Abstract
Introduction
The rapid diffusion of the surgical robot has been controversial because of the technology’s high costs and its disputed marginal benefit. Some, however, have suggested that adoption of the robot may have improved care for patients with renal malignancy by facilitating partial nephrectomy, an underutilized, technically challenging procedure believed to be less morbid than radical nephrectomy. We sought to determine whether institutional acquisition of the robot was associated with increased utilization of partial nephrectomy.
Methods
We used all payer data from 7 states to identify 21,569 nephrectomies. These patient-level records were aggregated to the hospital-level then merged with the American Hospital Association Annual Survey and publicly available data on timing of robot acquisition. We used a multivariable difference-in-difference model to assess at the hospital-level whether robot acquisition was associated with an increase in the proportion of partial nephrectomy, adjusting for hospital nephrectomy volume, year of surgery, and several additional hospital-level factors.
Results
In the multivariable-adjusted differences-in-differences model, hospitals acquiring a robot between 2001 and 2004 performed a greater proportion of partial nephrectomy in both 2005 (29.9% increase) and 2008 (34.9% increase). Hospitals acquiring a robot between 2005 and 2008 also demonstrated a greater proportion of partial nephrectomy in 2008 (15.5% increase). In addition, hospital nephrectomy volume and urban location were also significantly associated with increased proportion of partial nephrectomy.
Conclusions
Hospital acquisition of the surgical robot is associated with greater proportion of partial nephrectomy, an underutilized, guideline-encouraged procedure. This is one of the few studies to suggest robot acquisition is associated with improvement in quality of patient care.
Keywords: nephrectomy, nephron sparing surgery, renal cell carcinoma, robotics, outcomes
The rapid diffusion of the surgical robot has been a source of significant controversy.1 Despite the high costs of its purchase price ($1–$2.25 million), annual service contract ($140,000) and disposable instruments ($1500–$2000 per case),1–3 and little or no evidence suggesting benefit over traditional laparoscopic or open surgical techniques,4 the robot has been widely adopted for the treatment of an ever increasing number of urologic, gynecologic, cardiothoracic, colorectal, and head-and-neck surgical conditions.5 As a result of its popularity, adoption of the robot has been associated with changes in the delivery of health services such as increased rates of radical prostatectomy6–8 in regions with more surgical robots. It is unknown whether adoption of the surgical robot is associated solely with increased volume or whether it is associated with improvements in guideline-supported care.
Although open radical nephrectomy (removal of the entire kidney) has long been considered the gold standard treatment for renal tumors,9 more recent evidence suggests that partial nephrectomy (removal of the tumor only), may be taking its place. Several influential studies demonstrate that careful removal of the tumor with preservation of the healthy renal tissue not only achieves equivalent oncologic outcomes10–12 but also reduces the long-term risks of adverse cardiovascular events, chronic renal failure, and premature death.13–15 There is evidence to suggest that partial nephrectomy is being increasingly used in the United States. In light of the strong evidence of the procedure’s benefit, however, it is still considered underutilized.16–18 Partial nephrectomy was performed in only approximately 25% of cases in 2008,18,19 most likely because the surgery is technically challenging and may be complicated by increased intraoperative blood loss, ureteral obstruction, and postoperative urine leak.20
The surgical robot was initially adopted to facilitate radical prostatectomy, however, urologists soon found that the improved magnification and manual dexterity afforded by the robot could facilitate partial nephrectomy21,22 as well. Robotic partial nephrectomy is believed to have several advantages over traditional laparoscopic and open surgical techniques including less postoperative pain,23 shorter length of stay,23,24 and reduced intraoperative blood loss.24 Several recent reports have suggested that the adoption of the robot coincided with the diffusion of partial nephrectomy,25 possibly because of this decrease in perioperative complications. Specifically, Patel et al25 reported that use of robotic technology was associated with increased utilization of partial nephrectomy within the state of Maryland. Since this study analyzed data at a regional level, however, they were unable to determine association at an individual hospital level; simply describing trends at the regional level does not address the potential for ecologic fallacy.26 Even the studies which do analyze data at a hospital level do not follow hospitals longitudinally (before and after robot acquisition).27 There is a knowledge gap regarding the association between acquisition of the robot at the hospital level and the diffusion of partial nephrectomy. Determining the effect of the surgical robot on adoption of partial nephrectomy is a challenging problem which must account for hospital-level factors associated with partial nephrectomy utilization18,28 as well as important secular trends.16,17
In this study, we investigate whether acquisition of the surgical robot at the hospital level resulted in a greater proportion of partial nephrectomy at a given hospital. We hypothesized that robot acquisition would be associated with a greater hospital-level proportion of partial nephrectomy. If true, our findings would suggest that acquisition of the robot facilitated increased utilization of a guideline-supported procedure29 and likely contributed to improvement in the quality-of-care for patients with localized renal cell carcinoma. Conversely, if our hypothesis were rejected, associations reported in prior literature between robot acquisition and increased numbers of partial nephrectomies is probably an ecologic fallacy, where aggregate trends differ from local ones.26 The results of this study would be an important commentary on the role that reinvention30 plays in the diffusion of innovation and the impact it can have on improving the quality of care. Understanding the potential for unexpected benefits of technology diffusion should be extremely important to hospital administrators, policy experts, physicians, and patients as they consider the adoption of costly technology innovation in the face of increasing budgetary constraints.
