Abstract
Although high density lipoprotein-cholesterol (HDL-C) concentration is a negative risk factor for atherosclerotic cardiovascular disease (CVD), efforts to reduce CVD risk by raising HDL-C have not been uniformly successful. Many studies have shown that alcohol consumption, that increases plasma HDL-C concentration, reduces CVD incidence. However, recent genetic studies in large populations have not only removed HDL-C from the causal link between plasma HDL-C concentration and reduced CVD risk, but suggest that the association is weak as well. Herein we propose that the cardioprotective effects of alcohol are mediated by the interaction of its terminal metabolite, acetate, with the adipocyte-free fatty acid receptor-2, which elicits a profound antilipolytic effect that may increase insulin sensitivity without necessarily raising plasma HDL-C concentration.
Keywords: atherosclerosis, dyslipidemia, HDL, alcohol, acetate, FFAR2
Introduction
The underlying cause for most CVD is atherosclerosis, which begins with the transfer of low density lipoproteins (LDL) to the subendothelial space of the arterial wall where they undergo oxidative modification. Blood monocyte-derived macrophages within the subendothelium take up oxidized LDL and acquire a foamy aspect due to the intracellular accumulation of LDL-cholesteryl esters. High plasma LDL-cholesterol (LDL-C) levels and low plasma levels of HDL-C and major CVD lipid risk factors. Statins, which reduce plasma LDL-C concentrations, reduce CVD events in men and women with a range of plasma LDL-C concentrations and with other risk factors—diabetes, hypercholesterolemia, normocholesterolemia, angina, previous myocardial infarction (MI), and high-sensitivity C-reactive protein, an inflammatory marker[1]. There is a consensus that reducing plasma LDL-C levels is beneficial; the value of raising plasma HDL-C concentrations is under increasing scrutiny.
Raising Plasma HDL-C Concentrations is Cardioprotective
The French physician-scientist Michel Macheboeuf is the father of plasma lipoproteins. In his doctoral thesis, “Recherches sur les lipides, les stérols et les protéides du sérum et du plasma sanguinis,” he described the coprecipitation of lipids and proteins from horse serum [2]; the precipitate was later revealed as HDL. Nearly thirty years passed until Gofman and colleagues began the first prospective study to relate HDL subfractions to heart disease risk. After ten years follow-up they reported that ischemic heart disease was elevated in those with low plasma HDL concentrations, especially the larger, more cholesteryl ester-rich fraction, HDL2 [3]. This observation was confirmed by a 29 year follow-up of the study [4]. Later investigations of HDL shifted away from the tedious method of analytical ultracentrifugation used by Gofman to measuring plasma HDL-C concentrations. An early study showed that mean HDL-C levels were inversely associated with coronary heart disease (CHD), even after adjusting LDL-C and triglyceride (TG) levels [5]. This association was confirmed by the Framingham Heart Study [6]. Given the compelling cross-sectional and prospective data, tests were begun with the fibrate gemfibrozil, a peroxisome proliferator-activated receptor-alpha (PPARα) agonist that profoundly lowers plasma TG concentrations, while modestly increasing plasma HDL-C concentrations. Over a period of five years, gemfibrozil vs. placebo reduced plasma levels of total cholesterol (10%), non-HDL-C (14%), LDL-C (11%), and TG (35%), and increased mean HDL-C (11%). These changes were associated with a reduction (34%) in the incidence of coronary heart disease [7], a finding that was confirmed by the Veterans Affairs HDL Intervention Trial [8], an important study because it included patients with type 2 diabetes and metabolic syndrome. However, the cardiovascular benefit was more strongly correlated with insulin resistance than HDL-C. Moderate exercise and regular, moderate alcohol consumption increase the plasma HDL-C concentration nearly equally; however, addition of regular alcohol consumption does not add to the HDL-C-raising effect of moderate exercise.[9] Regular, moderate alcohol consumption is also associated with reduced CVD incidence and mortality [10], an effect initially assigned to attendant increased HDL-C [11]. Collectively, these studies revealed an inverse association between CVD and plasma HDL and HDL-C concentrations, and provided support for plasma HDL-C reduction as a cardioprotective measure.
Raising Plasma HDL-C Concentrations is not Cardioprotective
There is now a growing body of evidence that raising plasma HDL-C levels is not uniformly cardioprotective. Various studies, including genetic studies of several large cohorts, have put the “higher-HDL-is-better” hypothesis in doubt. For example, some patients with genetically elevated plasma levels of apolipoprotein AI (apo AI), the major protein component of HDL, and HDL-C, did not have a reduced risk for ischemic heart disease or MI[12]; an HDL-C–raising endothelial lipase variant was not associated with reduced MI [13]; controlling for HDL-C does not affect the magnitude of the negative relationship between alcohol intake and death from CVD [14]. According to these data, HDL is not mechanistically linked to the atheroprotective effects of alcohol ingestion.
