Abstract
Material safety data sheets (MSDSs) provide employers, employees, emergency responders, and the general public with basic information about the hazards associated with chemicals that are used in the workplace and are a part of every-day commerce. They are a primary information resource used by health, safety, and environmental professionals in communicating the hazards of chemicals and in making risk management decisions. Engineered nanomaterials represent a growing class of materials being manufactured and introduced into multiple business sectors. MSDSs were obtained from a total of 44 manufacturers using Internet search engines, and a simple ranking scheme was developed to evaluate the content of the data sheets. The MSDSs were reviewed using the ranking scheme, and categorized on the quality and completeness of information as it pertains to hazard identification, exposure controls, personal protective equipment (PPE), and toxicological information being communicated about the engineered nanomaterial. The ranking scheme used to evaluate the MSDSs for engineered nanomaterials was based on the determination that the data sheet should include information on specific physical properties, including particle size or particle size distribution, and physical form; specific toxicological and health effects; and protective measures that can be taken to control potential exposures. The first MSDSs for nanomaterials began to appear around 2006, so these were collected in the time period of 2007–2008. Comparison of MSDSs and changes over time were evaluated as MSDSs were obtained again in 2010–2011. The majority (67%) of the MSDSs obtained in 2010–2011 still provided insufficient data for communicating the potential hazards of engineered nanomaterials.
INTRODUCTION
Engineered nanomaterials are materials that are deliberately engineered and manufactured with at least one dimension between approximately 1 and 100 nanometers. This class of materials includes new entities such as carbon nanotubes (CNTs), carbon nanofibers (CNFs), fullerenes, and quantum dots, as well as the nanoscale form of established, familiar materials such as titanium dioxide, gold, and silver. The combined small size and large surface area of these chemicals at the nanoscale produce different or unique chemical and physical properties. These properties include increased or novel chemical and biologic reactivity, electrochemical and magnetic properties, light absorption, and color. Larger scale particles, including those of the same chemical composition, may not exhibit the same properties. In addition to exhibiting different chemical properties, nanoscale material may also exhibit discreet, unique morphological differences from their macroscale counterparts. For example, Figure 1 is an electron photomicrograph of a carbon nanotube and carbon nanofiber. Figure 2 is an electron photomicrograph of carbon black, a high-surface-area form of elemental carbon formed under a controlled combustion process. Both are forms of carbon, but carbon nanotubes and carbon nanofibers have very different chemical and physical properties from those of carbon black.
Figure 1.
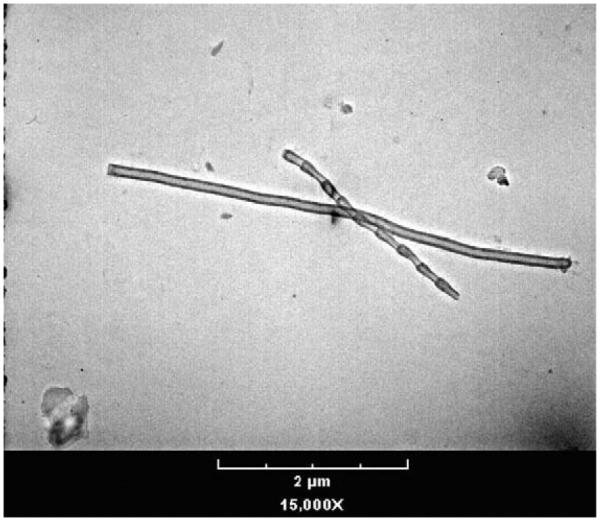
Electron Photomicrograph of a Carbon Nanotube and Carbon Nanofiber at 15,000× Magnification.
Figure 2.
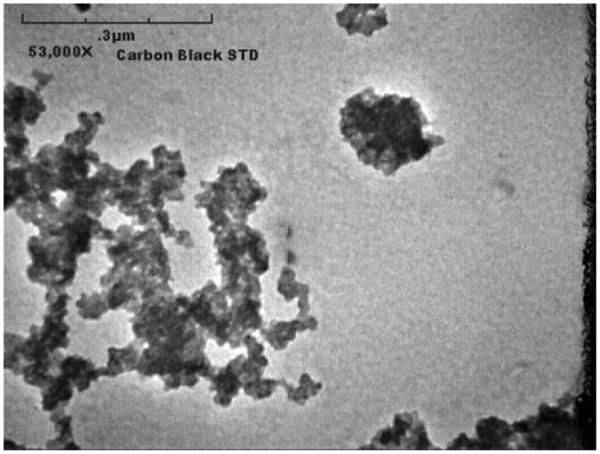
Electron Photomicrograph of Carbon Black at 53,000× Magnification.
