expressing wild-type CYP1A2 were the most sensitive, significant sensitivity was also conferred by cells expressing I386F after exposure t to the highest (10 μM) AFB1 concentration. Interestingly, we found that cells that express I386F do not exhibit sensitivity to AFB1 at lower concentrations (50 nM) of AFB1. Cells expressing C406Y were also semi-resistant to AFB1, compared to wild type, while cells expressing D348N were similar to wild type. These data support the notion that that persistent AFB1-associated DNA adducts, even at low concentration, can result in lethality if not repaired.
Figure 4.
Growth of rad4 rad51 cells expressing CYP1A2 (YB415), CYP1A2 C406Y (YB416), CYP1A2 I386F (YB418) and CYP1A2 D348N (YB417) during AFB1 exposure. 100 μl of log phase cells (approximately 10e5) were continuously exposed to 10 μM, 1 μM, 50 nM AFB1 or DMSO (solvent) in a 96-well plate and the experiment was done in triplicate. The optical density (A600) is plotted against time (0–24 h).
4. Discussion
The human genome contains 57 functional P450 genes, with important functions including caffeine metabolism, chemical detoxication, drug metabolism, lipid and hormone metabolism, and carcinogen activation [6,7,10]. With the rapid technological advances in genome sequencing, numerous P450 polymorphisms have been identified that are located in both introns and exons [5,11, 27, 35]. Epidemiological studies indicate that several polymorphisms are genetic risk factors for particular cancers that are associated with environment and diet: specifically, cancers of the lung, colon and liver [30,31,32,60,61]. One hypothesis is that these polymorphisms confer higher levels of metabolic carcinogen activation, thus rendering individuals more susceptible to environmental carcinogens [5,7,32,60,61].
Because multiple P450 genes are expressed in human liver cells, it is difficult to know whether a single P450 polymorphism confers altered levels of carcinogen activation. For example, there are rare polymorphisms that map in the coding sequence of CYP1A2 that differentially activate heterocyclic aryl amines (HAs) [37]. Therefore, we individually expressed different CYP1A2 polymorphisms in budding yeast, which does not contain P450 enzymes that activate aflatoxins. We studied the rare polymorphisms C406Y, D348N, and I386F, first found in the French population [34]. To determine whether expression of specific P450 alleles conferred differences in the metabolic activation of AFB1, we measured AFB1-associated DNA adducts, recombination frequencies, survival of a AFB1-sensitive DNA repair mutant and Rad51 foci formation. These genotoxic endpoints were chosen because they represent genetic instability phenotypes associated with AFB1-exposure [3]. Several different conclusions can be derived from our data. As expected, low levels of detectable AFB1-adducts correlated with low recombination and mutation frequencies and lack of AFB1-associated Rad51 foci; however, we did not observe significant differences between AFB1-associated recombination frequencies among cells expressing CYP1A2 C406Y and CYP1A2 D348N. Second, expression of CYP1A2 I386F, CYP1A2 C406Y and CYP1A2 conferred differences in AFB1-associated cytotoxicity as measured in a DNA repair mutant, defective in both recombinational and excision repair. Overall, these observations indicate that differences in the metabolic activation of AFB1 conferred by polymorphic P450 enzymes can be observed in budding yeast. This is the first study to characterize the metabolic activation of AFB1 conferred by P450 polymorphisms in budding yeast.
Our conclusions were derived using yeast strains containing CYP1A2 on high-copy number plasmids; AFB1-associated recombination events were not observed in strains containing CYP1A2 on the low-copy number plasmid pRS414. In addition, hOR was expressed from a chromosomal copy. Although expression of hOR on a multicopy plasmid does increase CYP1A2-associated MROD activity, it appears to only modestly affect genotoxic endpoints, consistent with previous studies [62]. High levels of hOR may be necessary for robust enzymatic activity in microsomal fractions measured in minutes, while genotoxic endpoints are measured after long exposure times, from 4 to 24 h. In addition, the yeast oxidoreductase, CPR, may also contribute to CYP1A2-mediated toxicity. We do not understand, however, why cells containing CYP1A2 D348N exhibit robust levels of AFB1-associated genotoxicity but low levels of MROD activities compared to yeast strains expressing wild type CYP1A2. .
