Abstract
Cylindrospermopsis raciborskii is among the most commonly recognized toxigenic cyanobacteria associated with harmful algal blooms (HAB) in freshwater systems, and specifically associated with multiple water-soluble toxins. Lipophilic metabolites from C. raciborskii, however, were previously shown to exert teratogenicity (i.e. inhibition of vertebrate development) in the zebrafish (Danio rerio) embryo model, specifically suggesting the presence of additional bioactive compounds unrelated to the currently known toxins. In the present study, a series of known teratogenic polymethoxy-1-alkenes (PMA) were identified, purified and chemically characterized from an otherwise well-characterized strain of toxigenic C. raciborskii. Although PMA have been previously identified in other cyanobacteria, this is the first time they have been identified from this recognized HAB species. Following their identification from C. raciborskii, the taxonomic distribution of the PMA was additionally investigated by chemical screening of a freshwater algal (i.e. cyanobacteria, green algal) culture collection. Screening suggests that these compounds are distributed among phylogenetically diverse taxa. Furthermore, parallel screening of the algal culture collection, using the zebrafish embryo model of teratogenicity, the presence of PMA was found to closely correlate with developmental toxicity of these diverse algal isolates. Taken together, the data suggest PMA contribute to the toxicity of C. raciborskii, as well as apparently several other taxonomically disparate cyanobacterial and green algal genera, and may, accordingly, contribute to the toxicity of diverse freshwater HAB.
Keywords: Cyanobacteria, Cylindrospermopsis raciborskii, Green algae (Chlorophyta), Polymethoxy-1-alkenes, Teratogenicity, Zebrafish embryo
1. Introduction
Several bioactive metabolites from cyanobacteria, particularly in association with so- called “harmful algal blooms” (HAB), are recognized toxins linked to human and animal intoxication, possible chronic health effects and impacts on aquatic ecosystems. Cylindrospermopsis raciborskii (Nostocales) is among the toxigenic cyanobacterial species most frequently linked to HAB in freshwater systems (Chorus et al., 1999; Rzymski and Poniedzialek, 2014). In particular, C. raciborskii has been associated with the production of multiple, water- soluble toxins, and specifically the hepatotoxic alkaloid, cylindrospermopsin (CYN), and the neurotoxic alkaloid, saxitoxin (STX) and its analogues.
Numerous prior studies, however, have suggested the presence of uncharacterized toxic metabolites from C. raciborskii. One such study, carried out by Saker et al. (2003) showed that intraperitoneal administration of cell extracts from four strains of C. raciborskii were toxic to mice even though there were no detectable amounts of CYN or STX. More recently, Berry et al., (2009) showed that lipophilic extracts from both CYN- and non-CYN-producing strain of the C. raciborskii were teratogenic (i.e., inhibited development) in the zebrafish embryo model. Taken together, these and other numerous studies (e.g. Nogueira et al., 2006; Acs et al., 2013; Jonas et al., 2014) have suggested that the presence of yet unidentified bioactive compounds may contribute to the toxigenicity of this species.
In order to identify toxic metabolites from C. raciborskii, the zebrafish (Danio rerio) embryo was used, as a model of vertebrate development, for identification of teratogenic metabolites from established, toxigenic cultures of this species. Owing to its numerous practical advantages, the zebrafish embryo has been extensively employed previously in the identification, including both screening and bioassay-guided fractionation studies, and subsequent characterization of toxic or otherwise bioactive compounds from various sources including, in particular, freshwater cyanobacteria (Oberemm et al., 1997; Papendorf et al., 1997; Oberemm et al., 1999; Lefebvre et al., 2004; Wright et al., 2006; Berry et al., 2007; Berry et al., 2009; El Ghazali et al., 2009; Rogers et al., 2011; Jaja-Chimedza et al., 2012a and 2012b; Acs et al., 2013; Jonas et al., 2014; Walton et al., 2014).
As described here, the teratogenic metabolites isolated and characterized in the present study (1-4) were specifically determined to be a series of polymethoxy-1-alkenes (PMA; Fig. 1). Although this is the first report of these toxic metabolites from C. raciborskii, PMA have, in fact, been previously isolated from both laboratory cultures and field collections of other cyanobacterial species (e.g. Mynderse and Moore, 1979; Mori et al., 1991a and 1991b; Banker et al., 2000; Jaja-Chimedza et al., 2012a), and their chemical structure has been proven by not only spectroscopic studies, but also total synthesis (Mori et al., 1991a and 1991b). Only recently, however, has toxicity (and specifically teratogenicity) been linked to these molecules (Jaja-Chimedza et al., 2012a).
