Abstract
BACKGROUND
Recent interest has demonstrated the nucleus accumbens (NAcc) as a potential target for the treatment of depression with deep brain stimulation (DBS).
OBJECTIVE
To demonstrate that DBS of the NAcc is an effective treatment modality for depression and that chemical and structural changes associated with these behavioral changes are markers of neuroplasticity.
METHODS
A deep brain stimulator was placed in the NAcc of male Wistar-Kyoto rats. Groups were divided into sham (no stimulation), intermittent (3 h/d for 2 weeks), or continuous (constant stimulation for 2 weeks). Exploratory and anxietylike behaviors were evaluated with the open-field test before and after stimulation. Tissue samples of the prefrontal cortex (PFC) were processed with Western blot analysis of markers of noradrenergic activity that included the noradrenergic synthesizing enzyme tyrosine hydroxylase. Analysis of tissue levels for catecholamines was achieved with high-performance liquid chromatography. Morphological properties of cortical pyramidal neurons were assessed with Golgi-Cox staining.
RESULTS
Subjects undergoing intermittent and continuous stimulation of the NAcc exhibited an increase in exploratory behavior and reduced anxietylike behaviors. Tyrosine hydroxylase expression levels were decreased in the PFC after intermittent and continuous DBS, and dopamine and norepinephrine levels were decreased after continuous stimulation. Golgi-Cox staining indicated that DBS increased the length of apical and basilar dendrites in pyramidal neurons of the PFC.
CONCLUSION
Deep brain stimulation induces behavioral improvement in and neurochemical and morphological alterations of the PFC that demonstrate changes within the circuitry of the brain different from the target area of stimulation. This observed dendritic plasticity may underlie the therapeutic efficacy of this treatment.
Keywords: DBS, Deep brain stimulation, Dendritic plasticity, Depression, Neuroplasticity, Nucleus accumbens
Deep brain stimulation (DBS) is a neurosurgical method that involves a lead or electrode being placed in a selected region of the brain.1 It is a reversible procedure that allows an adjustable treatment tailored to the patient. It has become a safe and effective treatment in various neurological disorders, including Parkinson disease and essential tremor.2–4 More recently, this approach has been applied for the treatment of obsessive-compulsive disorder, epilepsy, and depression.4
Depression is a major cause of disability worldwide5 and accounts for more than $83 billion in domestic costs in the United States alone.6 Lifetime prevalence varies widely, from 3% in Japan to 17% in the United States; however, in most countries, the number of people who suffer from depression at one time in their lives falls within an 8% to 12% range.7,8 Most patients are treated with antidepressant medications; some are treated with psychotherapy or counseling. A minority are treated with electroconvulsive therapy.9
The role of the prefrontal cortex (PFC) is important in understanding the nucleus accumbens (NAcc) and its circuitry. It has been shown that the NAcc core is involved in addiction and drug behavior and that the NAcc shell is involved in pleasure, fear behavior, and food intake.10,11 The NAcc has intricate connections with the PFC and limbic system. These connections are thought to be involved in the underlying symptomatology of depression. Therefore, DBS to the NAcc is a potential target in the treatment of depression, and its effects on the PFC become of interest.
Animal models of epilepsy have benefited from stimulation of the subthalamic nucleus, substantia nigra, and anterior thalamus.12–14 Rats maintained on a high-fat diet, as a model of obesity, experienced significant and sustained weight loss after continuous stimulation of the lateral hypothalamic nucleus.15 These models have led to an understanding of stimulation parameters and their possible effects on behavior. However, despite potential advances and therapeutic benefits of DBS, the cellular basis for the efficacy of this treatment remains elusive. Thus, studying the impact of DBS in preclinical models is important in establishing mechanisms underlying potential therapeutic improvements.
The Wistar-Kyoto rat strain has been shown to be a useful pre-clinical model exhibiting a depressive and anxietylike phenotype.16–18 This strain exhibits hormonal16,19 and behavioral17,18 abnormalities consistent with a depressive and anxious phenotype. Several studies have demonstrated differing responses to antidepressants, deficits in reward behavior, and changes in hormonal levels in this strain.20–22
Although DBS has been theorized to work by various mechanisms,23,24 neuroplasticity is a potential mechanism by which DBS results in therapeutic improvements.25 Neuroplasticity, also known as brain plasticity, can be defined as changes that occur in the organization of the brain as a result of experience.26 Areas related to memory formation such as the hippocampus and dentate gyrus are highly plastic and produce new neurons in a continuous fashion into adulthood.26 It has been shown that changes in long-term potentiation of cells induced by high-frequency stimulation of the subthalamic nucleus are a mechanism that determines the effectiveness of DBS in an animal model for the treatment of Parkinson disease.27 Thus, this study aimed to show a relationship between DBS-induced changes in behavior and potential neurochemical and morphological changes.
Owing to the importance of the NAcc in regulating motivated behaviors and mood control,28–31 the hypothesis that DBS of this region may be relevant in the treatment of depression was tested in the present study. Behavioral studies were conducted after stimulation of the NAcc, and neurochemical and morphological changes were subsequently assessed in the PFC.