Other studies have also failed to support the “higher HDL-C is better” hypothesis. Many patients with low HDL-C do not develop CVD, and vice versa. For example, the very high plasma HDL-C concentrations found in patients with cholesteryl ester transfer protein (CETP) deficiency do not commensurately reduce CVD incidence [15], and to date, tests of CETP inhibitors, which profoundly increase HDL-C levels [16, 17], have not reduced CVD events [17–19]. The CETP inhibitor torcetrapib disappointed because of unexpected toxicity and mortality [16], and dalcetrapib also failed due to a lack of clinically meaningful efficacy [17], perhaps scuttling the higher-HDL-is-better hypothesis. Mechanisms underlying the potential benefits of CETP inhibitors are difficult to sort out because of their concurrent LDL-C lowering effects.
Although niacin (also known as nicotinic acid or vitamin B3), which increases HDL-C levels, reduces CVD events and all-cause mortality [20], addition of niacin to a statin did not reduce CVD events [21]. In the AIM-HIGH Trial, niacin increased HDL-C and lowered TGs and LDL-C concentrations, but after three years the trial was stopped for futility[22]. There was no difference with respect primary composite end points at the end of three years. Thus, it appears doubtful that addition of niacin to a statin adds any benefit. Despite these findings, other niacin analogs and formulations are in various stages of development. Although other niacin analogs that do not cause flushing are in development, the disappointing results of niacin trials with statins put their futures in doubt.
HDL Function
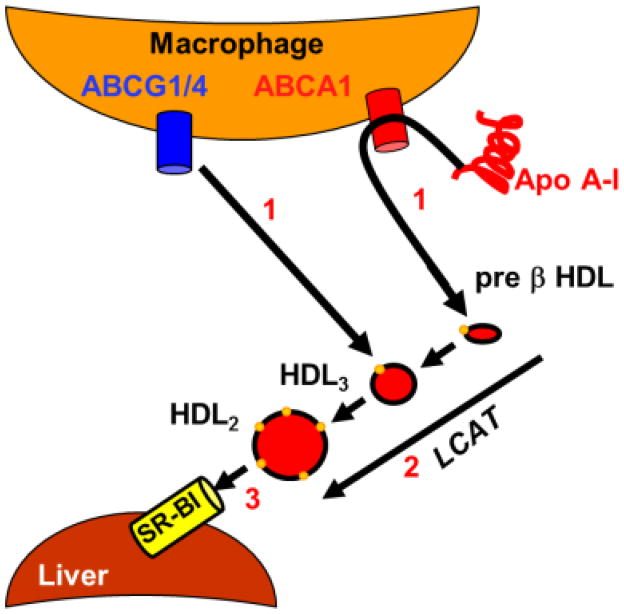
Given the failure of some interventions that raise plasma HDL-C concentrations to prevent atherosclerosis, other mechanistic strategies based on lipoprotein quality and function, have been tested. Most new strategies were designed to improve reverse cholesterol transport (RCT), the transfer of cholesterol from the arterial wall to the liver for disposal (Figure 1) [23]. All nucleated cells synthesize cholesterol, an essential lipid in membrane biogenesis, but of the major organs only the liver can dispose of it. In the context of atheroprotection, RCT begins with the transfer of macrophage-cholesterol in the arterial subendothelium to apo AI and HDL; transfer of cholesterol along with phospholipids to apo AI is mediated by the macrophage plasma membrane ATP-binding cassette transporter subfamily A, member 1 (ABCA1); direct cholesterol transfer to HDL is mediated by the macrophage plasma membrane ABCG4 transporter (Figure 1, Step 1). Both steps produce early forms of HDL, called nascent HDL, that diffuse to the plasma compartment, where in the second step, cholesterol is esterified by lecithin:cholesterol acyltransferase, producing mature HDL (Figure 1, Step 2). In the final step, HDL, lipids—cholesteryl ester, TGs, and some phospholipids, are selectively removed by hepatic scavenger receptor class B, member 1 (SR-BI), while HDL-apolipoproteins are excluded and released into the extracellular space (Figure 1, Step 3)[24]. New therapies amplifying one or more of the three RCT steps are in various stages of development.
Figure 1.
Current RCT Model.
Step 1, macrophage lipid efflux; step 2, esterification by lecithin:cholesterol acyl transferase (LCAT); step 3, selective hepatic uptake of HDL-lipids via SR-BI.
Although most RCT models begin with macrophage cholesterol efflux via the interaction of lipid-free apo AI with ABCA1, the concentration of lipid-free apo AI in plasma is low. This is not to say lipid-free apo AI does not occur in plasma but rather that its occurrence is transient. According to several methods, HDL resides in a kinetic trap from which it escapes via physicochemical perturbations [25]. In the process, HDL releases a large fraction of its apo AI as a lipid-free species [26, 27]. HDL is similarly disrupted by several plasma activities–CETP,[28] phospholipid transfer protein,[29] lecithin:cholesterol acyltransferase,[30] hepatic lipase,[31] and streptococcal serum opacity factor[32]; the free energy of activation for these processes are nearly the same as that for apo AI exchange between HDL[33] and some of them release only small amounts of lipid-free apo AI. However, serum opacity factor mediates release of nearly half of HDL-apo AI in vitro. However, in vivo little is detected in plasma suggesting that it rapidly reenters the HDL pool or is renally extracted[34]. Moreover, a small fraction could enter the subendothelial space to initiate macrophage RCT.