METHODS
We performed a hospital-level retrospective cohort study examining the change in the proportion of partial nephrectomy performed (ie, the numerator is partial nephrectomy and the denominator is all instances of partial or radical nephrectomy to treat renal cell carcinoma) as a function of surgical robot acquisition. This study was determined to be exempt from review by the New York University School of Medicine Institutional Review Board.
Our cohort was formed by combining data from 3 sources. We used the Health-care Cost and Utilization Project State In-patient Databases31 (HCUP-SID) from 7 states (AZ, FL, MD, NC, NY, NJ, and WA) in 2001, 2005, and 2008 to identify all nephrectomies performed for renal tumors. We aggregated nephrectomy data to the hospital level and then merged these with hospital characteristics from the American Hospital Association Survey32 and publicly available data from the internet (described below) documenting whether and when a surgical robot was purchased by any given hospital.
Selection of Cases
The HCUP-SID databases were queried for patients with the discharge diagnosis of renal neoplasm (ICD-9 code 189.0) in the years 2001, 2005, and 2008 (n = 48,946). Patients with a concomitant diagnosis of transitional cell carcinoma (n = 47) or end-stage renal disease (n = 2661) were excluded from the analysis, as they would have been inappropriate candidates for partial nephrectomy.33 Patients under the age of 18 years (n = 2068) and those with missing sociodemographic information (n = 798) were excluded. Data on the remaining 43,372 patients were examined to identify all instances of partial (ICD-9 procedure code 55.5×) or radical nephrectomy (ICD-9 procedure code 55.4). Overall, there were 21,569 instances of partial or radical nephrectomy during the study period.
Description of Variables
Dependent Variable of Interest
The dependent variable of interest for our analysis was the natural logarithm of the number of partial nephrectomies performed at a given hospital in 2005 and 2008. The proportion of partial nephrectomy for a given hospital was calculated by dividing the number of partial nephrectomies performed at a given hospital in a given year by the number of overall nephrectomies (partial+radical nephrectomies) performed at that hospital in the same year. Our primary outcome of interest was the hospital-level change in the proportion of partial nephrectomies performed in the time periods 2001–2004 and 2005–2008 attributable to the acquisition of the surgical robot. We controlled for the natural log of all nephrectomies (radical and partial nephrectomies) performed for renal cell carcinoma at that hospital in the given year. We adjusted for this because our principal question was whether robot acquisition was associated with increased utilization of partial nephrectomy over radical nephrectomy, rather than simply whether acquisition of the surgical robot was associated with total volume of partial nephrectomy.
The Independent Variable of Interest
The independent variable of interest was surgical robot acquisition. Using publicly available resources such as the website of Intuitive Surgical (the sole manufacturer of the surgical robot),5 press releases, newspapers articles, annual reports, and direct telephone contact with hospital personnel, we identified whether and when all hospitals had acquired a robot, as previously described.6 Acquisition was analyzed as a binary variable (acquired or not) in the 2 relevant time periods: 2001–2004 and 2005–2008.
Covariates
We analyzed the data for bivariate associations between the proportion of partial nephrectomy and relevant hospital characteristics such as mean age of hospital inpatients, percentage of female hospital inpatients, percentage of white hospital inpatients, percentage of privately insured hospital inpatients, hospital teaching status, urban or rural location, median Elixhauser Comorbidity Score34 of hospital inpatients, and hospital location by state (Table 1). Any characteristics with P < 0.20 were included in the final analysis along with hospital bed number which is a covariate known to affect both surgical robot adoption35 and the utilization of partial nephrectomy at the hospital level.36
TABLE 1.