MATERIALS AND METHODS
Animals
The animal procedures used were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University and conform with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Seventy-nine adult male Wistar-Kyoto strain rats (250–275 g; Charles River Laboratories International, Inc, Wilmington, Massachusetts) were used in the present study. Rats were caged individually on a 12-hour light schedule (lights on at 7 AM) in a temperature-controlled (20°C) colony room. Food and water were made freely available. Rats were allowed to acclimate to the animal housing facility for several days before the onset of the study. All efforts were made to use only the minimum number of animals necessary to produce reliable scientific data, and experiments were designed to minimize any animal distress.
Study Design
All rats were randomly divided into 3 experimental groups (15 rats per group). Each rat underwent behavioral testing via an open field before implantation. For the sham stimulation group, animals were sedated and underwent surgery with implantation of the electrode with no stimulation. Animals in the intermittent group were administered stimulation for the same 3 h/d for 2 weeks (14 days). The stimulation was turned on daily from 8 until 11 AM each day of the 14-day period. Animals in the continuous group were administered constant stimulation for 2 weeks (14 days). Continuous stimulation occurred 24 h/d for the full 14 days. Rats underwent behavioral evaluation after the stimulation period and were then exposed to isoflurane and euthanized by decapitation. Brains were removed and used for protein extraction and analysis.
DBS Implantation
Each rat underwent stereotactic placement of a 0.25-mm bipolar stimulating electrode (Plastics One Products) on the right side. In this study, DBS signifies the implantation of the electrode in a subcortical structure, the NAcc. Rats were initially anesthetized with a combination of ketamine hydrochloride (100 mg/kg) and xylazine (2 mg/kg) in saline and placed in a stereotaxic apparatus for surgery. Anesthesia was supplemented with isoflurane (Abbott Laboratories; 0.5–1.0% in air) via a specialized nose cone affixed to the stereotaxic frame (Stoelting Corp). The electrodes were placed via the coordinates determined by the Paxinos and Watson32 rat brain atlas (1.5 mm anterior from bregma, 0.8 mm medial/lateral, −7.4 mm ventral from the top of the skull) correlating with the shell of the NAcc. The electrode was secured with dental cement, and the incision was closed with staples.
Each rat was allowed a 7-day recovery period after implantation. After this period, they underwent stimulation via a continuous stimulus generator (Medtronic Screener model 3638). The stimulation parameters were as follows: frequency, 120 Hz; pulse width, 200 milliseconds; and strength, 2 V. The rats in the control group were connected to the generators, which were not turned on. Stimulation parameters were chosen on the basis of previous literature for DBS in rats for various study protocols.32
Open-Field Test
The open field was a square arena consisting of Plexiglas walls and floor measuring 43.2 × 43.2 × 30.5 cm. Rats were placed in the testing room 1 hour before behavioral tests. The behavioral analysis before and after stimulation was kept constant by ensuring that the same testing room, same lighting, and same analyses were performed during the same time period of the day by the same observer who was blinded to the experimental groups. The poststimulation behavioral test was done 3 hours after the last stimulation for both intermittent and continuous DBS.
The open-field test was used to evaluate the exploratory, anxiety, and depressionlike behavior of the rats. Rats were placed in the open-field test and recorded by the observer. They were observed for a 5-minute period in which their activity was measured on terms of the amount of quadrant changes and time spent on their hind legs as a marker of exploratory behavior or locomotor activity. Crossing of the midline was also analyzed and used as a marker of anxiety based on the difference of the rat spending time in either the periphery or center of the field test.
Histology
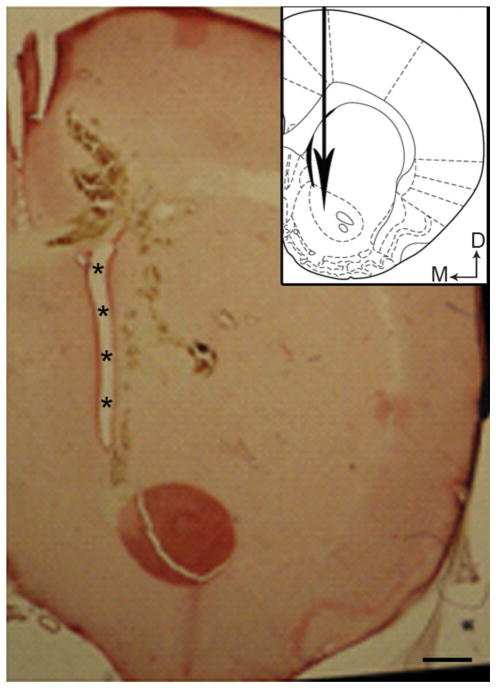
After the stimulation period and final behavioral analysis, the rats were immediately euthanized. The brain tissue was rapidly harvested for protein extraction and analysis. The electrode track was stained with Cresyl violet to confirm accurate placement of the electrode. The placement of the electrode targeted the NAcc at the anteroposterior level that corresponded to Figure 12 of the brain atlas of Paxinos and Watson32 (Figure 1). Rats that did not show optimal placement of the electrode were not included in the analysis.
FIGURE 1.