The unique properties of nanoscale materials make them attractive for a wide range of product and process improvement applications in medicine, energy, electronics, food and agriculture, and general material science. Because of all the potential applications being explored and developed, an increasing number of nanomaterials are becoming commercially available. These commercially available nanomaterials include nanoscale powders, solutions, and suspensions of nanoscale materials, as well as composite materials and devices having a nanostructure. According to Lux Research, governments, corporations, and venture capitalists worldwide spent $11.8 billion on nanotechnology research and development in 2006.1 It is estimated that by 2014, $2.6 trillion in manufactured goods – or about 15 percent of the total global output – will incorporate nanotechnology.1
Nanomaterials are being commercially incorporated into many consumer products, including sporting goods, batteries, electronics, pharmaceuticals, and cosmetics. Based on the nanotechnology consumer product inventory maintained by the Woodrow Wilson Project on Emerging Nanotechnologies (PEN), there are over 1300 manufacturer-identified nanotechnology-based consumer products currently on the market. Data obtained in March 2011 indicate that this inventory has grown by 521% since March 2006 (from 212 to 1317 products).2 Although these products are finished goods with nanoparticles incorporated into a matrix, most likely they were all produced with use of free, unbound engineered nanoparticles at some stage in their manufacture. These free, unbound, engineered nanoparticles (nanomaterials) are being manufactured and incorporated into products in the United States and throughout the world, thereby providing the potential for worker exposure. At the same time, there isa growing body of knowledge on the potential health hazard of engineered nanomaterials, based on the heightened biologic activity of these materials in toxicologic testing.
Since 1985, the Occupational Safety and Health Administration (OSHA) has required chemical manufacturers and importers to perform hazard determinations and complete material safety data sheets (MSDSs) on chemicals determined to be hazardous [OSHA Hazard Communication Standard (29 CFR 1910.1200)].3 OSHA requires that MSDSs contain the following categories of information: (1) manufacturer’s name and contact information; (2) chemical and common names of all hazardous ingredients; (3) physical/chemical characteristics of the agent or agents; (4) carcinogenicity potential; (5) emergency and first aid measures; (6) primary routes of entry; (7) exposure limits and toxicity information established by OSHA; (8) physical hazards and reactivity data, such as flammability or explosive reactivity; (9) health hazard data, such as medical symptoms or known diseases that can be aggravated by exposure; (10) precautions for handling and use, including appropriate hygienic practices, and the procedures to be used to clean up leaks and spills; (11) applicable control measures such as engineering controls, work practices, or personal protective equipment (PPE); and (12) date of preparation, updates, and reviews.4–6 Beyond requiring inclusion of this basic information, OSHA does not require or provide a standardized format for MSDSs. OSHA published a proposed rulemaking on September 30, 2009, to align its Hazard Communication Standard with the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). This proposed rule (not finalized as of February 10, 2012) includes 16 elements of a Safety Data Sheet (SDS); however, only 12 of the 16 sections will be required because 4 sections (ecological information, disposal considerations, transport information, and regulatory information) are outside of OSHA’s jurisdiction. Note that the preferred title for a MSDS will change to a Safety Data Sheet (SDS).
In order to convey the current OSHA-required information, many manufacturers use the MSDS format developed by the American National Standards Institute (ANSI) in 1993.7 This standard was created to provide information on MSDSs in a consistent manner and to make it easier to find information, regardless of the author or supplier. The ANSI format contains all 12 of the current OSHA-required informational categories as well as four additional categories: (1) toxicological information; (2) transport information; (3) disposal information; and (4) ecological information. The proposed OSHA rule on the GHS more closely aligns the OSHA SDS with the ANSI format.
Under the Hazard Communication Standard, employers must provide information to their employees about the hazardous chemicals to which they are exposed, by means of a hazard communication program; labels and other forms of warning; MSDSs; and information and training. One of the challenges in the United States has been that this is a performance standard with a number of different ways of achieving compliance. Distributors and manufacturers are also required to transmit MSDS and labeling information to their customers who are employers. This “downstream flow” of information ensures that each subsequent employer who receives the hazardous material is apprised of the hazards. This element of hazard communication, while still a performance requirement, resembles what is being required by the European Community Regulation on chemicals and their safe use (EC 1907/2006) through the Registration, Evaluation, Authorization and Restriction of Chemical substances (REACH).8
Manufacturers and importers in the European Union must provide their downstream users with the risk information they need to use a substance safely. This is accomplished via the classification and labeling system and SDSs. SDSs must also communicate information by using a variety of phrases that describe risk (R) or safety (S) concerns associated with the chemical. These statements are known as the R and S phrases. The GHS will provide a framework for the classification and labeling, adding some required content for the SDS and changing the way that some information is currently reported on U.S. MSDSs. As the United States adopts the GHS, the MSDS (SDS) format and content will become more harmonized and will include more information on the risks and hazards of chemicals on the basis of their intended use.