Bivariate Associations Between Hospital-level Covariates and Proportion of Partial Nephrectomy
| 2001 |
2005 |
2008 |
|||||
|---|---|---|---|---|---|---|---|
| Characteristics | %PN | %RN | %PN | %RN | %PN | %RN | P (Year) |
| Robot status | P < 0.01 (all years) | ||||||
| With robot | 18.0 | 82.1 | 27.3 | 72.7 | 33.1 | 66.9 | |
| Without robot | 9.3 | 90.8 | 15.8 | 84.2 | 20.2 | 79.8 | |
| Age | P < 0.01 (all years) | ||||||
| < 45 | 17.1 | 82.9 | 30.3 | 69.7 | 38.2 | 61.8 | |
| 45–64 | 14.9 | 85.1 | 24.8 | 75.2 | 31.8 | 68.2 | |
| 65–74 | 14.6 | 85.4 | 23.4 | 76.6 | 27.5 | 72.5 | |
| 75+ | 10 | 90 | 13.4 | 86.6 | 17.3 | 82.7 | |
| Sex | P = 0.05 (2001) | ||||||
| Male | 14.8 | 85.2 | 23.7 | 76.3 | 29.3 | 70.7 | P = 0.03 (2005) |
| Female | 13.0 | 87.0 | 21.5 | 78.5 | 27.2 | 72.8 | P = 0.04 (2008) |
| Race | P = 0.94 (2001) | ||||||
| White | 14.5 | 85.5 | 22.9 | 77.1 | 29.0 | 71.0 | P = 0.24 (2005) |
| Black | 14.0 | 86.0 | 25.8 | 74.2 | 29.4 | 70.6 | P = 0.89 (2008) |
| Other | 14.2 | 85.8 | 24.2 | 75.8 | 28.4 | 71.7 | |
| Elixhauser Score | P < 0.01 (all years) | ||||||
| 0 | 16.0 | 84.0 | 28.9 | 71.1 | 33.0 | 67.1 | |
| 1–2 | 14.0 | 86.0 | 22.4 | 77.6 | 28.6 | 71.4 | |
| 3+ | 10.1 | 89.9 | 13.9 | 86.1 | 22.1 | 77.9 | |
| Insurance | P < 0.01 (all years) | ||||||
| Public | 12.8 | 87.2 | 19.6 | 80.4 | 24.4 | 75.6 | |
| Private | 15.9 | 84.1 | 26.8 | 73.2 | 33.2 | 66.8 | |
| Other | 10.3 | 89.7 | 18.9 | 81.1 | 22.9 | 77.1 | |
| Teaching status | P < 0.01 (all years) | ||||||
| Nonteaching | 10.0 | 90.0 | 15.9 | 84.1 | 18.5 | 81.5 | |
| Teaching | 16.5 | 83.5 | 26.3 | 73.7 | 33.0 | 67.0 | |
| Location | P = 0.13 (2001) | ||||||
| Nonurban | 18.5 | 81.5 | 11.6 | 88.4 | 17.4 | 82.6 | P < 0.01 (2005) |
| Urban | 33.0 | 67.0 | 23.3 | 76.7 | 28.9 | 71.1 | P < 0.01 (2008) |
Statistical analysis performed using Pearson χ2 test.
%PN indicates number of partial nephrectomies/(number of partial+radical nephrectomies); %PN, number of partial nephrectomies/(number of partial+radical nephrectomies); PN, partial nephrectomy; RN, radical nephrectomy.
Statistical Analysis
We used a multivariable difference-in-difference model to estimate the independent effect of surgical robot acquisition on the change in the proportion of partial nephrectomy while controlling for confounding hospital-level characteristics and secular time trends. A difference-in-difference model compares the change in the variable of interest (proportion of partial nephrectomy) in a treatment group (hospitals who acquired the robot) to the change in a control group (hospitals which did not). The coefficient for each characteristic in the final regression model represents the marginal effect of that characteristic on change in the proportion of partial nephrectomy over time. We graphed the coefficients for our dependent variables of interest between 2001–2004 and 2005–2008.
Secondary Analyses
We performed several additional analyses to determine the robustness of our results. We hypothesized that after acquisition of the surgical robot, some time might have had to elapse before surgeons had undergone sufficient training on the new equipment to be able to perform robotic partial nephrectomy. We therefore tested the association by repeating the difference-in-difference model using new independent variables: robot acquisition between 2001–2003 and 2005–2007 (an 1-y lag). It was presumed that any observed lag time might be the result of the time needed to develop surgical facility with robotic surgery and partial nephrectomy.
Because hospitals acquiring surgical robots may also have engaged in other, capacity-building endeavors such as the hiring of additional surgeons or building of new operating rooms, we wanted to ensure that acquisition of a surgical robot was not associated with procedures for which the robot was unnecessary. We performed a falsification test37 to determine whether there was an association between acquisition of the surgical robot and rates of total knee replacement (ICD-9 code 81.54), a procedure which cannot employ the robot. We would expect to find no associations between robot acquisition and utilization of this procedure.
Because hospitals acquiring a surgical robot would presumably also have to recruit surgeons and ancillary staff trained to perform newer, technology-oriented procedures, we wanted to explore whether acquisition of the surgical robot might also be associated with increased utilization of other technology-intensive, but nonrobotic urologic procedures. We performed a second falsification test to determine the association between acquisition of the robot and changes in the utilization of percutaneous nephrolithotomy (ICD-9 codes 55.03, 55.04, 55.92), a technology-intensive, minimally invasive surgical procedure for the treatment of large kidney stones38 which might otherwise require open surgery. If we found an association between robot acquisition and utilization of percutaneous nephrolithotomy, it might suggest that the introduction of surgical personnel, rather than the robot, was the driver of the increased proportion of partial nephrectomy.
To evaluate the association between surgical outcomes and acquisition of the surgical robot, we repeated 2 additional difference-in-difference models with new dependent variables: mean hospital length of stay and hospital-level mortality for patients undergoing partial nephrectomy.
Statistical analyses were performed using SAS version 9.3 (Cary, NC). All P-values were 2-sided with statistical significance determined at the α = 0.05 level.