None of the rare polymorphisms conferred higher levels of AFB1-associated recombination, Rad51 foci or lethality; however, we did observe equivalent levels of AFB1-N7- Guanine adducts in AFB1-exposed cells expressing CYP1A2 D348N, an amino acid substitution that maps to the surface of the protein [37], compared to wild type CYP1A2. We did not measure other AFB1-associated DNA adducts, such as FAPY adducts, in cells expressing CYP1A2 variants; these DNA adducts could contribute to recombination by stalling DNA replication or indirectly generating DNA double-strand breaks. Thus, in order to directly correlate AFB1- associated DNA adducts with particular genotoxic effects, further studies are necessary to identify which AFB1-associated DNA adducts can initiate recombination events.
We found that measuring AFB1-resistant growth of the rad4 rad51 mutant was the best assay for detecting CYP1A2-mediated AFB1 cytotoxicity. AFB1 cytoxicity could be detected in cells exposed to nanomolar concentrations of AFB1, concentrations that are in the range of human exposure [56]. Although rad4 rad51 mutants expressing CYP1A2 I386F were mostly AFB1 resistant at low AFB1 concentrations, they were as sensitive as other cells expressing CYP1A2 when they were exposed at higher AFB1 concentrations. The differences were less apparent when higher numbers of cells (106) were exposed to AFB1; we attribute the atypical shape of the growth curves to the lower number of inoculated cells (105). These data may suggest that CYP1A2 alleles confer differential susceptibility to AFB1-in humans at lower carcinogen concentration.
Our data further illustrate that amino acid substitutions near the active site of CYP1A2 may alter the substrate specific of the modified CYP1A2, as suggested by Zhou et al. [37]. For example, the amino acid substitution present in CYP1A2 I386F replaces an aliphatic side chain with an aromatic side chain, while the amino acid substitution present in C406Y is thought to enhance the β1-sheet structure [37]. Both of these substitutions were previously shown to enhance the metabolic activation of specific HAs when the corresponding CYP1A2 variants were expressed in Escherichia coli [37]. Our studies would indicate that the CYP1A2 I386F greatly reduces but does not eliminate CYP1A2-mediated AFB1 activation, while the C406Y substitution may only slightly reduce the metabolic activation. These findings suggest that P450 polymorphisms that enhance human susceptibility to one class of carcinogens (HAs) may reduce susceptibility to hepatocarcinogens, such as AFB1. Of interest, then, would be to determine whether the cells expressing CYP1A2 variants exhibit enhanced genetic instability phenotypes after exposure to HAs [57].
The methodology presented in this study complements other approaches to characterize human CYP1A2 variants using higher eukaryotic and bacterial cells. The chief advantage of the yeast system is that the entire CYP1A2 protein can be expressed, the metabolic activation can be rapidly measured, and a variety of genotoxicity endpoints can be measured that mirror genotoxic events occurring in higher eukaryotic cells. In the future, combinations of P450 genes can be expressed in yeast. However, predicting whether one particular polymorphism confers an increased risk to AFB1 requires additional information concerning how the metabolic activation of AFB1 depends on the interaction and expression of additional P450 enzymes.
In this study, we have devised novel methods to characterize P450 metabolic activity conferred by amino acid polymorphisms in human P450 genes. The technique may be useful in studying other P450 polymorphisms, and will ultimately complement epidemiological studies in identifying which individuals may be susceptible to environmental carcinogens.
Supplementary Material
Supplemental Figure 1. Expression of human CYP1A2 in diploid yeast strains as detected by Western Blots and normalized to β-tubulin. A representative of two Western blots is shown. Lanes correspond to extracts from strains expressing CYP1A2 I386F (YB413), CYP1A2 D348N (YB412), CYP1A2 C406Y (YB411), CYP1A2 (YB410), as marked. The molecular weight markers are indicated. Each lane contains 100 μg of protein. CYP1A2 was detected in the upper panel, while β-tubulin was detected in the lower panel. The intensity of each band was measured by densitometry.