Bright-field photomicrograph of a representative coronal section showing the site of deep brain stimulation into the nucleus accumbens used in various experiments. Inset, a schematic diagram adapted from the rat brain atlas of Paxinos and Watson32 (1988) showing the region targeted for deep brain stimulation. The big arrow indicates the location where the electrode was placed; asterisks indicate lateral ventricle; small arrows indicate dorsal (D) and medial (M) orientation of the tissue sections. Scale bars = 100 μm.
Brain Harvesting
The PFC extends from the rostrocaudal segment of the cerebral cortex and is located at a level 5.2 mm anterior to bregma and extends 3.3 posterior to bregma.32 Anatomically forming the anterior part of the forebrain, the PFC is bounded medially by the median plane dividing the cerebral lobes; ventrally by the medial orbital cortex (5.2 mm anterior to bregma to 3.2 mm anterior to bregma), dorsal peduncular cortex (3.2 mm anterior to bregma to 2.2 mm anterior to bregma), or corpus callosum (1.7 mm anterior to bregma to 3.3 mm posterior from bregma); and caudally by the occipital area.32 In the present study, the PFC was microdissected (approximately covering the PFC area at a level 3.7 mm anterior to bregma extending at a level 1.7 posterior to bregma). This is commonly known as the medial PFC or infralimbic cortex.
Protein Extraction
Brain tissue was rapidly removed from each animal and put on ice after the final behavioral analysis. With the use of a trephine, the prefrontal brain region was microdissected from each animal. The PFC was homogenized with a pestle and extracted in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology) on ice for 20 minutes. Lysates were cleared by centrifugation at 13 000 rpm for 12 minutes at 41°C. Supernatants or protein extracts were diluted with an equal volume of Novex 2 tris-glycine sodium dodecyl sulfate sample buffer (Invitrogen) containing dithiothreitol (Sigma-Aldrich Inc). Protein concentrations of the undiluted supernatants were quantified with the bicinchoninic acid protein assay reagent (Pierce, Rockford, Illinois).
Western Blot Analysis
Cell lysates containing equal amounts of protein were separated on 4% to 12% tris-glycine polyacrylamide gels and then electrophoretically transferred to Immobilon-P polyvinylidene fluoride membranes (Millipore). Membranes were incubated in mouse anti–tyrosine hydroxylase (TH; Immunostar Inc) primary antibody overnight and then in alkaline phosphatase–conjugated secondary antibodies for 30 minutes to probe for the presence of proteins with a Western blotting detection system (Western Breeze Chemiluminescent Kit; Invitrogen). After incubation in a chemiluminescent substrate (Western Breeze Chemiluminescent Kit), blots were exposed to X-OMAT AR film (Kodak) for different lengths of time to optimize exposures. Tyrosine hydroxylase was readily detected by immunoblotting in rat PFC extracts. Immunoreactivity of TH was visualized as a single band that migrates at approximately 60 kDa. Blots were incubated in stripping buffer (Restore Stripping Buffer; Pierce) to disrupt previous antibody-antigen interactions and then reprobed with β-actin (1:5000 Sigma-Aldrich Inc) with 1-hour incubation to ensure proper protein loading. The density of each band was quantified with Un-Scan-It blot analysis software (Silk Scientific Inc). Tyrosine hydroxylase was normalized to β-actin immunoreactivity on each respective blot.
High-Pressure Liquid Chromatography
After the final behavioral analysis, PFC was sonicated in 1 mL of 0.1 mol/L perchloric acid. A 100-μL aliquot of the sonicated material was stored at 80°C for protein determination with a Pierce BCATM Protein Assay Kit (Thermo Scientific). The supernate was collected from centrifugation of the sonicated material at 13 000g for 15 minutes and stored at 70°C until catecholamine extraction was performed. Catecholamines were extracted by alumina extraction from 100 μL of the sonicated supernate as previously described.33 The eluted catechols were filtered through a 0.22 MillexGV syringe-driven filter, and detection was performed with the ESACoulochem II electrochemical detector (conditioning cell set at 350 mV, electrode 1 of analytical cell set at 90 mV, electrode 2 of analytical cell set at 300 mV; ESA). Phenomonex reverse-phase c18 Gemini column (150_4.6 mm, 3_C, 110 A; Phenomonex) and ScientificSoftware Inc were used for data collection and analysis. The catecholamines norepinephrine (NE) and dopamine (DA) were measured in the PFC. Catecholamine values were expressed as nanograms of catecholamine per milligram of protein. Data were adjusted to percent control, and values were expressed as the average percent change from control ± SEM. Experimental data were analyzed by GraphPad Prism (version 5.0; GraphPad Software Inc).
Golgi Impregnation and Morphological Analysis
The brains were prepared with the FD Rapid Golgi Stain kit (FD Neurotechnologies). Brains were placed in 20 mL Golgi-Cox solution (potassium dichromate, mercuric chloride, and potassium chromate), where they were stored in the dark for 14 days. After this incubation, sections containing PFC were obtained with a freezing microtome (100 μm; Micron HM550 cryostat; Richard-Allan Scientific), mounted onto gelatinized slides, and placed in coverslips. Individual neurons and their processes were visualized at × 100 with camera lucida. Dendritic length was measured in triplicate with National Institutes of Health Image J.