RESULTS
We identified 21,569 instances of nephrectomy performed for treatment of renal cell carcinoma in 2001, 2005, and 2008 (Table 2). Of these, 4827 (22.0%) were partial nephrectomies and 16,742 (78.0%) were radical nephrectomies. The proportion of partial nephrectomy increased over time from 14.1% in 2001 to 22.8% in 2005 to 28.5% in 2008. Hospitals adopting the robot between 2001 and 2004 performed a higher proportion of partial nephrectomy in 2005 (27.3%) and 2008 (37.5%) compared with those who did not (15.8% in 2005, 20.1% in 2008; Table 2). In 2008, hospitals that acquired the robot between 2005 and 2008 performed a higher proportion of partial nephrectomy (23.9%) than those not adopting the robot (20.1%) but a lower proportion than those adopting the robot in 2001–2004 (Table 2).
TABLE 2.
Overall Proportions of Partial and Radical Nephrectomy Stratified by Year and Hospital Robot Status
| Years | # Partial Nephrectomy (%) | # Radical Nephrectomy (%) | # All Nephrectomy | P |
|---|---|---|---|---|
| 2001 | 876 (14.1) | 5324 (85.9) | 6200 | |
| 2005 | 1731 (22.8) | 5848 (77.2) | 7579 | < 0.01 |
| Adopted 2001–2004 | 1263 (27.3) | 3357 (72.7) | 4620 | |
| Not adopted | 468 (15.8) | 2491 (84.2) | 2959 | |
| 2008 | 2220 (28.5) | 5570 (71.5) | 7790 | < 0.01 |
| Adopted 2001–2004 | 1284 (37.5) | 2144 (62.5) | 3428 | |
| Adopted 2005–2008 | 369 (23.9) | 1175 (76.1) | 1544 | |
| Not adopted | 567 (20.1) | 2251 (79.9) | 2818 | |
| Overall | 4827 (16.9) | 16742 (77.6) | 21569 |
Statistical analysis performed using Pearson χ2 test.
Year of surgery and the following hospital-level characteristics were statistically significant in the final model: mean age of hospital inpatients, fraction of female hospital inpatients, median Elixhauser Comorbidity Score of hospital inpatients, nephrectomy volume, hospital bed number, teaching status, urban location, and state of surgery. In the multivariable-adjusted difference-in-difference analysis, hospital-level acquisition of the surgical robot between 2001 and 2004 was associated with a 29.9% [95% confidence interval (CI), 10.7–49.1, P < 0.01] increase in the proportion of partial nephrectomy in 2005 and a 34.9% (95% CI, 14.0–55.8, P < 0.01) in the proportion of partial nephrectomy in 2008 as compared with the proportion in 2001 (Fig. 1). Acquisition of the robot in 2005–2008 was also associated with a smaller, although significant, 15.5% increase (95% CI, 1.4-29.7, P = 0.03) in the proportion of partial nephrectomy in 2008 as compared with 2001.
FIGURE 1.
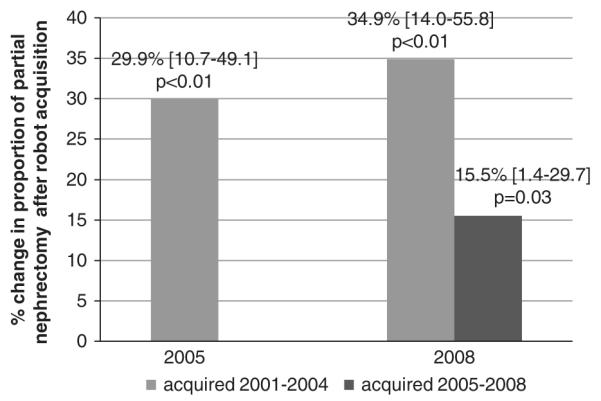
Multivariable adjusted marginal increase in proportion of partial nephrectomy associated with robot acquisition by year. Adjusted for hospital nephrectomy volume, year of surgery, urban location, teaching status, hospital bed number, and patient demographic information.
The lag-time analysis demonstrated that hospital-level robot acquisition between 2001 and 2003 was associated with a similar change in the proportion of partial nephrectomy in 2005 and 2008 as was acquisition between 2001 and 2004 (Appendix Table A1). Robot acquisition between 2005 and 2007 also was associated with a similar change in the hospital-level proportion of partial nephrectomy in 2008 as was acquisition between 2005 and 2008. There were no significant associations between robot acquisition and the utilization of either total knee replacement (Appendix Table A2) or percutaneous nephrolithotomy (Appendix Table A3) in our falsification tests.
There were no significant associations between either hospital length of stay or mortality following partial nephrectomy and robot acquisition (Appendix Table A3).
DISCUSSION
In the current, longitudinal hospital-level analysis, acquisition of the surgical robot was independently associated with a greater proportion of partial nephrectomy. Our multivariable-adjusted, difference-in-difference analysis demonstrated that acquisition of the surgical robot between 2001 and 2004 was independently associated with an increase in the proportion of partial nephrectomy (29.9% and 34.9% in 2005 and 2008 as compared with 2001). Later acquisition of the surgical robot between 2005 and 2008 was associated with a 15.5% marginal increase in the proportion of partial nephrectomy in 2008 as compared with 2001. Our findings suggest that hospitals acquiring the surgical robot were more likely to offer renal cell carcinoma patients this advanced and underutilized surgical technique.