Is Macrophage Cholesterol Efflux (MCE) a Marker of Atheroprotection?
Tangier disease patients present with elevated plasma TGs and the near absence of HDL [35]. In the context of Tangier disease, which is caused by a mutation in the ABCA1 gene [36], and the recapitulation of the Tangier disease phenotype in liver-specific ABCA1-null mice [37], hepatic ABCA1 appears to be central to most HDL formation. If plasma HDL-C levels are a negative CVD risk factor, one would expect high CVD rates in Tangier patients, something that is not observed[38]. Subsequent studies suggested that ABCA1 on macrophages was more important in atheroprotection. Some early studies of cellular cholesterol efflux from several non-macrophage cell types to various phospholipid acceptors showed that the rate-limiting step is desorption from the cell surface into the unstirred water layer around the cell [39]. Passive desorption from the cell surface was thought to be the major efflux mechanism for many years. Later, it was reported that incubation of apo AI with macrophage foam cells leads to cholesterol efflux that formed a nascent HDL particle, in the extracellular medium[40]. This was an important finding, given the defective cholesterol efflux from cells derived from patients with Tangier Disease, which, as mentioned above, is characterized by very low plasma HDL-C[41] and a mutation in the ABCA1 gene [36]. Other studies showed that cellular phospholipid (PL) and cholesterol efflux to human apo AI via ABCA1 produces a lipid poor, pre β1-HDL particle, which mediated efflux as effectively as lipid-free apo AI, ultimately giving rise to discoidal HDL [42]. Thus, pre β1-HDL is both a product and a ligand for cellular ABCA1-mediated lipid efflux.
Although ABCA1 is frequently cited as a cholesterol transporter, this is mechanistically not likely, because cholesterol diffuses freely across membranes without a need for ATP. However, the same cannot be said of phospholipid translocation, which is also part of the lipid efflux process that yields early forms of HDL such as pre β1-HDL; phospholipid translocation in the absence of energy input is slow [43]. However, phospholipids as the essential cholesterol-binding component of membranes and lipoproteins, support cholesterol translocation in an indirect way; ABCA1 activity increases the phospholipid content of the outer leaflet of a membrane bilayer[44], and in so doing, the cholesterol simply follows, and both associate with apo AI or nascent HDL and transfer to the extracellular compartment[40]. Thus, the phospholipid translocase activity of ABCA1 mediates concurrent phospholipid and cholesterol efflux to lipid-free apo AI and plasma pre-β1-HDL [45, 46].
The apparent failure of the “higher HDL-C is better” hypothesis provoked hypotheses about differential HDL functionality, which has been tested. Initially, investigators compared MCE to apo B-depleted serum from healthy volunteers with minimal carotid artery intima-media thickness, patients with angiographically confirmed coronary artery disease, and patients without such angiographically confirmed disease and found the following: Although HDL-C and apo AI were positive determinants of cholesterol efflux, they accounted for <40% of the observed variation. Importantly efflux capacity and carotid intima-media thickness were inversely correlated, even after adjusting for HDL-C levels. Somewhat paradoxically, efflux was also enhanced in patients with metabolic syndrome and low HDL-C levels [47]. This study supported the hypothesis that HDL quality may be as important as its plasma concentration[48], i.e., not all HDL is created functionally equal. Thus, the magnitude of efflux to serum is a functional metric of HDL that is inversely correlated with carotid intima-media thickness, independently of the HDL cholesterol level.
Another study (The Dallas Heart Study) investigated the association of cholesterol efflux capacity with incident atherosclerotic CVD outcomes[49]. This study was important because it was conducted in a large, multiethnic population cohort. In a fully adjusted model that included traditional risk factors, HDL-C level, and HDL particle concentration, CVD risk in the highest quartile of cholesterol efflux capacity was 67% lower than that of the lowest quartile. Once again, they concluded that plasma cholesterol efflux capacity is a biomarker that quantifies the initial RCT step that is inversely associated with the incidence of CVD events. Unexpectedly, a third study[50] found that the magnitude of MCE to apo B-depleted serum was associated with increased prospective risk for MI, stroke, and death and that most macrophages-cholesterol was destined for the lipoprotein deficient fraction.
A small study of MCE to plasma, from obese patients with metabolic syndrome in a weight loss program, found the following [51]; First, at early time points, ~75% of the MCE associates with HDL, but transfers to the apo B-containing lipoproteins at later times, where mostly resides on LDL. Second, MCE in obese patients with metabolic system is greater than in normal weight healthy controls. However, following weight-loss, the magnitude of efflux is the same as control [51]. In this context, reducing MCE by weight loss may not have much atheroprotective value when there is concurrent robust reduction of hypertension, total cholesterol, LDL-C, non HDL-C, apo B and insulin resistance. The higher CVD risk in diabetic and metabolic syndrome patients compared to control non-diabetic subjects suggests that the perhaps negative effects of reduced cholesterol efflux to plasma is not as important as reducing the traditional CVD risk factors, especially the number of apo B-containing lipoproteins.