Data Analysis
Statistical analyses were performed with GraphPad InStat software (GraphPad Software Inc). For comparison of 2 groups in experiments, an unpaired Student t test was used to analyze data. One-way analysis of variance, followed by post-hoc Newman-Keuls multiple comparisons test, was used to analyze differences among 3 independent groups. Averages are expressed as mean ± SEM. Differences were considered to be statistically significant when P < .05.
RESULTS
DBS Increases Exploratory Behavior and Reduces Anxietylike Behavior in an Open-Field Paradigm
To investigate whether DBS of the NAcc exerts an effect on depression and anxietylike behaviors in rats, an assessment of behavior in the open-field test was carried out. Open field is a traditional paradigm used to assess locomotor or exploratory activity and anxietylike behaviors wherein the inherent drive to explore a novel environment is opposed by the tendency to stay in protective areas. The rodent that exhibits anxietylike behaviors avoids the unsafe and aversive space of the inner fields and thus spends more time in the outer perimeter of the field.34 Data obtained were based on the amount of quadrant changes and time spent on the rat’s hind legs as a marker of exploratory behavior or locomotor activity. Crossing of the midline was also analyzed and used as a marker of anxiety based on the difference in time that the rat spent in the periphery or the center of the field test.
Shown in the Table is the average value (n = 15 rats per group) of each criterion analyzed in the open-field test. These data are used to determine the exploratory nature of the rat and the level of anxiety of the rat during the test as a marker of depressive and anxietylike behaviors. Assessment was conducted for 5-minute intervals before and after stimulation.
TABLE.
Behavioral Analysis With an Open-Field Test
| Quadrant Changes, n | Time on Hind Legs, s | Crossing the Midline, n | |
|---|---|---|---|
| Control | 24.3 ± 1.38 | 35.1 ± 2.35 | 1.1 ± 0.32 |
| Intermittent | 19.9 ± 2.19 | 25.9 ± 4.20 | 1.3 ± 0.41 |
| Continuous | 22.1 ± 2.0 | 31.9 ± 2.90 | 1.1 ± 0.30 |
| Postimplantation | |||
| Control | 15.88 ± 1.37 | 21.56 ± 1.77 | 0.1 ± 0.09 |
| Intermittent | 23.9 ± 3.06a | 36.6 ± 6.61b | 1.79 ± 0.53b |
| Continuous | 28.3 ± 1.91b | 45.5 ± 4.89b | 2.3 ± 0.51b |
| Difference | |||
| Control | −8.42 | −13.54 | −1 |
| Intermittent | 4 | 10.7 | 0.5 |
| Continuous | 6.2 | 13.6 | 1.2 |
P < .01.
P < .001 vs control.
The behavioral data obtained from the control rats that received sham stimulation of the NAcc showed a significant decrease (P < .001) in all parameters analyzed compared with the prestimulation time point. Specifically, these subjects exhibited increases in depression and anxietylike behaviors that were manifested as a decrease in locomotor and exploratory behaviors in the open-field test, as well as decreased crossing of midline (Table). Wistar-Kyoto rats that received DBS of the NAcc showed significant increases (P < .05) in exploratory behavior and crossing of midline, indicative of a reduction in depression and anxietylike behavior.
When the 3 treatment groups were compared with each other, preimplantation baseline behavior showed no significant difference across the treatments, as shown in the Table. Post-implantation data showed a significant difference across all groups (P < .01), with the largest difference seen when either the intermittent or continuous stimulation group was compared with the control group. Control rats continued to exhibit depressionlike and anxietylike behaviors compared with either the intermittently or continuously stimulation groups (P < .01). There was a significant decrease (P < .01) in quadrant changes and less time spent on hind legs (P < .001). In addition, the sham-stimulated control group demonstrated almost no tendency to cross the midline, and there was a significant decline (P < .001) in exploratory behavior and an increase in anxiety. The intermittent and continuous stimulation groups showed a significant increase (P < .01) in quadrant changes and time spent on hind legs, demonstrating increased exploratory behavior compared with preimplantation and sham-stimulated controls. There was also a significant increase (P < .001) in crossing the midline, indicating less anxiety. A larger change was observed in the continuous stimulation group than in the intermittent stimulation group compared with all preimplantation values. These data demonstrate that there was a more significant behavioral response to treatment with continuous stimulation compared with intermittent stimulation.
DBS Alters the Levels of TH Expression in the PFC
In the present study, the PFC was microdissected (approximately covering the PFC area at a level 3.7 mm anterior to bregma extending at a level 1.7 posterior to bregma), and TH protein expression after DBS was assessed with Western blot analysis. Prefrontal cortex extracts from rats (n = 4 rats per group) that were euthanized after continuous stimulation showed a significant decrease (P < .05) in TH expression levels compared with the intermittent stimulation group and sham-stimulated control group (Figure 2). There was also a decrease in TH expression level after intermittent stimulation. However, it did not reach a statistically significant difference.
FIGURE 2.
Western blot analysis of tyrosine hydroxylase (TH) expression in the prefrontal cortex (PFC) after deep brain stimulation/sham stimulation of the nucleus accumbens of Wistar-Kyoto rats. Expression of TH in the PFC of the animals is expressed as a fold change from the control mean when the control equals 1.0 ± SEM. [beta]-Actin immunoblotting was used as a control to verify equal protein loading. After continuous stimulation, TH was significantly decreased (*P < .05) compared with the sham stimulation group.