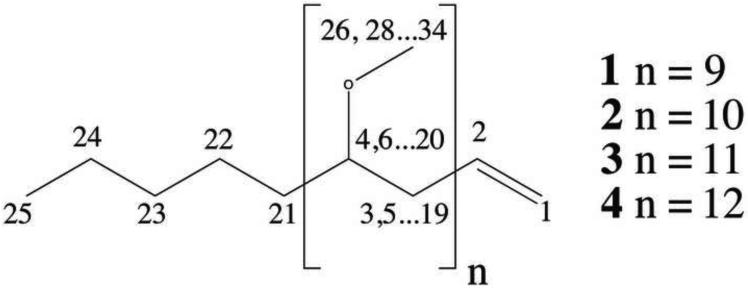
Fig. 1.
Chemical structure of PMA variants (1-4) identified in the present study. The numbers on the structure correspond to compound 2 (C34H68O9), where n = 9.
Following the present identification of these teratogenic metabolites from C. raciborskii, and previous identification from other cyanobacterial species, the present study additionally endeavored to investigate the phylogenetic distribution of these metabolites among freshwater algae. To this end, a culture collection of freshwater cyanobacteria, as well as taxonomically distinct green algae, was evaluated by a chemical screening (i.e. liquid chromatography/mass spectrometry [LC-MS]) approach. The results with respect to the phylogenetic distribution of the PMA among freshwater algae, and relevance to cyanobacterial HAB, are subsequently discussed.
2. Methods and Materials
2.1. Cyanobacterial and algal culture material
Cultures of C. raciborskii AQS was provided by Centro de Investigacão Marinhae Ambiental (Laboratory of Ecotoxicology) at the University of Porto (Portugal), and cultured as previously described (Berry et al., 2009). The isolate was originally isolated and characterized from aquaculture ponds in Australia (Saker and Eaglesham, 1999). In addition to C. raciborskii, established cultures of ninety-seven taxonomically diverse freshwater cyanobacteria and green algae (Table S1 of Supplementary Data) were grown, as previously described (Berry et al., 2007; Gantar et al., 2008; Berry et al., 2009), and more or less identically to C. raciborskii AQS - except in smaller (i.e. 250 mL) culture volumes - for chemical screening (see 2.6. Screening of cyanobacterial and green algal cultures for PMA). In each case, algal biomass from cultures was harvested after three to four weeks by centrifugation, and subsequently freeze-dried for extraction.
2.2. Zebrafish embryo teratogenicity assay
The zebrafish (Danio rerio) embryo was used, as a model of vertebrate development, to evaluate teratogenicity of C. raciborskii extracts, and subsequent chemical fractions. Maintenance and breeding of the adult zebrafish, as well as the zebrafish embryo teratogenicity assay, were done as previously described (Berry et al., 2009; Jaja-Chimedza et al., 2012a). Embryos thus exposed to extracts, and subsequent fractions (see 2.3. Purification of compounds 1-4), were evaluated by light microscopy over 6 days post-fertilization [dpf] to assess developmental toxicity. All breeding and bioassays involving zebrafish were conducted under protocols approved by the FIU Institutional Animal Care and Use Committee (IACUC), and performed by trained investigators.