Both OSHA and ANSI formats require information on health hazard data and control measures. The creation and use of engineered nanomaterials are part of an emerging technology, however, and a growing body of toxicological evidence suggests that nanomaterials behave differently than their macroscale counterparts. It stands to reason, then, that increased or unique chemical reactivity exhibited by nanomaterials might lead to increased biological activity, as well as potentially increased toxicity.
In 2005, the National Institute for Occupational Safety and Health issued a draft guidance document on employee exposure to nanomaterials. This document was subsequently updated and finalized in 2009. The document recommends preventing or reducing exposures with use of a hierarchy of controls, based on the increasing amount of research indicating adverse health effects on animals – and, therefore, potential health effects in humans.9
Experimental studies with rodents and in vitro mammalian cell cultures have shown that the toxicity of ultrafine particles or nanoparticles is greater than that of the same mass of larger particles of similar chemical composition.10–18 In laboratory animal exposure studies, single-walled carbon nanotubes (SWCNTs) have been observed to be more fibrogenic than an equal mass of either ultrafine carbon black or fine quartz.19,20 On the basis of their findings in mice, Shvedova et al. estimated that workers may be at risk of developing lung lesions if they were exposed to SWCNTs over a period of 20 days at the current OSHA permissible exposure limit (PEL) for graphite (5 mg/m3).19 Lam et al. provided similar estimates and suggested that the graphite PEL should not be used (e.g., on MSDSs) as a safe concentration for workers exposed to CNTs.20,21 Sargent et al. determined that in vitro exposure to SWCNTs can disrupt formation of the mitotic spindle within cells and could therefore disrupt cell division.22 In addition, it has been found that SWCNTs bind to G-C-rich DNA sequences in the chromosomes (telomeres) that could potentially lead to conformational changes and destabilize the DNA.23 The toxicity of SWCNTs may be linked to the effects that they have on DNA, cell division, and the potential for aneuploidy.
In laboratory animal exposure studies performed by Porter et al., multiwall carbon nanotubes (MWCNTs) have been found to cause pulmonary fibrosis and granulomatous lung inflammation that persisted throughout a 56-day post-exposure period.24 Additionally, pleural penetration was observed at 56 days after exposure in two of four mice. However, the significance of this observation will require further and more extensive investigations to assess if pleural penetration by MWCNT results in any adverse health outcomes.
Inhalation exposure to metal nanomaterials, such as silver or gold, have demonstrated the materials’ potential to move easily through cell membranes, accumulate in areas outside of the respiratory tract, in laboratory animal exposure studies.25,26
Occupational exposure levels (OELs) associated with the largerform bulk metal materials probably won’t be sufficient to protect workers from negative health effects resulting from exposure to the nanosized form. Generally, OELs suggest levels of exposure that most employees may have – up to 8 h per day, 40 h per week, for a working lifetime – without experiencing adverse health effects. However, not all employees will be protected from adverse health effects, even if their exposures are maintained below these levels. A small percentage may experience adverse health effects because of individual susceptibility, a pre-existing medical condition, and/or a hypersensitivity reaction (allergy). Therefore, even OELs for bulk, macrosize materials are not able to provide complete protection for every individual.
In addition to exposure by inhalation, nanoparticles might also be introduced to the body through dermal absorption. Studies have indicated that nanoparticles are capable of penetrating the outermost layer of skin cells.27–29 Shukla et al. demonstrated that titanium dioxide nanoparticles produce reactive oxygen species, deplete glutathione, and increase lipid peroxidase within human epidermal cells.30 This indicates that titanium dioxide nanoparticles induce oxidative stress within the cell.
As further recognition of the known differences between macroscale material and nanoscale material, the United States Environmental Protection Agency (EPA) noted under the Toxic Substances Control Act (TSCA) that carbon nanotubes are distinct chemical substances different from other forms of carbon (e.g., graphite).31
At present, there are no enforceable national or international occupational exposure standards specific to engineered nanomaterials. NIOSH published a recommended exposure limit (REL) of 0.3 mg/m3 for ultrafine titanium dioxide.32 NIOSH also has issued a draft REL of 7 μg/m3 for carbon nanotubes and carbon nanofibers, measured as elemental carbon (EC), for an 8-h respirable-mass airborne concentration.