Given that there seems to be some consensus about the value of MCE measurement, as most find a positive correlation of MCE with various measures of CVD, some conceptual and technical short-comings also exist. First, as one might suspect, it would be better to conduct MCE on fresh, recently collected samples, not stored samples in which the functional integrity of the lipoproteins can be compromised. Recognizing that studies of large cohorts cannot be otherwise conducted, it is essential to perform confirmatory follow-up studies in a well-controlled cohort, in which MCE is measured in freshly collected never-frozen samples. Second, it is preferable to measure MCE directly using a wet assay for cholesterol instead of radio- or fluorescent-labeled cholesterol, which only give efflux but not influx rates; it is important to know the balance of cholesterol between lipoproteins and macrophages at the end of the assay, since net influx and efflux affect intracellular metabolism in opposing ways [52]. Any correspondence between the efflux rates for radio- and fluorescent-labeled cholesterol only testifies to the rates being controlled by the same thermodynamic forces. Notably, MCE measured with fluorescent-labeled cholesterol is seven times faster than when measured with radiolabeled cholesterol. Third, patients carrying the apo AIMilano variant have low plasma HDL-C concentrations but less CVD. Despite this, MCE in WT apo AI and the apo AIMilano variant was not different, nor was MCE to reassembled HDL (rHDL) comprising WT and apo AIMilano.[53]. Lastly there is a conceptual issue. The two large studies[47, 54] measured MCE using apo B-depleted plasma; hypothetically, this would measure MCE mainly to HDL. If removing cholesterol from macrophages is an atheroprotective measure, why not measure transfer to dilute whole plasma, given that LDL is a major depot for MCE, even though HDL is the initial acceptor [51]. Moreover, given its much longer plasma lifetime compared to LDL, why would MCE to HDL be more atheroprotective that transfer to shorter-lived LDL? As mice in which the HDL receptor, SR-BI, has been deleted, have high HDL-C but develop atherosclerosis, improving HDL-C clearance may be more atheroprotective than increased MCE.
Atheroprotective Therapeutic Strategies via Enhanced MCE
As HDL and apo AI are the major MCE acceptors, mimetics of both have been tested as potential therapeutics. One of the former is rHDL, comprising native human plasma apo AI and phospholipids. This mimetic can have several advantages, such as Apo AI would not pose any immunological challenges; rHDL can be formulated to maximize efflux by using phospholipids with high cholesterophilicity and without any cholesterol, which would readily transfer back to tissues. Efflux to rHDL is a function of phospholipid composition, increasing with increased acyl chain length and saturation [55]. Similar trends have been reported for the binding of cholesterol to phospholipids [56], so that the thermodynamic determinant of MCE is the cholesterophilicity of the acceptor phospholipids. These correlations provided the rationale for several potential therapies that use rHDL. Many MCE tests have been conducted with rHDL, produced by the spontaneous association of apo AI with some saturated phospholipids [57], or by a detergent removal method [58], and several rHDL formulations have been tested for their anti atherogenic effects. A single injection of one of these, CSL-111, a rHDL comprising human apo AI and soybean phosphatidylcholine, into mice, was followed by a dose- and time-dependent increase of plasma apo AI, pre-β HDL, total cholesterol, and triglycerides [59]. Tests in humans, which were encouraging but unimpressive, showed that CSL-111 but not placebo infusion into patients reduced atheroma volume. A recent formulation, which contains less residual cholate, has a lower potential for liver toxicity [60].
Alcohol Consumption and HDL
Daily moderate alcohol consumption is one of the few measures that simultaneously increase plasma HDL-C concentrations and reduce CVD death; however at high rates of consumption, other causes of death increase [61]. There are two lines of thought about the mechanisms that link alcohol consumption to reduced CVD. One widely held view is that cardioprotection is due to alcohol-induced increases in HDL-C [62, 63], which would enhance multiple salutary effects of HDL as an anti-oxidant, anti-inflammatory agent, and, especially as an acceptor of macrophage cholesterol efflux. This hypothesis is supported by the observation that persons carrying the loss-of-function allele for alcohol dehydrogenase type 1C (ADH1C) have profoundly elevated plasma HDL-C levels and less CVD [64]. Given that ADH1C catalyzes the conversion of alcohol to acetaldehyde, its inhibition would maintain body alcohol content for a longer time, during which it elicits a direct HDL-raising effect by an unknown mechanism. It would also suggest that the effect of alcohol on HDL metabolism is direct and not mediated by its metabolites, acetaldehyde and acetate.
Given the modest effect of plasma HDL-C levels in the adjusted hazard ratios for CVD death among alcohol consumers [14], other mechanisms must also be considered. Alcohol or one of its metabolites could be cardioprotective via an HDL-independent process, i.e., the mechanisms that raise HDL-C and prevent CVD could be distinct. One mechanism involves the terminal metabolite of alcohol, acetate. Acetate exerts a transient but profound effect on mammalian lipid metabolism. Alcohol ingestion profoundly reduces plasma non esterified fatty acids (NEFA), an effect that is recapitulated by its terminal metabolite, acetate [65]; this effect is likely due to the inhibition of adipose tissue-lipolysis. This conclusion is consistent with studies showing that acetate but not alcohol or acetaldehyde, inhibits epinephrine-induced lipolysis in isolated rat adipocytes [66]. While alcohol is usually thought to increase plasma TGs, particularly among hypertriglyceridemic patients, this effect is only seen in normolipemic persons in which the alcohol-induced lipemia is mild [67]. However, when alcohol is consumed with a fat-containing meal, a profound lipemic response that exceeds the sum of the fat and alcohol consumed separately is observed [68]; this effect has been attributed to lipoprotein lipase (LPL) inhibition [69]. Thus, alcohol produces the unusual state in which both adipose tissue lipolysis and LPL are inhibited.