Highlights for Review.
Genotoxic endpoints distinguish the activity of P450 polymorphic enzymes in yeast.
CYP1A2 I386F confers poor activation of aflatoxin B1 in budding yeast.
DNA damage-associated foci are biomarkers for confirming aflatoxin activation.
Acknowledgments
This research was supported by grants P30ES003819 (PE) and 1R21ES015954 (MF) from the National Institutes of Health. We thank Kathleen Chamberlin for her comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild CP. Environmental exposure measurement in cancer epidemiology. Mutagenesis. 2009;24:117–125. doi: 10.1093/mutage/gen061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wogan GN, Kensler TW, Groopman JD. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Addit Contam Part A Chem Anal Control Expos Risk Assess. 2012;29:249–57. doi: 10.1080/19440049.2011.563370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen HM, Ong CN. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat Res Rev Genet Toxicol. 1996;366:23–44. doi: 10.1016/s0165-1110(96)90005-6. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–43. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solus JF, Arietta BJ, Harris JR, Sexton DP, Steward JQ, McMunn C, Ihrie P, Mehall JM, Edwards TL, Dawson EP. Genetic variation in eleven phase I drug metabolism genes in an ethnically diverse population. Pharmacogenomics. 2004;5:895–931. doi: 10.1517/14622416.5.7.895. [DOI] [PubMed] [Google Scholar]
- 6.Ioannides C, Lewis DF. Cytochromes P450 in the bioactivation of chemicals. Curr Top Med Chem. 2004;4:1767–1788. doi: 10.2174/1568026043387188. [DOI] [PubMed] [Google Scholar]
- 7.Omura T. Forty years of cytochrome P450. Biochem Biophys Res Commun. 1999;266:690–698. doi: 10.1006/bbrc.1999.1887. [DOI] [PubMed] [Google Scholar]
- 8.Martin CN, Garner RC. Aflatoxin B1-oxide generated by chemical or enzymatic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature. 1977;267:863–865. doi: 10.1038/267863a0. [DOI] [PubMed] [Google Scholar]
- 9.Smela ME, Hamm ML, Henderson PT, Harris CM, Harris TM, Essigmann JM. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2002;99:6655–6660. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 11.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–37. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- 13.Boobis AR, Gooderham NJ, Rich KJ, Zhao K, Edwards RJ, Murray BP, Lynch AM, Murray S, Davies DS. Enzymatic studies of the activation of heterocyclic food mutagens in man. Princess Takamatsu Symp. 1995;23:134–144. [PubMed] [Google Scholar]
- 14.Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Ann Rev Pharmacol Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Guengerich FP. Cytochrome P450 activation of arylamines and heterocyclic amines. Annu Rev Pharmacol Toxicol. 2005;45:27–49. doi: 10.1146/annurev.pharmtox.45.120403.100010. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher EP, Kunze KL, Stapleton PL, Eaton DL. The kinetics of aflatoxin B1 oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol Appl Pharmacol. 1996;141:595–606. doi: 10.1006/taap.1996.0326. [DOI] [PubMed] [Google Scholar]
- 17.Guengerich FP, Parikh A, Turesky RJ, Josephy PD. Inter-individual differences in the metabolism of environmental toxicants: cytochrome P450 1A2 as a prototype. Mutat Res. 1999;428:115–124. doi: 10.1016/s1383-5742(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 18.Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, Nebert DW. Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci U S A. 1996;93:1671–1676. doi: 10.1073/pnas.93.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dragin N, Uno S, Wang B, Dalton TP, Nebert DW. Generation of ‘humanized’ hCYP1A1_1A2_Cyp1a1/1a2(−/−) mouse line. Biochem Biophys Res Commun. 2007;359:635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landi MT, Sinha R, Lang NP, Kadlubar FF. Human cytochrome P4501A2.IARC. Sci Publ. 1999;148:173–195. [PubMed] [Google Scholar]