This result confirms the hypothesis generated by previous studies examining the regional-level relationship between the surgical robot and partial nephrectomy.25 A patient diagnosed in 2001 was nearly 5× more likely to undergo partial nephrectomy than one diagnosed in 1988.17 Between 2003 and 2008, there was a 90% increase in the annualized rate of partial nephrectomy per 100,000 individuals between 2003 and 2008.18 Our data confirmed the presence of this trend: the year 2008 was independently associated with a 25.7% increase in the proportion of partial nephrectomy as compared with 2001. Previous studies examining the associations between robotic acquisition and utilization of partial nephrectomy did not adjust for these secular trends.19 Without exploring this question at the hospital level, the diffusion of nearly any innovation during this time could appear to have an association with increased proportion of partial nephrectomy. Our difference-in-difference model demonstrates that surgical robot acquisition was associated with an increase in the proportion of partial nephrectomy independent of this secular trend. Furthermore, prior studies failed to account for important hospital-level and environmental characteristics such as age,16,17 sex,16,28 comorbidity,18,28 teaching status,18,28 urban location,18,28 and nephrectomy volumes18,28 which have previously been demonstrated to have an association with partial nephrectomy utilization independent of the surgical robot. Our multivariable model also found several other hospital-level characteristics to be associated with changes in the proportion of partial nephrectomy including nephrectomy volume, fraction of female hospital inpatients, mean age of hospital inpatients, median Elixhauser Comorbidity Score of hospital inpatients, and urban location (Table 3). Failing to adjust for these relevant hospital-level and environmental characteristics precludes a thorough understanding of the structures, processes, and outcomes that affect quality of care.39–41
TABLE 3.
Multivariable Differences-In-Differences Model to Determine the Association Between Hospital-level Acquisition of the Surgical Robot and Changes in Proportion of Partial Nephrectomy*
| Hospital Characteristics | Marginal Change in Proportion of Partial Nephrectomy Compared With 2001 (95% CI) |
P |
|---|---|---|
| Robot acquisition 2001–2004 | In 2005: +29.9% (10.7 to 49.1) | < 0.01 |
| In 2008: +34.9% (14.0 to 55.8) | < 0.01 | |
| Robot acquisition 2005–2008 | In 2008: +15.5% (1.4 to 29.7) | 0.03 |
| Year 2005 | +19.8% (14.0 to 25.7) | < 0.01 |
| Year 2008 | +25.7% (18.8 to 32.5) | < 0.01 |
| Nephrectomy volume (per 1% increase) | +0.66% (0.58 to 0.71) | < 0.01 |
| Fraction of female hospital inpatients (per 1% increase) | −0.17% (−0.30 to −0.04) | 0.01 |
| Mean age of hospital inpatients | −0.6% (−1.1 to −0.1) | 0.01 |
| Median Elixhauser Comorbidity Score of hospital inpatients | −5.6% (−8.6 to −2.6) | < 0.01 |
| No. beds | +0.01% (−0.01 to 0.04) | 0.16 |
| Teaching | +4.22% (−2.1 to 10.6) | 0.19 |
| Urban | −7.5% (−14.3 to −0.8) | 0.03 |
| Fraction of privately insured hospital inpatients (per 1% increase) | −0.08% (−0.22 to 0.06) | 0.25 |
Nephrectomy volume: number of all nephrectomies (partial or radical) performed for renal cell carcinoma at a given hospital per year.
Fraction of female patients: the percentage of all patients who were of the female sex at a given hospital.
Median Elixhauser Comorbidity Score of patients: the median Elixhauser Comorbidity Score of all patients at a given hospital.
Fraction of privately insured patients: the percentage of patients at a given hospital who were privately insured.
Each hospital characteristic was determined at the hospital level.
Although the utilization of partial nephrectomy has been generally increasing with time, these changes have been slow.19 This observation is consistent with previous studies suggesting that surgeons are reluctant to alter established practice to conform to new scientific evidence.42–44 Paradoxically, surgeons are often keen to adopt new technology; for example, laparoscopic cholecystectomy and fundoplication were adopted so rapidly that within 5 years, >50% of both surgeries were performed laparoscopically.45,46 The rapid diffusion of the new technology was also associated with lower thresholds for surgery and resulted in increased numbers of cholecystectomy.47 Diffusion of the surgical robot proceeded similarly. By 2009, 85% of patients undergoing radical prostatectomy had robotic surgery.1 In the case of prostate cancer, it has been suggested that adoption of the surgical robot might have steered patients away from active surveillance or other guideline-supported prostate cancer management strategies.48,49 By contrast, our findings suggest that in the case of renal cell carcinoma, the surgical robot facilitated performance of partial nephrectomy. Thus, the desire to adopt new technology promoted increased performance of partial nephrectomy, an underutilized, guideline-supported procedure. If new surgical technology could be designed with the provision of evidence-based care in mind, it might be an effective intervention for surgeon behavior change.