Given the cardioprotective effects of alcohol, it is tempting to add alcohol to one’s diet. However, the potential risks associated with such a recommendation likely outweigh the benefits in persons with hepatic dysfunction, or a potential for addiction or psychosocial disorders. Moreover, alcohol consumption increases the incidence of cancers of the upper gastrointestinal tract [70, 71]. Thus, alternatives that raise plasma HDL-C in a cardioprotective way are needed. One possible mechanism for inhibition of adipose tissue lipolysis has emerged from recent studies of colonic fermentation, which produces short chained fatty acids that are ligands for several free fatty acid receptor-2 (FFAR2, also known a G-protein coupled receptor 43). FFAR2, a receptor for acetate, is likely involved in immune and inflammatory responses because it is highly expressed in immune cells [72, 73]. More relevant to lipid metabolism, FFAR2 is also expressed in mouse adipocytes, which in response to acetate, inhibits lipolysis, an effect not seen in adipocytes from FFAR2-null mice [74]. FFAR2-null mice are obese on a normal diet, whereas mice with adipose tissue-specific FFAR2 overexpression remain lean even on a high-fat diet [75]. These studies suggested that FFAR2 reduces adipose tissue insulin sensitivity while increasing insulin sensitivity in liver and skeletal muscle [76].
Acetate as CVD Therapy
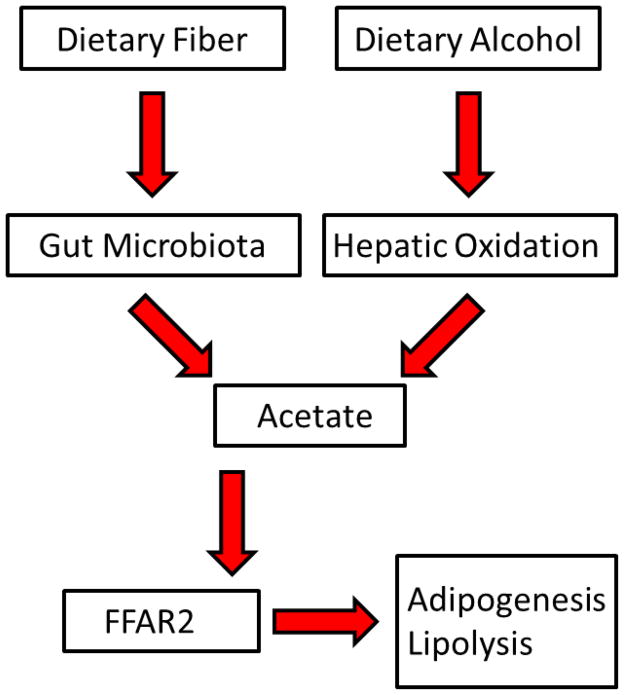
Several lines of evidence support a beneficial role of acetate in CVD. After alcohol ingestion, plasma acetate levels increase up to 20-fold, to near millimolar concentrations [77, 78]. Dietary acetic acid reduces serum cholesterol and TG in rats fed a cholesterol-rich diet [79]. Ingestion of acetic acid as vinegar by rats lowers blood pressure, an effect that has not been tested in humans [80]. Some speculate that the risk of fatal ischemic heart disease in humans is lowered by the regular consumption of acetate as vinegar and oil salad dressings [80]. It is interesting to further speculate that perhaps the observed effects of alcohol on lipids, blood pressure, and CVD, are at least in part mediated through its conversion to acetate and activation of FFAR2. Before the introduction of modern hypoglycemic agents, diabetics used vinegar teas to control symptoms [80]. Several trials have revealed that the addition of small amounts of domestic vinegar (~25g) to food, or vinegar taken with a meal, reduce the glycemic index of carbohydrate-rich food in people with and without diabetes [81–83]. This also has been expressed as lower glycemic index ratings in the region of 30% [84, 85]. Vinegar improves insulin sensitivity in normal and metabolic syndrome subjects[86], an effect that would be expected to reduce net adipocyte lipolysis, thereby lowering plasma NEFA and TG levels. Moderate daily alcohol intake is also associated with lower insulin secretion [87]. Thus, acetate from alcohol, or from dietary fiber which is converted to acetate by gut microbiota [88], could be salutary and operate through its effects via adipose tissue-FFAR2. The hypothetical connection of alcohol and fiber with adipocyte biology is shown in Figure 2. As potential anti metabolic syndrome, anti diabetic and cardioprotective agents, acetate may offer other advantages over alcohol. Tests in humans showing that oral acetate raises HDL-C might establish acetate as the HDL-C-raising effect of alcohol, and suggest that acetate ingestion by humans is cardioprotective.