- 21.Kalow W, Tang BK. Caffeine as a metabolic probe: exploration of the enzyme-inducing effect of cigarette smoking. Clin Pharmacol Ther. 1991;49:44–48. doi: 10.1038/clpt.1991.8. [DOI] [PubMed] [Google Scholar]
- 22.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptormediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen BB, Brix TH, Kyvik KO, Brøsen K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics. 2002;12:473–478. doi: 10.1097/00008571-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Klein K, Winter S, Turpeinen M, Schwab M, Zanger UM. Pathway-targeted pharmacogenomics of CYP1A2 in human liver. Front Pharmacol. 2010;1:129. doi: 10.3389/fphar.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeya K, Jaiswal AK, Owens RA, Jones JE, Nebert DW, Kimura S. Human CYP1A2: sequence, gene structure, comparison with the mouse and rat orthologous gene, and differences in liver 1A2 mRNA expression. Mol Endocrinol. 1989;3:1399–1408. doi: 10.1210/mend-3-9-1399. [DOI] [PubMed] [Google Scholar]
- 26.Nukaya M, Bradfield C. Conserved genomic structure of the CYP1A1 and CYP1A2 loci and their dioxin responsive elements cluster. Biochem Pharmacology. 19?;77:654–659. doi: 10.1016/j.bcp.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.http://www.cypalleles.ki.se/cyp1a2.htm
- 28.Browning SL, Tarekegn A, Bekele E, Bradman N, Thomas MG. CYP1A2 is more variable than previously thought: a genomic biography of the gene behind the human drugmetabolizing enzyme. Pharmacogenet Genomics. 2010;20:647–664. doi: 10.1097/FPC.0b013e32833e90eb. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Wang H, Xie W, Liang R, Wei Z, Zhi L, Zhang X, Hao B, Zhong S, Zhou G, Zhang L, Gao X, Zhu Y, He F. Association of CYP1A2 genetic polymorphisms with hepatocellular carcinoma susceptibility: a case control study in a high-risk region of China. Pharmacogenet Genomics. 2006;16:219–227. doi: 10.1097/01.fpc.0000194424.20393.c6. [DOI] [PubMed] [Google Scholar]
- 30.Olivieri EH, da Silva SD, Mendonça FF, Urata YN, Vidal DO, Faria Mde A, Nishimoto IN, Rainho CA, Kowalski LP, Rogatto SR. CYP1A2*1C, CYP2E1*5B, and GSTM1 polymorphisms are predictors of risk and poor outcome in head and neck squamous cell carcinoma patients. Oral Oncol. 2009;45:e73–79. doi: 10.1016/j.oraloncology.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Jiao L, Li Y, Doll MA, Hein DW, Bondy ML, Evans DB, Wolff RA, Lenzi R, Pisters PW, Abbruzzese JL, Hassan MM. Polymorphisms of cytochrome P4501A2 and N-acetyltransferase genes, smoking, and risk of pancreatic cancer. Carcinogenesis. 2006;27:103–111. doi: 10.1093/carcin/bgi171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moonen H, Engels L, Kleinjans J, Kok T. The CYP1A2-164A-->C polymorphism (CYP1A2*1F) is associated with the risk for colorectal adenomas in humans. Cancer Lett. 2005;229:25–31. doi: 10.1016/j.canlet.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Chevalier D, Cauffiez C, Allorge D, Lo-Guidice JM, Lhermitte M, Lafitte JJ, Broly F. Five novel natural allelic variants - 951A>C, 1042G>A (D348N), 1156A>T (I386F), 1217G>A (C406Y) and 1291C>T (C431Y) - of the human CYP1A2 gene in a French Caucasian population. Hum Mutat. 2001;17:355–356. [PubMed] [Google Scholar]