OELs for macroscale materials are often expressed as a concentration, such as μg/m3, which may not be appropriate or even measurable for nanoscale materials. The current draft NIOSH REL of 7 μg/m3 EC for an 8-h respirable-mass airborne concentration is based on the lowest airborne EC concentration that can be accurately measured by NIOSH method 5040, but it has been noted that adverse lung effects have the potential to occur with exposure levels below this recommended level. In addition, whereas the NIOSH RELs are mass-based, current research indicates that mass and bulk chemistry may be less important than particle number, size and shape, surface area, and surface chemistry (or activity) as indicators of biologic activity for some nanostructured materials.10–12,18 Research is ongoing with regard to the relative importance of these different exposure metrics and how to best characterize exposures to nanomaterials in the workplace.9
Given this uncertainty and the growing body of literature on engineered nanomaterials, it is important to effectively communicate useful hazard information in a manner that is informative and protective and to give risk managers information that will assist them in making prudent risk management decisions. A survey conducted in 2007 by researchers at the University of Massachusetts, Lowell, indicated that MSDSs from suppliers are the preferred source of risk information for nanotechnology firms.33
This report presents findings from the review of a small collection of MSDSs for engineered nanomaterials to evaluate the effectiveness of those data sheets in accomplishing the goal of providing both workers and managers with the most accurate health and safety information on nanomaterials.
METHODS
Obtaining Material Safety Data Sheets
A sample of 59 MSDSs specific to engineered nanomaterials (neat nanomaterials or chemical mixtures containing a nanomaterial, not including nanoengineered composites or finished goods) was obtained from 32 manufacturers by means of Internet search engines during 2007–2008. In 2010–2011, a random sample of an additional 21 MSDSs specific to engineered nanomaterials was obtained. In addition, 23 of the original 2007–2008 nanomaterial MSDSs were re-collected in 2010–2011 for comparison purposes to determine whether improvements and revisions had occurred. These MSDSs were obtained with use of Internet search engines, as well as verbal and written requests, and were all evaluated on the basis of the same ranking scheme.
Reviewing Material Safety Data Sheets
The MSDSs were evaluated by means of a set of basic questions intended to determine whether their content was informative and protective, specifically as it pertains to hazard identification, exposure controls, PPE, and toxicological information. MSDSs were categorized according to the following four questions:
Did the MSDS indicate that the material is in the nanometer size range (<100 nm) by using numerical references or ranges?
Did the MSDS contain an OEL for the larger or bulk form (macroscale) of the material, and was there any guidance given on whether this OEL may or may not be protective for the nanomaterial?
Did the MSDS include specific toxicological data or information on the nanomaterial or indicate that nanomaterials may have different toxicities than larger particles of the same material?
Did the MSDS advise the use of protective measures, such as engineering controls, appropriate respiratory protection, and non-permeable gloves, when there is the potential for exposure?
The following paradigm was used to evaluate the collected MSDSs:
If the MSDS was deficient in only one of the above categories, it was classified as satisfactory.
If it was deficient in two categories, it was classified as in need of improvement.
If it was deficient in more than two categories, it was classified as in need of significant improvement.
Statistical Analysis
MSDSs produced by the same manufacturer are likely to have the same language and similar information. The resulting lack of statistical independence among such data sheets would invalidate commonly used tests for statistical significance.34 This issue was addressed by randomly choosing one MSDS per manufacturer when multiple MSDSs were available.
Descriptive statistics and tests were performed with use of Graph Pad-QuickCalcs software (http://www.graphpad.com/quickcalcs/index.cfm).35 Proportions were compared with 2 μ 2 tables and Fisher’s exact test. All tests were 1-tailed and used an α of 0.05.
RESULTS
Study Sample
Fifty-nine MSDSs from 32 different manufacturers were initially obtained in 2007–2008. For purposes of this study, duplicates were removed and only one product per manufacturer was randomly identified for analysis, leaving 32 MSDSs for this evaluation. In the 2010–2011 period, 65 MSDSs were originally obtained. Duplicates were removed, and only one product per manufacturer was randomly identified for analysis, leaving 21 original MSDSs and 23 for comparison with the 2007–2008 MSDSs. A total of 44 independent manufacturers’ MSDSs were available following the 2010– 2011 search. Table 1 provides a summary of the characteristics of each MSDS and the answers obtained from the four categorization questions.
Table 1.
Characteristics and Summary of Questions Addressed in MSDSs.
| MSDS Characteristic | Number (%) of MSDSs |
||
|---|---|---|---|
| 2007–2008 n = 32 |
2010–2011 n = 21 |
2010–2011, Updated from 2007–2008 n = 23 |
|
| Contained bulk OEL (macroscale)a | 19 (59.4%) | 16 (76.2%) | 11 (47.8%) |
| Specified that material is nanometer range, using numbersb | 6 (18.8%) | 4 (19.0%) | 6 (26.1%) |
| Specified nanomaterial toxicological data for productc | 6 (18.8%) | 6 (28.6%) | 3 (13.0%) |
| Advised using engineering controls and/or PPEd | 22 (68.8%) | 20 (95.2%) | 15 (65.2%) |
| No original or revision date | 7 (22.0%) | 2 (9.5%) | 3 (13.0%) |
| Year of last review: mean (median), range | 2005 (2005), | 2009 (2010), | 2008 (2008), |
| 2003–2008 | 2005–2011 | 2004–2011 | |
Did the MSDS contain an OEL for the larger or bulk form (macroscale) of the material, and was there any guidance given on whether this OEL may or may not be protective for the nanomaterial?