Figure 2.
Hypothetical Model Diet-Induced Inhibition of Lipolysis via Acetate-FFAR2 Signaling.
Acetate, derived from dietary fiber and hepatic oxidation of dietary alcohol, binds to adipocyte FFAR2 thereby regulating adipogenesis and lipolysis.
Concluding remarks and future perspectives
Many open questions about atheroprotection remain (See Outstanding Questions Box). The higher-HDL-is-better hypothesis is supported by epidemiological evidence linking low plasma levels of HDL-C to premature CVD. However, support for the hypothesis is weakened by genetic studies showing little or no correlation between the distribution of HDL-C modifying genes and differences in CVD prevalence. Moreover, interventions that raise plasma HDL-C levels do not appear to prevent CVD and even in some cases where there is a positive effect on HDL-C, it could be explained by parallel drug-induced changes in another plasma analyte, usually reduced plasma triglyceride levels. Two challenges remain: The first is to identify the mechanisms underlying the cardioprotective effects of some HDL-raising interventions. The second is to use this information to develop new HDL therapies that cardioprotect irrespective of their effects on plasma HDL-C concentrations.
Trends.
A large body of epidemiological evidence shows that plasma HDL-C concentrations are negatively correlated with the incidence of atherosclerosis.
CETP inhibitors and HDL genes that increase plasma HDL-C concentrations do not cardioprotect.
The functional qualities of HDL are more relevant to cardioprotection than plasma HDL-C concentrations.
Alcohol consumption is associated with reduced cardiovascular disease without a profound increase in plasma HDL-C concentrations.
Alcohol is converted to acetate, which elicits an antilipolytic effect via a G-protein coupled receptor (FFAR2).
New interventions that improve HDL functionality without necessarily increasing its concentration should be identified and validated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Henry J. Pownall, Email: HJPownall@houstonmethodist.org, Houston Methodist Research Institute and Weill Cornell Medical College, 6670 Bertner Avenue, Houston TX 77030.
Antonio M. Gotto, Jr., Email: amg2004@med.cornell.edu, Houston Methodist Research Institute and Weill Cornell Medical College, 1305 York Avenue, New York, NY, USA.
References
- 1.Ridker PM. The JUPITER trial: results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes. 2009;2:279–285. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 2.Macheboeuf M. Recherches sur les phosphoaminolipides et les sterides du serum et du plasma sanguins: I. Entrainement des phospholipids, des sterols et des sterides par les diverses fractions au cours du fractionnement des proteides du serum. Bull Soc Chim Biol. 1930;223:99. [Google Scholar]
- 3.Gofman JW, et al. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. doi: 10.1161/01.cir.34.4.679. [DOI] [PubMed] [Google Scholar]
- 4.Williams PT, Feldman DE. Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman’s Livermore Cohort. Atherosclerosis. 2011;214:196–202. doi: 10.1016/j.atherosclerosis.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelli WP, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–772. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 6.Gordon T, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 7.Frick MH, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 8.Rubins HB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 9.Hartung GH, et al. Effect of alcohol intake on high-density lipoprotein cholesterol levels in runners and inactive men. JAMA. 1983;249:747–750. [PubMed] [Google Scholar]
- 10.Ronksley PE, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer RD, et al. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation. 1992;85:910–915. doi: 10.1161/01.cir.85.3.910. [DOI] [PubMed] [Google Scholar]
- 12.Haase CL, et al. Genetically Elevated Apolipoprotein A-I, High-Density Lipoprotein Cholesterol Levels, and Risk of Ischemic Heart Disease. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0450. [DOI] [PubMed] [Google Scholar]
- 13.Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012 doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnus P, et al. Controlling for high-density lipoprotein cholesterol does not affect the magnitude of the relationship between alcohol and coronary heart disease. Circulation. 2011;124:2296–2302. doi: 10.1161/CIRCULATIONAHA.111.036491. [DOI] [PubMed] [Google Scholar]
- 15.Zhong S, et al. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GG, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 18.Tall AR, et al. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27:257–260. doi: 10.1161/01.ATV.0000256728.60226.77. [DOI] [PubMed] [Google Scholar]
- 19.Zhao HP, Xiang BR. Discontinued cardiovascular drugs in 2013 and 2014. Expert Opin Investig Drugs. 2015;24:1083–1092. doi: 10.1517/13543784.2015.1051619. [DOI] [PubMed] [Google Scholar]
- 20.The coronary drug project. Findings leading to further modifications of its protocol with respect to dextrothyroxine. The coronary drug project research group. JAMA. 1972;220:996–1008. [PubMed] [Google Scholar]