DBS Alters the Levels of NE and DA Expression in the PFC
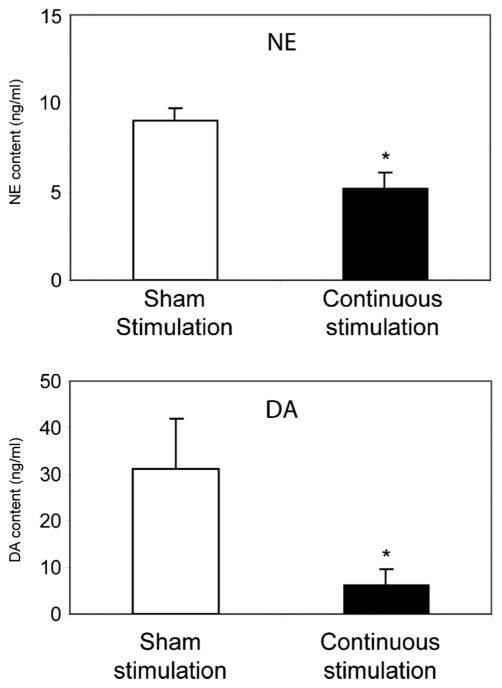
We assessed DA and NE content in the PFC after continuous stimulation using high-performance liquid chromatography. Dopamine and NE were chosen for testing secondary to being downstream in the biosynthetic pathway for catecholamines and should be directly correlated with the effects observed with TH (Figure 3). This aimed to determine whether the decreased levels of the converting enzyme TH after continuous stimulation observed on Western blot analysis (Figure 2) correlated with a decrease in the production of its neurotransmitters in the biosynthetic pathway for catecholamines. Statistical analysis showed that PFC extracts from rats (n = 4–5 rats per group) that were euthanized after continuous stimulation demonstrated a significant decrease in the content of NE (P < .05) and DA (P < .05) compared with sham-stimulated control (Figure 4).
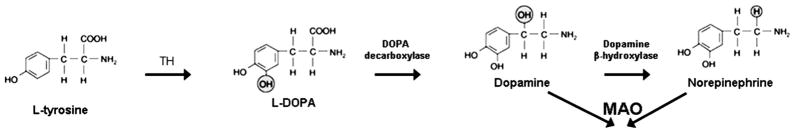
FIGURE 3.
Biosynthetic pathway for catecholamine. MAO, monoamine oxidase.
FIGURE 4.
Dopamine (DA) and norepinephrine (NE) levels in the prefrontal cortex of Wistar-Kyoto rats are altered after deep brain stimulation/sham stimulation of the nucleus accumbens. Values are mean ± SEM of 4 rats per group. *P < .05 vs sham stimulation.
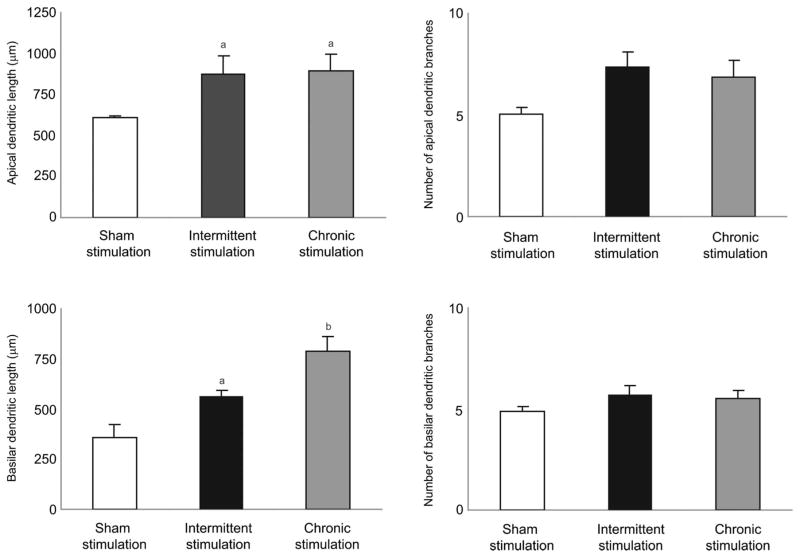
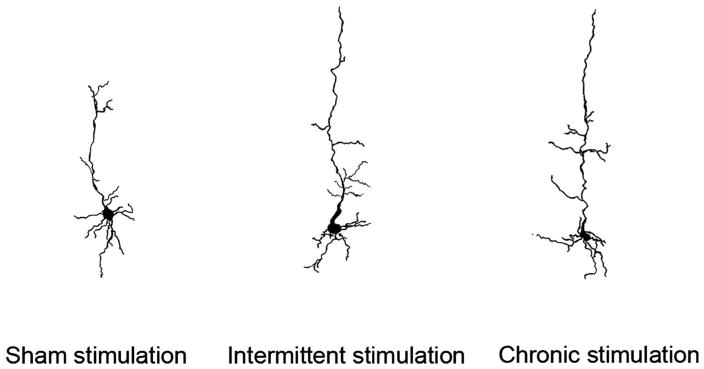
DBS Increases Dendritic Length in Layer V Pyramidal Cells in the PFC
Statistical analysis showed that intermittent and continuous stimulation for 14 days in the NAcc significantly increased apical (P < .01) and basilar (P < .01) dendritic length in the layer V pyramidal cells in the PFC (Figure 5). However, no significant difference was observed in the number of basilar dendritic branches in the 3 groups studied. Apical dendritic length did not differ significantly between the intermittent and continuous stimulation groups. Figure 6 presents representative camera lucida drawings of Golgi-Cox–stained neurons found in PFC in the sham-stimulated and intermittent and continuous stimulation groups. These drawings clearly illustrate that the length of the dendrites and the length of the branches have increased with both intermittent and continuous stimulation compared with the sham-stimulated control group. Therefore, DBS of the NAcc influences dendritic plasticity in the PFC of Wistar-Kyoto rats.