Did the MSDS indicate that the material is in the nanometer size range (<100 nm), using numerical references or ranges?
Did the MSDS include specific toxicological data or information for the nanomaterial or reference that nanomaterials may have different toxicities than larger particles of the same material?
Did the MSDS advise the useofengineering controls and/or appropriate respiratory protection (e.g., N95 or P100) and nonpermeable gloves when the potential for exposure exists?
Categorization of MSDSs
Categorization of the effectiveness of the 2007–2008 MSDSs concluded that 21.8% were satisfactory, 40.6%were in need of improvement, and 37.5% were in need of significant improvement. The most common deficiencies noted in the 2007–2008 MSDSs were the lack of toxicological data specific to the nanomaterial and the failure either to identify the material as being nanometer-sized or to list a particle size distribution showing the size range. Both deficiencies were noted in 26 (81.3%) of the 2007–2008 MSDSs. Nineteen (59.4%) of the MSDSs contained OELs for the bulk material without providing guidance on the ability of the OEL to protect against the nanoscale material. Twenty-two (68.8%) of the MSDSs recommended control of dust through either the use of engineering controls or the use of appropriate respirators and non-permeable gloves.
A total of 23 of the 32 nanomaterial MSDSs originally obtained in 2007– 2008 were recollected in 2010–2011 (several of the initial 32 manufacturers no longer had an Internet address). The 2010–2011 updated MSDSs were categorized as follows: four (17.4%) were ranked satisfactory, eight (34.8%) were ranked in need of improvement, and 11 (47.8%) were ranked in need of significant improvement. Comparison with the original MSDS data obtained for the same products/manufacturers showed an overall decrease in the percent of MSDSs ranked satisfactory or in need of improvement and an increase in the number of MSDSs ranked in need of significant improvement. This could be because some of the more accurate MSDSs produced in 2007–2008 were not available in 2010–2011. The difference in the number of MSDSs that were ranked satisfactory was not statistically significant (p = 0.6615). A comparison of the results for each of the four questions asked about each MSDS indicated that the changes noted in the MSDSs between 2007–2008 and 2010–2011 were not statistically significant.
The most common deficiency in the 21 new MSDSs (from 2010 to 2011) was a failure to specify that the material is in the nanometer size range (<100 nm) by using numerical references or ranges; this occurred in 17 (81.0%) of the MSDSs. Additionally, 15 (71.4%) of the MSDSs failed to include toxicological data or information specific to the nanomaterial or to reference that nanomaterials may have different toxicities than larger particles of the same material. A comparison of the 21 independent 2010–2011 MSDSs to the 32 independent 2007–2008 MSDSs indicated that there was an overall increase in the number of MSDSs ranked as satisfactory and a decrease in those ranked in need of improvement or in need of significant improvement. The difference in the number of MSDSs ranked satisfactory was not a statistically significant improvement (p = 0.5439). Comparison of the results for each of the four answers sought within each MSDS 59.4% indicated that the changes noted in the information available were not statistically significant, with one exception. Ninety-five percent of MSDSs from 2010 to 2011 recommended using engineering controls and/or personal protective equipment to protect workers handling nanomaterials, which was found to be a statistically significant improvement (p = 0.0478). The mean review and/or revision date for the 2010–2011 MSDSs was 2009, which could indicate that companies had reviewed current information about the potential health effects of nanomaterial exposure before they updated the MSDSs.
There was no relationship between language addressing the four questions used in this study and the date on which the information in the MSDS had last been reviewed or updated. It was not clear whether a literature review had been performed as part of the last review date listed on the MSDSs. Eleven (47.8%) of the 2007–2008 versus 2010–2011 comparison MSDSs had not been updated since the year 2007. Five (21.7%) of the 2007–2008 versus 2010–2011 comparisons had both no change in status and no revision date listed.
The majority of the MSDSs provided insufficient information for communicating the potential hazards of engineered nanomaterials. Table 2 summarizes the percent rankings of satisfactory, in need of improvement, and in need of significant improvement with the 2007–2008, 2010–2011, and 2007–2008 versus 2010–2011 comparison MSDSs.
Table 2.