- 21.Boden WE, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 22.Investigators AH, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 23.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 24.Shen WJ, et al. Scavenger receptor class B type I (SR-BI): a versatile receptor with multiple functions and actions. Metabolism. 2014;63:875–886. doi: 10.1016/j.metabol.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta R, et al. Human plasma high-density lipoproteins are stabilized by kinetic factors. J Mol Biol. 2003;328:183–192. doi: 10.1016/s0022-2836(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 26.Pownall HJ, et al. Speciation of human plasma high-density lipoprotein (HDL): HDL stability and apolipoprotein A-I partitioning. Biochemistry (Mosc) 2007;46:7449–7459. doi: 10.1021/bi700496w. [DOI] [PubMed] [Google Scholar]
- 27.Pownall HJ. Remodeling of human plasma lipoproteins by detergent perturbation. Biochemistry (Mosc) 2005;44:9714–9722. doi: 10.1021/bi050729q. [DOI] [PubMed] [Google Scholar]
- 28.Rye KA, et al. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J Biol Chem. 1997;272:3953–3960. doi: 10.1074/jbc.272.7.3953. [DOI] [PubMed] [Google Scholar]
- 29.Settasatian N, et al. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J Biol Chem. 2001;276:26898–26905. doi: 10.1074/jbc.M010708200. [DOI] [PubMed] [Google Scholar]
- 30.Liang HQ, et al. Remodelling of reconstituted high density lipoproteins by lecithin: cholesterol acyltransferase. J Lipid Res. 1996;37:1962–1970. [PubMed] [Google Scholar]
- 31.Kee P, et al. Metabolism of apoA-I as lipid-free protein or as component of discoidal and spherical reconstituted HDLs: studies in wild-type and hepatic lipase transgenic rabbits. Arterioscler Thromb Vasc Biol. 2002;22:1912–1917. doi: 10.1161/01.atv.0000038485.94020.7f. [DOI] [PubMed] [Google Scholar]
- 32.Gillard BK, et al. Serum opacity factor unmasks human plasma high-density lipoprotein instability via selective delipidation and apolipoprotein A-I desorption. Biochemistry (Mosc) 2007;46:12968–12978. doi: 10.1021/bi701525w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handa D, et al. Kinetic and thermodynamic analyses of spontaneous exchange between high-density lipoprotein-bound and lipid-free apolipoprotein A-I. Biochemistry (Mosc) 2015;54:1123–1131. doi: 10.1021/bi501345j. [DOI] [PubMed] [Google Scholar]
- 34.Bisoendial RJ, et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman HN, Fredrickson DS. Tangier disease (familial high density lipoprotein deficiency). Clinical and genetic features in two adults. Am J Med. 1965;39:582–593. doi: 10.1016/0002-9343(65)90081-1. [DOI] [PubMed] [Google Scholar]
- 36.Brooks-Wilson A, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 37.Timmins JM, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iatan I, et al. Effect of ABCA1 mutations on risk for myocardial infarction. Curr Atheroscler Rep. 2008;10:413–426. doi: 10.1007/s11883-008-0064-5. [DOI] [PubMed] [Google Scholar]
- 39.Rothblat GH, Phillips MC. Mechanism of cholesterol efflux from cells. Effects of acceptor structure and concentration. J Biol Chem. 1982;257:4775–4782. [PubMed] [Google Scholar]
- 40.Lyssenko NN, et al. Factors controlling nascent high-density lipoprotein particle heterogeneity: ATP-binding cassette transporter A1 activity and cell lipid and apolipoprotein AI availability. FASEB J. 2013;27:2880–2892. doi: 10.1096/fj.12-216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawn RM, et al. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duong PT, et al. Characterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. J Lipid Res. 2008;49:1006–1014. doi: 10.1194/jlr.M700506-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homan R, Pownall HJ. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim Biophys Acta. 1988;938:155–166. doi: 10.1016/0005-2736(88)90155-1. [DOI] [PubMed] [Google Scholar]
- 44.Quazi F, Molday RS. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem. 2013;288:34414–34426. doi: 10.1074/jbc.M113.508812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JD, et al. ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid Res. 2004;45:635–644. doi: 10.1194/jlr.M300336-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Gillotte KL, et al. Removal of cellular cholesterol by pre-beta-HDL involves plasma membrane microsolubilization. J Lipid Res. 1998;39:1918–1928. [PubMed] [Google Scholar]
- 47.Khera AV, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton JA, Deckelbaum RJ. Crystal structure of CETP: new hopes for raising HDL to decrease risk of cardiovascular disease? Nat Struct Mol Biol. 2007;14:95–97. doi: 10.1038/nsmb0207-95. [DOI] [PubMed] [Google Scholar]
- 49.Rohatgi A, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li XM, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasudevan M, et al. Modest diet-induced weight loss reduces macrophage cholesterol efflux to plasma of patients with metabolic syndrome. J Clin Lipidol. 2013;7:661–670. doi: 10.1016/j.jacl.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picardo M, et al. Partially reassembled high density lipoproteins. Effects on cholesterol flux, synthesis, and esterification in normal human skin fibroblasts. Arteriosclerosis. 1986;6:434–441. doi: 10.1161/01.atv.6.4.434. [DOI] [PubMed] [Google Scholar]
- 53.Weibel GL, et al. Wild-type ApoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler Thromb Vasc Biol. 2007;27:2022–2029. doi: 10.1161/ATVBAHA.107.148403. [DOI] [PubMed] [Google Scholar]