- 35.Saito Y, Hanioka N, Maekawa K, Isobe T, Tsuneto Y, Nakamura R, Soyama A, Ozawa S, Tanaka-Kagawa T, Jinno H, Narimatsu S, Sawada J. Functional analysis of three CYP1A2 variants found in a Japanese population. Drug Metab Dispos. 2005;12:1905–1910. doi: 10.1124/dmd.105.005819. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Z, Dalton TP, Jin L, Wang XB, Tsuneoka Y, Shertzer HG, Deka R, Nebert DW. Toward evaluation of function in genetic variability: characterizing human SNP frequencies and establishing BAC transgenic mice carrying the human CYP1A1_CYP1A2 locus. Human Mutation. 2005;25:196–206. doi: 10.1002/humu.20134. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Josephy PD, Kim D, Guengerich FP. Functional characterization of four allelic variants of human cytochrome P450 1A2. Arch Biochem Biophys. 2004;422:23–30. doi: 10.1016/j.abb.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Palma BB, Silva E, Sousa M, Vosmeer CR, Lastdrager J, Rueff J, Vermeulen NP, Kranendonk M. Functional characterization of eight human cytochrome P450 1A2 gene variants by recombinant protein expression. Pharmacogenomics J. 2010;10:478–488. doi: 10.1038/tpj.2010.2. [DOI] [PubMed] [Google Scholar]
- 39.Sansen S, Yano JK, Reynald RL, Schoch GA, Griffin KJ, Stout CD, Johnson EF. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J Biol Chem. 2007;282:14348–14355. doi: 10.1074/jbc.M611692200. [DOI] [PubMed] [Google Scholar]
- 40.Sengstag C, Weibel B, Fasullo M. Genotoxicity of aflatoxin B1: evidence for a recombination-mediated mechanism in Saccharomyces cerevisiae. Cancer Res. 1996;56:5457–5465. [PMC free article] [PubMed] [Google Scholar]
- 41.Keller-Seitz M, Certa U, Sengstag C, Wurgler F, Sun M, Fasullo M. Transcriptional response of the yeast to the carcinogen aflatoxin B1: Recombinational repair involving RAD51 and RAD1. Mol Biol Cell. 2004;15:4321–4336. doi: 10.1091/mbc.E04-05-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fasullo M, Sun M, Egner P. Stimulation of sister chromatid exchanges and mutation by aflatoxin B1-DNA adducts in Saccharomyces cerevisiae requires MEC1 (ATR), RAD53, and DUN1. Mol Carcinog. 2008;47:608–615. doi: 10.1002/mc.20417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fasullo M, Chen Y, Bortcosh W, Sun M, Egner PA. Aflatoxin B1-associated DNA adducts stall S phase and stimulate Rad51 foci in Saccharomyces cerevisiae. J Nucleic Acids. 2010;1:456487. doi: 10.4061/2010/456487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke D, Dawson D, Stearns T. A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Press; New York: 2000. Methods in yeast genetics. [Google Scholar]
- 45.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 1 Short Protocols in Molecular Biology. 4. Vol. 1. Wiley; New York: 1999. [Google Scholar]
- 47.Fasullo MT, Bennett T, AhChing P, Koudelik J. The Saccharomyces cerevisiae RAD9 checkpoint reduces the DNA damage-associated stimulation of directed reciprocal translocations. Mol Cell Biol. 1998;18:1190–2000. doi: 10.1128/mcb.18.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fasullo MT, Davis RW. Recombination substrates designed to study recombination between unique and repetitive sequences in vivo. Proc Natl Acad Sci USA. 1987;84:6215–6219. doi: 10.1073/pnas.84.17.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fasullo M, Dave P, Rothstein R. DNA-damaging agents stimulate the formation of directed reciprocal translocations in Saccharomyces cerevisiae. Mutat Res. 1994;314:121–133. doi: 10.1016/0921-8777(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 50.Dong Z, Fasullo M. Multiple recombination pathways for spontaneous and DNA damage-associated sister chromatid exchange in Saccharomyces cerevisiae: Role of RAD1 and the RAD52 epistasis group genes. Nucleic Acids Res. 2003;31:2576–2585. doi: 10.1093/nar/gkg352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fung CW, Mozlin AM, Symington LS. Suppression of the double-strand-break-repair defect of the Saccharomyces cerevisiae rad57 mutant. Genetics. 