Our study has several strengths. Our clinical question is very important, as the underutilization of partial nephrectomy represents a significant quality-of-care concern.17 We were able to study this question using a large population-based sample of nephrectomies from the HCUP-SID databases. Our final cohort of 21,569 nephrectomies represents the largest in the literature examining this question to date. Furthermore, the inclusion of patients of all ages and insurance types in the SID helps avoid some of the selection biases inherent in Medicare or VA cohorts. Moreover, our study is unique in that it demonstrates that robot acquisition is associated with increased utilization of partial nephrectomy over radical nephrectomy (proportion). As the incidence of renal cell carcinoma has increased with time as has the incidence of all nephrectomies (Table 2), other studies which focus only on the number of partial nephrectomies performed are confounded by the possibility that robot acquisition is associated with increased performance of both partial and radical nephrectomies (with no resultant change in the proportion of partial nephrectomy).
Our study also has several limitations. Our retrospective design and observational data precludes a definite determination of causality. We can only conclude that robot acquisition was significantly associated with increased utilization of partial nephrectomy in our multivariable model. Our difference-in-difference model, however, overcomes much of the bias typically present in observational studies because we simulated a control group of robot nonacquirers. This design allowed us to adjust for changes in secular trends which would normally be a significant limitation of observational data. Secondly, although our falsification test does partially allay the concern of endogeneity, it cannot dispel this notion entirely. Another limitation is the use of ICD-9 coding to identify cases. We were unable to differentiate whether any particular partial nephrectomy occurred via the open, pure laparoscopic or robotic-assisted modality and cannot definitively conclude whether the greater proportion of partial nephrectomy occurred only because of increased performance of robotic partial nephrectomy or also due to a “spillover benefit” of increased open partial nephrectomy by nonrobotic surgeons trying to keep up with their peers. Although the ability to accurately determine which technique was employed in the performance of partial nephrectomy might be an important observation, our primary question was whether robot acquisition was associated with increased provision of evidence-based care. Thus, our finding of a greater of proportion of partial nephrectomy (by any technique) associated with robot acquisition is a more important observation than proof of the tautology that “a hospital with a surgical robot performs more robotic partial nephrectomy.” As the HCUP-SID database does not provide any pathologic data, we are not able to determine whether the partial nephrectomies performed were appropriate, as certain pathologic criteria might contraindicate partial nephrectomy. This weakness, however, would be likely to bias our results toward the null hypothesis as we include in our analysis a group of patients with tumors which could never have been treated with partial nephrectomy. Our data source also excludes operative parameters or long-term complications. Although certainly important, this topic is outside the scope of our discussion as it has been previously demonstrated that robotic and laparoscopic partial nephrectomies result in similar perioperative outcomes.50 Despite these limitations, our study provides compelling evidence that hospital-level adoption of the surgical robot was independently associated with a greater proportion of partial nephrectomy.
The unexpected benefits of surgical robot acquisition on the management of renal tumors are an example of “reinvention” or the manner in which a new innovation is altered in the process of its adoption and implementation after its initial development.30 Although the surgical robot was not developed or initially marketed for the management of renal tumors, our results suggest that its acquisition was associated with an increased utilization of partial nephrectomy, an underutilized, guideline-supported procedure. Although hospitals and physicians have been criticized for adopting this unproven technology early in its development,1 it appears that the greatest increase in proportion of partial nephrectomy was observed among those earliest adopters. This example suggests that the benefits from early adoption of new technology might not be apparent to policy makers, researchers, or physicians a priori and that there might be some benefit to ensuring access to new technology for select institutions. This is potentially a very important lesson in the political debate over the use of comparative effectiveness data for the approval of new, unproven technology. Although much research and data are clearly necessary before widespread adoption, policy mechanisms like Medicare payments for technology adoption, should remain in place to encourage some degree of limited technology adoption, even if the technology may not immediately appear cost-effective.
Acknowledgments
The Louis Feil Charitable Lead Trust, United States Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (CDP-12-254). Dr Makarov is a VA HSR&D Career Development awardee (CDA 11-257) at the Manhattan VA. National Cancer Institute Cancer Center Support Grant (5 P30 CA016087-31).
APPENDIX
TABLE A1.
Results of the Lag Time Analysis to Determine the Association Between Adoption of the Surgical Robot and Changes in the Proportion of Partial Nephrectomy
| Hospital Characteristics | Marginal Change in Proportion of Partial Nephrectomy as Compared With Proportion in 2001 (95% CI) |
P |
|---|---|---|
| Robot acquisition 2001–2003 | In 2005: +26.0% (5.5–46.4) | 0.01 |
| In 2008: +30.3% (0.30–60.4) | < 0.01 | |
| Robot acquisition 2005–2007 | In 2008: +20.0% (6.8–33.1) | 0.048 |
CI indicates confidence interval.
TABLE A2.