- 54.Rohatgi A, et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N Engl J Med. 2014 doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson WS, et al. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem. 1995;270:5882–5890. doi: 10.1074/jbc.270.11.5882. [DOI] [PubMed] [Google Scholar]
- 56.Niu SL, Litman BJ. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pownall HJ, et al. Kinetics of lipid--protein interactions: interaction of apolipoprotein A-I from human plasma high density lipoproteins with phosphatidylcholines. Biochemistry (Mosc) 1978;17:1183–1188. doi: 10.1021/bi00600a008. [DOI] [PubMed] [Google Scholar]
- 58.Matz CE, Jonas A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J Biol Chem. 1982;257:4535–4540. [PubMed] [Google Scholar]
- 59.Chen Z, et al. Reconstituted HDL elicits marked changes in plasma lipids following single-dose injection in C57Bl/6 mice. J Cardiovasc Pharmacol Ther. 2012;17:315–323. doi: 10.1177/1074248411426144. [DOI] [PubMed] [Google Scholar]
- 60.Krause BR, Remaley AT. Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol. 2013;24:480–486. doi: 10.1097/MOL.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 61.Thun MJ, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 62.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev. 1993;15:328–351. doi: 10.1093/oxfordjournals.epirev.a036124. [DOI] [PubMed] [Google Scholar]
- 63.Gaziano JM, Buring JE. Alcohol intake, lipids and risks of myocardial infarction. Novartis Found Symp. 1998;216:86–95. doi: 10.1002/9780470515549.ch7. discussion 95–110. [DOI] [PubMed] [Google Scholar]
- 64.Hines LM, et al. Alcohol consumption and high-density lipoprotein levels: the effect of ADH1C genotype, gender and menopausal status. Atherosclerosis. 2005;182:293–300. doi: 10.1016/j.atherosclerosis.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Abramson EA, Arky RA. Acute antilipolytic effects of ethyl alcohol and acetate in man. J Lab Clin Med. 1968;72:105–117. [PubMed] [Google Scholar]
- 66.Nilsson NO, Belfrage P. Effects of acetate, acetaldehyde, and ethanol on lipolysis in isolated rat adipocytes. J Lipid Res. 1978;19:737–411. [PubMed] [Google Scholar]
- 67.Pownall HJ, et al. Effect of moderate alcohol consumption on hypertriglyceridemia: a study in the fasting state. Arch Intern Med. 1999;159:981–987. doi: 10.1001/archinte.159.9.981. [DOI] [PubMed] [Google Scholar]
- 68.Wilson DE, et al. The enhancement of alimentary lipemia by ethanol in man. J Lab Clin Med. 1970;75:264–274. [PubMed] [Google Scholar]
- 69.Pownall HJ. Dietary ethanol is associated with reduced lipolysis of intestinally derived lipoproteins. J Lipid Res. 1994;35:2105–2113. [PubMed] [Google Scholar]
- 70.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 71.Rehm J, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Poul E, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 73.Nilsson NE, et al. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. doi: 10.1016/s0006-291x(03)00488-1. [DOI] [PubMed] [Google Scholar]
- 74.Hong YH, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 75.Kimura W, Mossner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol. 1996;20:177–184. doi: 10.1007/BF02803766. [DOI] [PubMed] [Google Scholar]
- 76.Kimura I, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lundquist F, et al. Ethanol metabolism and production of free acetate in the human liver. J Clin Invest. 1962;41:955–961. doi: 10.1172/JCI104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siler SQ, et al. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr. 1999;70:928–936. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 79.Fushimi T, et al. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr. 2006;95:916–924. doi: 10.1079/bjn20061740. [DOI] [PubMed] [Google Scholar]
- 80.Johnston CS, Gaas CA. Vinegar: medicinal uses and antiglycemic effect. MedGenMed. 2006;8:61. [PMC free article] [PubMed] [Google Scholar]
- 81.Liljeberg H, Bjorck I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur J Clin Nutr. 1998;52:368–371. doi: 10.1038/sj.ejcn.1600572. [DOI] [PubMed] [Google Scholar]
- 82.Johnston CS, et al. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care. 2004;27:281–282. doi: 10.2337/diacare.27.1.281. [DOI] [PubMed] [Google Scholar]
- 83.Leeman M, et al. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J Clin Nutr. 2005;59:1266–1271. doi: 10.1038/sj.ejcn.1602238. [DOI] [PubMed] [Google Scholar]
- 84.Sugiyama M, et al. Glycemic index of single and mixed meal foods among common Japanese foods with white rice as a reference food. Eur J Clin Nutr. 2003;57:743–752. doi: 10.1038/sj.ejcn.1601606. [DOI] [PubMed] [Google Scholar]
- 85.Ostman EM, et al. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74:96–100. doi: 10.1093/ajcn/74.1.96. [DOI] [PubMed] [Google Scholar]
- 86.Vu CN, et al. Altered relationship of plasma triglycerides to HDL cholesterol in patients with HIV/HAART-associated dyslipidemia: further evidence for a unique form of metabolic syndrome in HIV patients. Metabolism. 2013;62:1014–1020. doi: 10.1016/j.metabol.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown AG, et al. Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J Chem Soc Perkin. 1976;1:1165–1170. [PubMed] [Google Scholar]
- 88.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]