Summary of MSDS Ranking, Based on Simple Ranking Scheme.
| MSDS: Date Collected | Ranking: No. (%) |
||
|---|---|---|---|
| Satisfactory | In Need of Improvement | In Need of Significant Improvement | |
| 2007–2008, n = 32 | 7 (21.8) | 13 (40.6) | 12 (37.5) |
| 2010–2011, n = 21 | 7 (33.3) | 10 (47.6) | 4 (19.1) |
| 2007–2008, recollected in 2010–2011, n = 23 |
4 (17.4) | 8 (34.8) | 11 (47.8) |
In addition to reviews of data sheet content against the questions developed for this evaluation, some of the language used to communicate possible safety or hazard issues was evaluated. MSDS statements considered vague and ineffective included the following:
“There are no reports of adverse health effects on this material from customers and operators in our plant.”
“No known toxicological effects.”
“We are not aware of any reported health hazards for this product.”
“Non-hazardous.”
“Hazard description – not applicable.”
These statements would seem to indicate that current research on nanomaterials was not reviewed by the manufacturer prior to making the MSDS available for employee and customer use.
DISCUSSION
The overwhelming majority of MSDSs did not clearly communicate the potential hazards of engineered nanoparticles in a way that was informative and protective. Of the reviewed MSDSs, 61% of those representing a mixture failed to identify which of the ingredients were nanosized. The nanoscale ingredient was made clearly evident in single-chemical MSDSs, but it was difficult to discern nanoscale ingredients in MSDSs pertaining to mixtures. More than half of the MSDSs included or referred to OELs for the bulk or larger-sized form of the chemical in question, even though the MSDSs pertained to the nanoscale version of the chemical. One half of the 26 MSDSs for carbonaceous materials (carbon nanotubes, carbon nanofibers, and fullerenes) referred to the OSHA OEL for the respirable fraction of synthetic graphite (5 mg/m3), carbon black (3.5 mg/m3), or for total nuisance particulates (15 mg/m3) without any indication that these may not be fully protective. Three (11.5%) of the MSDSs for carbonaceous materials indicated that there was no OEL available, and 10 (38.5%) made no reference whatsoever to an OEL. Referencing the bulk chemical exposure limit or the total particulate exposure limit in the absence of supporting data or conditional language may be misleading because there is currently no evidence to suggest that these levels are protective for the nanoscale material.
Many nanomaterial MSDSs also lacked toxicological data or listed toxicological data for the respective bulk substance. This may suggest that the same toxicological effects occur for both forms of the chemical, which may not necessarily be true. Knowledge of the size of particles that employees are being exposed to could assist in the selection of appropriate personal protection or engineering controls as well as provide a better understanding of potential health effects and risks.
This study is limited by a small sample size. Although there are many product MSDSs that contain information on nanomaterials, MSDSs produced by the same manufacturer are likely to have the same language and similar information. Because of this, the approach of randomly selecting one MSDS per manufacturer from among multiple available MSDSs was taken to render each MSDS statistically independent. The sample size in a survey can affect the precision of an estimate of prevalence.32 The final 2010–2011 sample size of 44 MSDSs (23 updated and 21 new) indicated a 25% prevalence of the satisfactory ranking. With an α of 0.05, the population of MSDSs yields a precision of ±12.6%. This indicates that if all nanomaterial MSDSs were reviewed, the prevalence of a satisfactory ranking would be between 12% and 38%. Even if 38% of the MSDSs were found to be ranked as satisfactory, there is still a 62% possibility that an individual using the information on the MSDS in the workplace may not be adequately informed or protected, because of a lack of knowledge regarding safety practices, toxicology, or health effects of the product.
CONCLUSIONS
In conclusion, this study revealed that important information is not being developed or included on current nanomaterial MSDSs. The consequences of not providing adequate information is that workers may have exposure to nanomaterials that are known to have the potential to cause cellular damage and respiratory and pulmonary disease in animal studies, and that may migrate to other areas of the body following inhalation or dermal exposure.19,20,22–26,30 If workers are not able to obtain accurate, up-to-date information from the MSDS related to the nanomaterial in use, they may be less likely to use appropriate engineering controls or PPE to reduce exposures that pose health risks. In addition, when the employer’s primary source of information is an MSDS that fails to indicate a potential hazard to employees, then the employer may not be willing to integrate appropriate engineering controls or enforce the use of PPE.