Results of the Falsification Test to Determine the Association Between Adoption of the Surgical Robot and Changes in the Proportion of Partial Nephrectomy
| Hospital Characteristics | Marginal Change in Utilization of Total Knee Replacements as Compared With 2001 (95% CI), P |
Marginal Change in Utilization of Percutaneous Nephrolithotomy as Compared With 2001 (95% CI), P |
|---|---|---|
| Robot acquisition 2001–2004 | In 2005: +3.4% (−8.2 to 14.9), P = 0.57 | In 2005: +1.4% (−12.3 to 15.1), P = 0.84 |
| In 2008: −3.4% (−20.2 to 13.4), P = 0.69 | In 2008: −3.6% (−19.0 to 11.9), P = 0.65 | |
| Robot acquisition 2005–2008 | In 2008: −0.4% (−12.2 to 11.3), P = 0.94 | In 2008: +2.1% (−11.7 to 15.9), P = 0.76 |
CI indicates confidence interval.
TABLE A3.
Results of the Postoperative Outcomes Analysis to Determine the Association Between Adoption of the Surgical Robot and Changes in Postpartial Nephrectomy Surgical Outcomes
| Hospital Characteristics | Marginal Change in Length of Stay (d) Following Partial Nephrectomy as Compared With 2001 (95% CI), P |
Marginal Change in Mortality Following Partial Nephrectomy as Compared With 2001 (95% CI), P |
|---|---|---|
| Robot acquisition 2001–2004 | In 2005: +0.20 (0.81 to 1.21), P = 0.70 | In 2005: +3.7% (−0.5 to 7.9), P = 0.09 |
| In 2008: 0.58 (−0.48 to 1.63), P = 0.29 | In 2008: +0.2% (−3.2 to 3.6), P = 0.89 | |
| Robot acquisition 2005–2008 | In 2008: +0.58 (−0.54 to 1.70), P = 0.31 | In 2008: −0.9% (−2.6 to 0.9), P = 0.35 |
CI indicates confidence interval.
Footnotes
D.V.M. is a consultant for Castlight Health. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Kolata G. Results Unproven, Robotic Surgery Wins Converts. The New York Times. 2010 Feb 14; Health. [Google Scholar]
- 2.Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy: cost comparison of open, laparoscopic and robot assisted techniques. J Urol. 2004;172(4 pt 1):1431–1435. doi: 10.1097/01.ju.0000139714.09832.47. [DOI] [PubMed] [Google Scholar]
- 3.Carreyrou J. [Accessed May 4, 2010];Surgical robot examined in injuries. Wall Street Journal. 2010 Available at: http://online.wsj.com/article/SB10001424052702304703104575173952145907526.html. [Google Scholar]
- 4.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009:1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed May 2, 2013];da Vinci® Procedures. 2013 Available at: http://www.davincisurgery.com/da-vinci-surgery/da-vinci-procedures/
- 6.Makarov DV, Yu JB, Desai RA, et al. The association between diffusion of the surgical robot and radical prostatectomy rates. Med Care. 2011;49:333–339. doi: 10.1097/MLR.0b013e318202adb9. [DOI] [PubMed] [Google Scholar]
- 7.Stitzenberg KB, Wong YN, Nielsen ME, et al. Trends in radical prostatectomy: centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuner JM, See WA, Pezzin LE, et al. The association of robotic surgical technology and hospital prostatectomy volumes: increasing market share through the adoption of technology. Cancer. 2012;118:371–377. doi: 10.1002/cncr.26271. [DOI] [PubMed] [Google Scholar]
- 9.Robson CJ. Radical nephrectomy for renal cell carcinoma. J Urol. 1963;89:37–42. doi: 10.1016/S0022-5347(17)64494-X. [DOI] [PubMed] [Google Scholar]
- 10.Leibovich BC, Blute M, Cheville JC, et al. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 11.Becker F, Siemer S, Humke U, et al. Elective nephron sparing surgery should become standard treatment for small unilateral renal cell carcinoma: long-term survival data of 216 patients. Eur Urol. 2006;49:308–313. doi: 10.1016/j.eururo.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RH, Siddiqui S, Lohse CM, et al. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol. 2009;182:2601–2606. doi: 10.1016/j.juro.2009.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Archiv Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulabon LM, Lowrance WT, Russo P, et al. Trends in renal tumor surgery delivery within the United States. Cancer. 2010;116:2316–2321. doi: 10.1002/cncr.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175(3 pt 1):853–857. doi: 10.1016/S0022-5347(05)00422-2. discussion 858. [DOI] [PubMed] [Google Scholar]
- 18.Kim SP, Shah ND, Weight CJ, et al. Contemporary trends in nephrectomy for renal cell carcinoma in the United States: results from a population based cohort. J Urol. 2011;186:1779–1785. doi: 10.1016/j.juro.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Patel SG, Penson DF, Pabla B, et al. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. J Urol. 2012;187:816–821. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 20.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 21.Stifelman MD, Caruso RP, Nieder AM, et al. Robot-assisted laparoscopic partial nephrectomy. JSLS. 2005;9:83–86. [PMC free article] [PubMed] [Google Scholar]