2009;181:1195–1206. doi: 10.1534/genetics.109.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foiani M, Marini F, Gamba D, Lucchini G, Plevani P. The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol Cell Biol. 1994;14:923–933. doi: 10.1128/mcb.14.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dyavaiah M, Rooney JP, Chittur SV, Lin Q, Begley TJ. Autophagy-dependent regulation of the DNA damage response protein ribonucleotide reductase 1. Mol Cancer Res. 2011;4:462–475. doi: 10.1158/1541-7786.MCR-10-0473. [DOI] [PubMed] [Google Scholar]
- 54.Egner PA, Groopman JD, Wang JS, Kensler TW, Friesen MD. Quantification of aflatoxin-B1-N7-Guanine in human urine by high-performance liquid chromatography and isotopedilution tandem mass spectrometry. Chem Res Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 56.Pompon D, Louerat B, Bronine A, Urban P. Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 1996;272:51–64. doi: 10.1016/s0076-6879(96)72008-6. [DOI] [PubMed] [Google Scholar]
- 57.Eugster HP, Bärtsch S, Würgler FE, Sengstag C. Functional co-expression of human oxidoreductase and cytochrome P450 1A1 in Saccharomyces cerevisiae results in increased EROD activity. Biochem Biophys Res Commun. 1992;185:641–647. doi: 10.1016/0006-291x(92)91673-e. [DOI] [PubMed] [Google Scholar]
- 58.Guo Y, Breeden LL, Zarbl H, Preston BD, Eaton DL. Expression of a human cytochrome p450 in yeast permits analysis of pathways for response to and repair of aflatoxin-induced DNA damage. Mol Cell Biol. 2005;25:5823–5833. doi: 10.1128/MCB.25.14.5823-5833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller-Seitz M. PhD Thesis. Swiss Federal Institute of Technology; Zurich, Switzerland: 2001. The role of recombination in aflatoxin B1-induced DNA damage in Saccharomyces cerevisiae. [Google Scholar]
- 60.Silvestri L, Sonzogni L, De Silvestri A, Gritti C, Foti L, Zavaglia C, Leveri M, Cividini A, Mondelli MU, Civardi E, Silini EM. CYP enzyme polymorphisms and susceptibility to HCV-related chronic liver disease and liver cancer. Int J Cancer. 2003;104:310–317. doi: 10.1002/ijc.10937. [DOI] [PubMed] [Google Scholar]
- 61.Johansson I, Ingelman-Sundberg M. Genetic polymorphism and toxicology - with emphasis on cytochrome P450. Toxicol Sci. 2011;120:1–13. doi: 10.1093/toxsci/kfq374. [DOI] [PubMed] [Google Scholar]
- 62.Sengstag C, Würgler FE. DNA recombination induced by aflatoxin B1 activated by cytochrome P450 1A enzymes. Mol Carcinog. 1994;(4):227–235. doi: 10.1002/mc.2940110408. [DOI] [PubMed] [Google Scholar]
- 63.Venkateswarlu K, Lamb DC, Kelly DE, Manning NJ, Kelly SL. The N-terminal membrane domain of yeast NADPH-cytochrome P450 (CYP) oxidoreductase is not required for catalytic activity in sterol biosynthesis or in reconstitution of CYP activity. J Biol Chem. 1998;(273):4492–4496. doi: 10.1074/jbc.273.8.4492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Expression of human CYP1A2 in diploid yeast strains as detected by Western Blots and normalized to β-tubulin. A representative of two Western blots is shown. Lanes correspond to extracts from strains expressing CYP1A2 I386F (YB413), CYP1A2 D348N (YB412), CYP1A2 C406Y (YB411), CYP1A2 (YB410), as marked. The molecular weight markers are indicated. Each lane contains 100 μg of protein. CYP1A2 was detected in the upper panel, while β-tubulin was detected in the lower panel. The intensity of each band was measured by densitometry.