FIGURE 5.
Mean dendritic length (μm) and number of dendritic branches in the nucleus accumbens (NAcc) of rats after brain stimulation/sham stimulation of the NAcc of Wistar-Kyoto rats. Values are mean ± SEM of 4 rats per group. *P < .05 vs sham stimulation.
FIGURE 6.
Representative camera lucida drawings of the nucleus accumbens (NAcc) after deep brain stimulation/sham stimulation of the NAcc of Wistar-Kyoto rats.
DISCUSSION
The present study provides the first in vivo evidence that DBS of the NAcc reduces anxiety and depression in an animal model via an open-field test. These results correlate with a recent report demonstrating that DBS in the NAcc can reduce anhedonia in patients with treatment-resistant depression.35 Moreover, DBS of the NAcc induces neurochemical and morphological modifications in the PFC that may partly explain the neural mechanism by which DBS exerts its effects and that the locations of changes in the brain circuitry are yet to be defined. This study set out to correlate behavioral changes directly with structural changes within this circuit. By establishing changes in the circuit, we can demonstrate that the effects of DBS may be upstream or downstream from its target, may cause neuroplasticity, and may not be directly at the area of stimulation. Deep brain stimulation may have either excitatory or inhibitory effects that may lead to changes in a different location of the brain.
Methodological Considerations
Inherent caveats exist with respect to Western blot analysis experiments. These caveats include the accuracy of sampling of the region of interest and the comparison of equal protein quantities across treatment groups. To circumvent the variability in tissue excision, a single investigator obtained the brain samples for each experiment. Moreover, to ensure equivalent loading of protein, blots were reprobed with β-actin, and results were normalized to this internal standard. β-Actin expression was comparable across the treatment groups examined.
Some effects may not have been visualized such as the absence of change in TH expression levels with intermittent stimulation that could be attributed to the short stimulation and observation in the conduct of DBS. The stimulation period lasted for only 2 weeks, which may not have been long enough to demonstrate all the changes that were occurring. This could partly explain the similar dendritic branching among the groups studied. It would also be interesting to examine how long these effects on behavior, neurochemical, and structural changes would last after a period of stimulation. Shi et al29 suggested that there may be highly plastic neural networks that may undergo lasting effects with DBS.
The NAcc as a Target for DBS Treatment
The NAcc is known to integrate limbic and cortical inputs, which then sends projections to the basal forebrain and in turn sends back projections into the PFC.36,37 Neurochemical manipulation of the NAcc has been linked to changes in pleasure.30,38 Anhedonia, which is the inability to experience pleasure from previously pleasurable activities, is a prominent clinical feature of depression.39,40 In patients suffering from extremely resistant forms of depression that did not respond to pharmacotherapy, psychotherapy, and electroconvulsive therapy, DBS via bilateral electrodes placed in the NAcc significantly reduced anhedonia.35 Although effectively reducing anhedonia, DBS of the NAcc did not produce any neurological and psychological side effects.35 Therefore, DBS of the NAcc can be a potential target for the treatment of depression to alleviate the symptoms of anhedonia.
DBS in the NAcc Reduces Anxietylike Behavior and Alters Catecholamine Levels in the PFC
Our present behavioral data are consistent with previous reports showing that Wistar-Kyoto rats exhibit depressionlike behaviors.16–18 An increase in depressionlike behaviors in control subjects most likely results from rats being individually housed throughout the experimental conditions. Previous data have shown that single rat housing is considered a mild stress,41 indicating that sham-stimulated rats may have experienced this mild stress that resulted in decreases in locomotor and exploratory behaviors. More important, our behavioral data support previous clinical reports on bilateral DBS of the NAcc that showed immediate effects on ratings of depression in patients with treatment-resistant depression.35
We specifically demonstrated in an open-field test that although sham-stimulated control rats continued to manifest signs of depression and anxiety, rats that received either intermittent or continuous DBS showed a significant reduction of depression and anxietylike behaviors as evidenced by quadrant changes, time spent on hind legs, midline crossing, exploratory behavior, and reduced anxiety compared with preimplantation values and controls. There was a more significant behavioral response to treatment with continuous stimulation as opposed to intermittent stimulation. Control rats continued to be more depressed with age. The open-field test is usually used to measure locomotor behavior, and although useful in our experiment, its interpretation of the behavior change is somewhat limited secondary to correlating behavioral change with depression and anxiety. The open-field test has also been used as a measure of anxiety as supposed to being a sole indicator of depression. That being said, the true significance of the behavioral data lies in that the control group worsened in all areas tested for behavioral analysis while the stimulation groups improved. This finding demonstrates that DBS of the NAcc not only halted the progression of the disease process but improved it, consistent with evidence showing that neuronal adaptation is disrupted in mood disorders and that treatments for depression would need to enhance neuroplasticity.42 The present study also determines whether the behavioral changes after stimulation of the NAcc lead to changes in neural circuits, specifically the PFC.