2.3. Purification of compounds 1-4
Following the tentative identification of PMA in extracts, and subsequent bioactive fractions, from C. raciborskii AQS, purification of 1-4 was achieved by a method adapted from previous isolation of PMA from cyanobacteria (i.e. Aphanizomenon ovalisporum; Jaja-Chimedza et al., 2012a). Briefly, freeze-dried biomass was twice extracted in chloroform overnight, and subsequently filtered extracts were pooled and concentrated to dryness in vacuo. The pooled crude extracts (resuspended in hexane) were separated by flash-chromatography on a normal phase (Silica Gel 60Å, Commercial 40-63μm) column with a stepwise gradient of ethyl acetate in hexane (10%, 20%, 40%, and 100% ethyl acetate); the bioactive fraction eluted with 100% ethyl acetate. This bioactive fraction was subsequently separated by reversed-phase (Phenomenex Luna 5 μm C18 100 Å LC Column, 250 × 4.6 mm) high-performance liquid chromatography (HPLC) using a solvent gradient starting at 50% acetonitrile in water up to 100% acetonitrile for 37 min) with detection based on UV absorbance (200 nm). A bioactive fraction with a retention time of 20-24 min contained a mixture of 1-4. From this mixture, each compound was further purified by HPLC (isocratic, 65:35 acetonitrile:water) with the compounds eluting in order of increasing molecular weight (as subsequently determined by LC- MS).
2.4. Chemical characterization of compounds 1-4
Once purified, compounds 1-4 were chemically characterized by mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. Low-resolution MS analyses were performed on a Thermo TSQ Quantum Access ESI/triple quadrupole (QqQ) instrument, coupled to a Thermo Accela UHPLC (i.e. LC-MS), and high-resolution mass spectrometric (HRMS) analyses were performed on a Thermo LTQ ESI-Orbitrap. Compound 2 (as one of the most abundant congener) was most extensively characterized by NMR including one-dimensional (1H and 13C-DEPT-135) and two-dimensional homonuclear (COSY) and heteronuclear (HMQC and HMBC) experiments on a Bruker AVANCE 400 MHz instrument. Analysis of the other three congeners was limited to 1H-NMR and 2-D homonuclear COSY (3-4) experiments. However, interpretation of this data, as for 2 (see 3.2. Chemical Characterization of Compounds 1-4), was sufficient - in conjunction with the previously published chemical characterization data for the PMA - to assign structures for these congeners.
4,6,8,10,12,14,16,18-octamethoxy-1-tricosene (1)
white amorphous solid, 1H NMR (400 MHz, benzene-d6) 3.16 (s, 3H), 3.21 (s, 3H), 3.22 (s, 3H), 3.24 (s, 3H), 3.25 (s, 3H), 3.27 (s, 3H), 3.28 (s, 3H), 3.28 (s, 3H), 5.07 (dd, 1H, J = 10,2 Hz), 5.10 (dd, 1H, J = 17,2 Hz), 5.90 (ddt, 1H, J = 17,10,7 Hz). HRESIMS m/z 563.4489 (M+H)+ (calculated for C31H63O8, 563.4478)
4,6,8,10,12,14,16,18,20-Nonamethoxy-1-pentacosene (2)
white amorphous solid, 1H NMR (400 MHz, benzene-d6) δ 0.91 (t, 3H, J = 7.1 Hz), 1.30 (m, 4H), 1.42 (m, 2H), 1.56 (m, 2H), 1.67 (m, 2H), 1.81 (m, 6H), 2.03 (m, 8H), 2.30 (m, 2H), 3.16 (s, 3H), 3.21 (s, 3H), 3.22 (s, 3H), 3.24 (s, 3H), 3.25 (s, 3H), 3.27 (s, 3H), 3.27 (s, 3H), and 3.28 (s, 6H), 3.37 (m, 2H), 3.64 (m, 7H), 5.07 (dd, 1H, J = 10,2 Hz), 5.10 (dd, 1H, J = 17,2 Hz), 5.90 (ddt, 1H, J = 17,10,7 Hz); 13C NMR (100 MHz, benzene-d6) δ 14.6, 23.4, 25.4, 32.8, 34.3, 38.3, 38.5, 38.6, 38.9, 38.9 (3C), 39.0 (4C), 52.2, 56.3, 56.3 (3C), 56.4 (2C), 56.5, 75.9, 76.0 (2C), 76.1, 76.1, 77.9, 78.4, 117.3, 135.6. HRESIMS m/z 621.4900 (M+H)+ (calculated for C34H69O9, 621.4897)