In preparing MSDSs for engineered nanoparticles, product manufacturers should address the degree of current knowledge and all potential concerns that employees and/or health and safety personnel may have. It is recommended that MSDSs for engineered nanomaterials include the following information:
manufacturer’s name and contact information,
chemical and common names of all hazardous ingredients,
physical/chemical characteristics of the agent or agents (for example, an indication that the material is or contains a nanomaterial and the particle size or particle size distribution of the nanomaterial),
carcinogenicity potential,
emergency and first aid measures,
primary routes of entry,
exposure limits established by OSHA, NIOSH, American Conference of Governmental Industrial Hygienists, or European Union communities (if there is no OEL for the nanomaterial, then indicate that the OELs for the macroscale bulk material should be considered minimally protective for the nanometersized material),
physical hazards and reactivity data, such as flammability or explosive reactivity,
health hazard data, such as medical symptoms or known diseases that can be aggravated by exposure,
precautions for handling and use, including appropriate hygienic practices and procedures for cleaning up leaks and spills (note that wet methods are preferred for cleanup of dry powder nanomaterials, and if a vacuum is used, it should be exhausted through a HEPA filter; dry sweeping and blowing of materials should be prohibited),
applicable control measures such as engineering controls, work practices, or PPE (for example, recommend that worker exposure be minimized by institution of a program of engineering controls such as exhausted enclosures or local exhaust ventilations; by good work practices, such as separation of the handling area from the rest of the facility; or by use of PPE such as protective clothing, safety glasses or goggles, chemical-resistant gloves, and respirators selected on the basis of the NIOSH respirator selection logic36),
toxicological information (for instance, an explanation that the characteristics of nanoparticles, such as small particle size and increased surface area, may contribute to potentially greater toxicity and biological activity than larger particles of the same chemical composition; in addition, the most recent toxicity information from an annual or more frequent literature search [OSHA mandates that newly found information on chemical hazards be added to MSDSs within 3 months of discovering such information]),
transport information,
disposal information,
ecological information,
date of preparation.
MSDSs for nanomaterials can be improved by conducting an extensive scientific literature review on the nanomaterial. The emphasis of this review should be discovering the latest toxicologic data, epidemiological findings, measurement techniques, engineering controls, and regulatory status so that the MSDS can be revised with the best, most current data. This will provide MSDS users with a knowledge base that will allow them to develop effective precautionary measures to control exposure and protect worker safety and health. It would also be beneficial to cite the publications that particular revisions are based upon so that additional information can be easily accessed if needed. Until the completeness and accuracy of information on MSDSs are ensured, the safety and health of workers using nanomaterial products cannot be fully protected.
ACKNOWLEDGEMENTS
The authors thank Bruce Lippy of The Lippy Group; Janet Carter, David O’Conner and Maureen Ruskin of U.S. OSHA for their review of the draft manuscript.
Contributor Information
Adrienne Eastlake, Environmental Data Validation, Pittsburgh, PA, United States.
Laura Hodson, National Institute for Occupational Safety and Health (NIOSH), Education and Information Division (EID), Cincinnati, OH, United States, (Tel.: +1 5135338526; lhodson@cdc.gov).
Charles Geraci, National Institute for Occupational Safety and Health (NIOSH), Education and Information Division (EID), Cincinnati, OH, United States.
Carlos Crawford, Environmental Data Validation, Pittsburgh, PA, United States.
REFERENCES
- 1.Lux Research . The Nanotech Report. 5th Lux Research; New York: 2007. [Google Scholar]
- 2.Woodrow Wilson Project on Emerging Nanotechnologies Consumer Products Inventory. http://www.nanotechproject.org/inventories/consumer/updates/(accessed 7/1/2011)
- 3.Occupational Safety and Health Administration U.S. Code of Federal Regulations, 42 CFR 1910.1200.
- 4.Fluke C. Material safety data sheets. J. Health Mater. Manage. 1993;11:64–67. [PubMed] [Google Scholar]
- 5.Kolp P, Sattler B, Blayney M, Sher-wood T. Comprehensibility of material safety data sheets. Am. J. Ind. Med. 1993;23:135–141. doi: 10.1002/ajim.4700230119. [DOI] [PubMed] [Google Scholar]
- 6.Solomon CJ. Understanding and using the MSDS. AAOHN J. 1998;36:376–379. [PubMed] [Google Scholar]
- 7.American National Standards Institute (ANSI) American National Standard for Hazardous Industrial Chemicals: Material Safety Data Sheets – Preparation. New York; ANSI. ANSI Z400. 1-1993; [Google Scholar]
- 8.European Parliament Council Regulation (EC) 1907/2006 Registration, Evaluation, Authorization and Restriction of Chemical substances (REACH) Dec 18, 2006.