It is anatomically established that the NAcc receives afferent projections from the PFC.43–45 The PFC is known for its vital role in cognitive and behavioral processes, including arousal, attention, cognition, motivation, working memory, and vigilance.46 The release of the catecholamines NE and DA is related to arousal states that consequently have profound effects on cognitive and behavioral processes involving PFC functions.47,48 The connections of the PFC and NAcc as part of the limbic circuit are very sensitive to changes to neurochemical environment, so any minute changes in catecholamine generate profound effects on the activity of PFC.49 Indeed, the role of the medial PFC is important in understanding the NAcc and its circuitry, therefore making it also important to understand the neuroadaptations in the PFC after DBS of NAcc.
Using Western blotting and high-performance liquid chromatography, we determined the levels of TH and of NE and DA, respectively, after DBS of the NAcc. Western blot analysis demonstrated a decrease in expression of TH in the PFC after both intermittent and continuous stimulation, although statistical significance was observed only in continuous stimulation. To determine whether the decreased levels of the converting enzyme TH correlated with a decrease in the production of its neuro-transmitters in the biosynthetic pathway for catecholamines, we evaluated the levels of DA and NE. The levels of DA and NE were significantly decreased in the PFC with continuous stimulation compared with controls, demonstrating a correlation with the decreased levels of TH. This was not observed in the intermittent stimulation group in that the levels of DA and NE mimicked those of the control group. This may be secondary to the less pronounced response in TH levels seen in Western blot analysis for intermittent stimulation. There may be a threshold in TH levels that needs to be breached before there are changes of levels in these neurotransmitters. We can also hypothesize that this is why there was not as large a response in behavior changes in the intermittent stimulation group as in the continuous stimulation group.
DBS in the NAcc Induces Neuroplasticity in the PFC
The results of the present study showed that neurochemical alterations were evident in the expression levels of TH, DA, and NE. To determine whether these neurochemical changes lead to morphological changes in neuronal structure, dendritic growth and branching were examined as markers for neuroplasticity. As a fundamental mechanism of neuronal adaptation, neuro-plasticity is altered in stress paradigm in animals. For instance, continuous restraint stress significantly reduced the length and number of branches of apical dendrites but not of basilar dendrites of the pyramidal neurons of lamina II to III of the medial PFC.10,11 Stress also caused atrophy of the hippocampal neurons.50 An evaluation with Golgi-Cox staining demonstrated that the length of the apical and basilar dendrites of the pyramidal neurons of layer V of medial PFC increased with both intermittent and continuous stimulation compared with the control group. These data confirm the behavioral data in an open field in which rats that received intermittent and continuous stimulation exhibited reduced depressive and anxietylike behavior. Although the neurochemical data were consistent with significant reductions in TH, NE, and DA in PFC only in continuous but not in intermittent stimulation, it is likely that the reduction of TH, NE, and DA, even though not statistically significant, may have efficiently induced behavioral changes and morphological changes in apical and basilar dendrites. Further investigation is needed to elucidate these data. Nevertheless, the present morphological data indicate that DBS of the NAcc influences dendritic plasticity in the PFC.
CONCLUSION
Our present results highlight DBS of the NAcc as a potential treatment modality for depression. In addition, our results present DBS of the NAcc as a mechanism that induces defined neurochemical changes and structural dendritic changes in the PFC that demonstrate neuroplasticity in the circuitry of the brain. It was shown that the effects of DBS not only may be at the area of stimulation or placement of the electrode but also can lead to changes either upstream or downstream in the circuits of the brain. This further solidifies the idea that long-term structural changes occur with DBS. Understanding DBS and the brain as a circuit may be a link to unlocking the brain’s ability to undergo neuroplasticity. The neurochemical alterations in the expression levels of TH, DA, and NE, as well as the dendritic neuroplasticity observed in the cortical areas, may underlie the behavioral changes after DBS of the NAcc shell.
ABBREVIATIONS
- DA
dopamine
- DBS
deep brain stimulation
- NAcc
nucleus accumbens
- NE
norepinephrine
- PFC
prefrontal cortex
- TH
tyrosine hydroxylase
Footnotes
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord. 2006;21(Suppl 14):S247–S258. doi: 10.1002/mds.20959. [DOI] [PubMed] [Google Scholar]
- 2.Gildenberg PL. Evolution of neuromodulation. Stereotact Funct Neurosurg. 2005;83(2–3):71–79. doi: 10.1159/000086865. [DOI] [PubMed] [Google Scholar]
- 3.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichmann T, Delong MR. Deep brain stimulation for neurologic and neuro-psychiatric disorders. Neuron. 2006;52(1):197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. [Accessed May 2010];The World Health Report 2001—Mental Health: New Understanding, New Hope. Available at: http://www.who.int/whr/2001/en/whr01_en.pdf.