- 22.Gettman MT, Blute ML, Chow GK, et al. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. 2004;64:914–918. doi: 10.1016/j.urology.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Oh J, Hong SK, et al. Open versus robot-assisted partial nephrectomy: effect on clinical outcome. J Endourol. 2011;25:1181–1185. doi: 10.1089/end.2010.0670. [DOI] [PubMed] [Google Scholar]
- 24.Benway BM, Bhayani SB, Rogers CG, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866–872. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Patel HD, Mullins JK, Pierorazio PM, et al. Trends in renal surgery: robotic technology is associated with increased use of partial nephrectomy. J Urol. 2012;189:1229–1235. doi: 10.1016/j.juro.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Kievit RA, Frankenhuis WE, Waldorp LJ, et al. Simpson’s paradox in psychological science: a practical guide. Front Psychol. 2013;4:513. doi: 10.3389/fpsyg.2013.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kardos SV, Gross CP, Shah ND, et al. Association of type of renal surgery and access to robotic technology for kidney cancer: results from a population-based cohort. BJU Int. 2014;114:549–554. doi: 10.1111/bju.12711. [DOI] [PubMed] [Google Scholar]
- 28.Hollenbeck BK, Taub DA, Miller DC, et al. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 29.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Rogers E. Diffusion of Innovations. 5th ed Free Press; New York: 2003. [Google Scholar]
- 31.HCUP State Inpatient Databases (SID) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2008. [Google Scholar]
- 32.American Hospital Association . American Hospital Association Annual Survey. American Hospital Association; Washington, DC: 2010. [Google Scholar]
- 33.Campbell SC, Lane BR. Malignant Renal Tumors. 10th ed Volume 11. Saunders; Philadelphia, PA: 2012. [Google Scholar]
- 34.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Ulmer WD, Prasad SM, Kowalczyk KJ, et al. Factors associated with the adoption of minimally invasive radical prostatectomy in the United States. J Urol. 2012;188:775–780. doi: 10.1016/j.juro.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Bjurlin MA, Walter D, Taksler GB, et al. National trends in the utilization of partial nephrectomy before and after the establishment of AUA guidelines for the management of renal masses. Urology. 2013;82:1283–1289. doi: 10.1016/j.urology.2013.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy P. A Guide to Econometrics. The MIT Press; Cambridge, MA: 2003. [Google Scholar]
- 38.Preston MA, Blew BD, Breau RH, et al. Survey of senior resident training in urologic laparoscopy, robotics and endourology surgery in Canada. Can Urol Assoc J [Journal de l’Association des Urologues du Canada] 2010;4:42–46. doi: 10.5489/cuaj.09036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donabedian A, Wheeler JR, Wyszewianski L. Quality, cost, and health: an integrative model. Med Care. 1982;20:975–992. doi: 10.1097/00005650-198210000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Rubenstein LV, Mittman BS, Yano EM, et al. From understanding health care provider behavior to improving health care: the QUERI framework for quality improvement. Quality Enhancement Research Initiative. Med Care. 2000;38(suppl 1):I129–I141. [PubMed] [Google Scholar]
- 41.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 42.Stirrat GM. Ethics and evidence based surgery. J Med Ethics. 2004;30:160–165. doi: 10.1136/jme.2003.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melis M, Karl RC, Wong SL, et al. Evidence-based surgical practice in academic medical centers: consistently anecdotal? J Gastrointest Surg. 2010;14:904–909. doi: 10.1007/s11605-010-1175-1. [DOI] [PubMed] [Google Scholar]
- 44.Brennan MF. Is nil per os still appropriate for patients undergoing upper gastrointestinal surgery? Nat Clin Pract Gastroenterol Hepatol. 2008;5:660–661. doi: 10.1038/ncpgasthep1279. [DOI] [PubMed] [Google Scholar]
- 45.Fendrick AM, Escarce JJ, McLane C, et al. Hospital adoption of laparoscopic cholecystectomy. Med Care. 1994;32:1058–1063. doi: 10.1097/00005650-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Finlayson SR, Laycock WS, Birkmeyer JD. National trends in utilization and outcomes of antireflux surgery. Surg Endosc. 2003;17:864–867. doi: 10.1007/s00464-002-8965-9. [DOI] [PubMed] [Google Scholar]
- 47.Escarce JJ, Chen W, Schwartz JS. Falling cholecystectomy thresholds since the introduction of laparoscopic cholecystectomy. JAMA. 1995;273:1581–1585. [PubMed] [Google Scholar]
- 48.Schroeck FR, Kaufman SR, Jacobs BL, et al. The impact of technology diffusion on treatment for prostate cancer. Med Care. 2013;51:1076–1084. doi: 10.1097/MLR.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellison JS, Montgomery JS, Wolf JS, Jr, et al. A matched comparison of perioperative outcomes of a single laparoscopic surgeon versus a multisurgeon robot-assisted cohort for partial nephrectomy. J Urol. 2012;188:45–50. doi: 10.1016/j.juro.2012.02.2570. [DOI] [PubMed] [Google Scholar]