- 9.NIOSH . Approaches to Safe Nanotechnology: Managing the Health and Safety Concerns Associated with Engineered Nanomaterials. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, Ohio: 2009. DHHS (NIOSH) Publ. No. 2009-125. [Google Scholar]
- 10.Oberdörster G, Ferin J, Gelein R, Soderholm SC, Finkelstein J. Role of the alveolar macrophage in lung injury – studies with ultrafine particles. Environ. Health Perspect. 1992;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberdörster G, Ferin J, Lehnert BE. Correlation between particle-size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994;102(S5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberdörster G, Ferin J, Soderholm S, Gelein R, Cox C, Baggs R, Morrow PE. Increased pulmonary toxicity of inhaled ultrafine particles: due to lung overload alone? Ann. Occup. Hyg. 1994;38(Suppl. 1):295–302. [Google Scholar]
- 13.Lison D, Lardot C, Huaux F, Zanetti G, Fubini B. Influence of particle surface area on the toxicity of insoluble manganese dioxide dusts. Arch. Toxicol. 1997;71(12):725–729. doi: 10.1007/s002040050453. [DOI] [PubMed] [Google Scholar]
- 14.Tran CL, Cullen RT, Buchanan D, Jones AD, Miller BG, Searl A, Davis JMG, Donaldson K. Contract Research Report 216/1999. Health and Safety Executive; Suffolk, UK: 1999. Investigation and prediction of pulmonary responses to dust. Part II; Investigations into the Pulmonary Effects of Low Toxicity Dusts. [Google Scholar]
- 15.Tran C, Buchanan LD, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal. Toxicol. 2000;12(12):1113–1126. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- 16.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001;175(3):191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 17.Barlow PG, Clouter-Baker AC, Donaldson K, MacCallum J, Stone V. Carbon black nanoparticles induce type II epithelial cells to release chemotaxins for alveolar macrophages. Part. Fiber Toxicol. 2005;2(14) doi: 10.1186/1743-8977-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffin R, Tran L, Brown D, Stone V, Donaldson K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol. 2007;19(10):849–856. doi: 10.1080/08958370701479323. [DOI] [PubMed] [Google Scholar]
- 19.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D. Unusual inflammatory and fibrogenic pulmonary responses to single walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289(5):L698–L708. doi: 10.1152/ajplung.00084.2005. Epub. [DOI] [PubMed] [Google Scholar]
- 20.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 21.Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 22.Sargent L, Shvedova A, Hubbs A, Salisbury J, Benkovic S, Kashon M, Lowry D, Murray A, Kisin E, Friend S, McKinstry K, Battelli L, Reynolds S. Induction of aneuploidy by single-walled carbon nanotubes. Environ. Mol. Mutagen. 2009;50:708–717. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Peng Y, Ren J, Qu X. Carboxyl-modified single-walled carbon nanotubes selectively induce human telomeric i-motif formation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19658–19663. doi: 10.1073/pnas.0607245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter DW, Hubbs AF, Mercer RR, Nianqiang W, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen B, Tsuruoka S, Endo M, Castranova V. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Lankveld DPK, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, Van Eijkeren JCH, Geertsma RE, De Jon WH. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials. 2010;31:8350–8361. doi: 10.1016/j.biomaterials.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Gopinath P, Gogoi SK, Chattopadhyay A, Gosh SS. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. J. Nanobiotechnol. 2008;19 doi: 10.1088/0957-4484/19/7/075104. Art.No.075104. [DOI] [PubMed] [Google Scholar]
- 27.Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Serry W. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol. Appl. Skin Physiol. 1999;12:247–256. doi: 10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- 28.Bennat C, Muller-Goymann CC. Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int. J. Cosmet. Sci. 2000;22:271–283. doi: 10.1046/j.1467-2494.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 29.Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, DePree K, Adkins EJ. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ. Health Perspect. 2003;111:1202–1208. doi: 10.1289/ehp.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla R, Sharma V, Pandey A, Singh S, Sultana S, Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro. 2011;25:231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Federal Register U.S. EPA Toxic Substances Control Act Inventory Status of Carbon Nanotubes. Oct 31, 2008. 2008.
- 32.Current Intelligence Bulletin 62 . Occupational Exposure to Titanium Dioxide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, Ohio: DHHS (NIOSH) Publ. No. 2011-160. [Google Scholar]
- 33.Lindberg JE, Quinn MM. A survey of environmental, health and safety risk management information needs and practices among nanotechnology firms in the Massachusetts region. Dec; PEN Brief No. 1. 2007, www.nanotechproject.org/publications/archive/a_survey_environmental_health_safety/
- 34.Elston R, Johnson W. Essentials of Biostatistics. Vol. 46. Davis Company; Philadelphia, FA: 1994. pp. 169–176. [Google Scholar]
- 35.GraphPadQuickCalcs Software Fisher’s and Chi-square. 2002–2005 http://www.graphpad.com/quickcalcs/index.cfm (accessed 8/12/11)
- 36.NIOSH Respirator Selection Logic. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, Ohio: 2005. DHHS (NIOSH) Pub. No. 2005-100. [Google Scholar]