- 6.Gelenberg AJ. The prevalence and impact of depression. J Clin Psychiatry. 2010;71(3):e06. doi: 10.4088/JCP.8001tx17c. [DOI] [PubMed] [Google Scholar]
- 7.Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12(1):3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 9.Ciapparelli A, Dell’Osso L, Tundo A, et al. Electroconvulsive therapy in medication-nonresponsive patients with mixed mania and bipolar depression. J Clin Psychiatry. 2001;62(7):552–555. doi: 10.4088/jcp.v62n07a09. [DOI] [PubMed] [Google Scholar]
- 10.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 11.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Hamani C, Hodaie M, Chiang J, et al. Deep brain stimulation of the anterior nucleus of the thalamus: effects of electrical stimulation on pilocarpine-induced seizures and status epilepticus. Epilepsy Res. 2008;78(2–3):117–123. doi: 10.1016/j.eplepsyres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Mirski MA, Ziai WC, Chiang J, Hinich M, Sherman D. Anticonvulsant serotonergic and deep brain stimulation in anterior thalamus. Seizure. 2009;18(1):64–70. doi: 10.1016/j.seizure.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Shi LH, Luo F, Woodward D, Chang JY. Deep brain stimulation of the substantia nigra pars reticulata exerts long lasting suppression of amygdala-kindled seizures. Brain Res. 2006;1090(1):202–207. doi: 10.1016/j.brainres.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 15.Sani S, Jobe K, Smith A, Kordower JH, Bakay RA. Deep brain stimulation for treatment of obesity in rats. J Neurosurg. 2007;107(4):809–813. doi: 10.3171/JNS-07/10/0809. [DOI] [PubMed] [Google Scholar]
- 16.Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuro-endocrinology. 1995;20(8):879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of anti-depressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22(2):191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 18.Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85(1):57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 19.Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27(3):303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 20.Malkesman O, Braw Y, Weller A. Assessment of antidepressant and anxiolytic properties of NK1 antagonists and substance P in Wistar Kyoto rats. Physiol Behav. 2007;90(4):619–625. doi: 10.1016/j.physbeh.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Rauhut AS, Zentner IJ, Mardekian SK, Tanenbaum JB. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93(1–2):177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8(11):925–932. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- 23.Hemm S, Wardell K. Stereotactic implantation of deep brain stimulation electrodes: a review of technical systems, methods and emerging tools. Med Biol Eng Comput. 2010;48(7):611–624. doi: 10.1007/s11517-010-0633-y. [DOI] [PubMed] [Google Scholar]
- 24.Leiphart JW, Valone FH. Stereotactic lesions for the treatment of psychiatric disorders. J Neurosurg. 2010;113(6):1204–1211. doi: 10.3171/2010.5.JNS091277. [DOI] [PubMed] [Google Scholar]
- 25.Hammond C, Ammari R, Bioulac B, Garcia L. Latest view on the mechanism of action of deep brain stimulation. Mov Disord. 2008;23(15):2111–2121. doi: 10.1002/mds.22120. [DOI] [PubMed] [Google Scholar]
- 26.Urakubo H, Honda M, Tanaka K, Kuroda S. Experimental and computational aspects of signaling mechanisms of spike-timing-dependent plasticity. HFSP J. 2009;3(4):240–254. doi: 10.2976/1.3137602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen KZ, Zhu ZT, Munhall A, Johnson SW. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse. 2003;50(4):314–319. doi: 10.1002/syn.10274. [DOI] [PubMed] [Google Scholar]
- 28.Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu HY, Jin J, Tang JS, et al. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13(1):40–46. doi: 10.1111/j.1369-1600.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- 30.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do muopioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17(10):2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York, NY: Academic Press; 1986. [Google Scholar]
- 33.Eisenhofer G, Goldstein DS, Stull R, et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32(11):2030–2033. [PubMed] [Google Scholar]
- 34.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 35.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 36.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell P, Lavin A, Enquist LW, Grace AA, Card JP. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci. 1997;17(6):2143–2167. doi: 10.1523/JNEUROSCI.17-06-02143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 39.Argyropoulos SV, Nutt DJ. Anhedonia and chronic mild stress model in depression. Psychopharmacology (Berl) 1997;134(4):333–336. doi: 10.1007/s002130050458. [DOI] [PubMed] [Google Scholar]
- 40.Rush AJ, Weissenburger JE. Melancholic symptom features and DSM-IV. Am J Psychiatry. 1994;151(4):489–498. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- 41.Wu HH, Wang S. Strain differences in the chronic mild stress animal model of depression. Behav Brain Res. 2010;213(1):94–102. doi: 10.1016/j.bbr.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 42.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 43.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338(2):255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 44.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308(2):249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 45.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320(2):145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 46.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 47.Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(suppl 1):16–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- 48.Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137(3):1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 49.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]