4,6,8,10,12,14,16,18,20,22-Decamethoxy-1-heptacosene (3)
white amorphous solid, 1H NMR (400MHz, benzene-d6) δ 0.91 (t, 3H, J = 7.1 Hz), 1.30 (m, 4H), 1.42 (m, 2H), 1.55 (m, 2H), 1.67 (m, 2H), 1.81 (m, 7H), 2.02 (m, 9H), 2.30 (m, 2H), 3.16 (s, 3H), 3.21 (s, 3H), 3.22 (s, 3H), 3.24 (s, 3H), 3.25 (s, 3H), 3.27 (s, 3H), 3.27 (s, 3H), 3.28 (s, 9H), 3.37 (m, 2H), 3.65 (m, 8H), 5.07 (dd, 1H, J = 10,2 Hz), 5.10 (dd, 1H, J = 17,2 Hz), 5.90 (ddt, 1H, J = 17,10,7 Hz). HRESIMS m/z 679.5312 (M+H)+ (calculated for C37H75O10, 679.5316)
4,6,8,10,12,14,16,18,20,22,24-Undecamethoxy-1-nonacosene (4)
white amorphous solid, 1H NMR (400MHz, benzene-d6) δ 0.91 (t, 3H, J = 7.1 Hz), 1.30 (m, 4H), 1.44 (m, 2H), 1.58 (m, 2H), 1.68 (m, 2H), 1.81 (m, 8H), 2.04 (m, 10H), 2.30 (m, 2H), 3.16 (s, 3H), 3.21 (s, 3H), 3.22 (s, 3H), 3.24 (s, 3H), 3.25 (s, 3H), 3.27 (s, 6H), 3.28 (s, 12H), 3.37 (m, 2H), 3.65 (m, 9H), 5.07 (dd, 1H, J = 10,2 Hz), 5.10 (dd, 1H, J = 17,2 Hz), 5.90 (ddt, 1H, J = 17,10,7 Hz). HRESIMS m/z 737.5733 (M+H)+ (calculated for C40H81O11, 737.5734)
2.5. Screening of cyanobacterial and green algal cultures for PMA and teratogenicity
Ninety-seven algal isolates (see Table S1 of Supplementary Data) from a culture collection of freshwater cyanobacteria and green algae (Chlorophyta) were extracted and fractionation, based on the previously optimized method for C. raciborskii, and screened by LC- MS for PMA. Briefly, freeze-dried culture material (~100 mg) for each strain was extracted twice in chloroform, using freeze-thaw to disrupt cells. Extracts were filtered, concentrated in vacuo and re-suspended in 1 mL of chloroform. Resulting extracts were fractionated using solid- phase extraction (SPE) cartridges (Supelclean LC-Si, bed weight: 100mg, volume: 1 mL) whereby extracts were loaded onto pre-conditioned cartridges, and three eluted fractions were collected: 40% ethyl acetate in hexane, 100% ethyl acetate, and 100% methanol. All 3 fractions were analyzed for the presence of PMA by LC-MS (Thermo Accela UHPLC) with a reversed- phase column (Phenomenex Kinetex 2.6 μm C18 100 Å LC Column 100 × 4.6 mm) using an acetonitrile gradient starting with 50% acetonitrile in water for 5 min followed by a gradient up to 100% acetonitrile over 15 min, and 100% acetonitrile for 5 min for a total run time of 25 min.
The PMA - and particularly the variants (1-4) characterized from C. raciborskii in the present study - were subsequently detected by heated electrospray ionization (HESI)-MS, and selected reaction monitoring (SRM) using a Thermo TSQ Quantum Access mass spectrometer. The variants were specifically detected based on retention time and presence of the corresponding molecular ion in full scan mode, as well as the sodium adduct ([M+Na]+), and relevant mass transition associated with loss of a methoxy group ([M-32+Na]+), in SRM mode, as follows: m/z 585 > 553 (1), m/z 643 > 611 (2), m/z 701 > 669 (3) and m/z 759 > 727. As a “positive control”, C. raciborskii AQS (from the present study), and the previously characterized PMA-producing strain, A. ovalisporum APH (Jaja-Chimedza et al., 2012a), were evaluated in parallel with screened isolates. The relevant HESI-MS/SRM parameters were as follows:
| Spray Voltage: 4000 V | Scan Width: 2.0 u |
| Capillary Temperature: 370 °C | Scan Time: 0.201 s |
| Vaporizer Temperature: 375°C | Q1/Q3 Peak Width: 0.7 u |
| Sheath Gas (N2): 40 units | Collision Energy: 50 eV |
| Auxiliary Gas (N2): 10 units | Polarity: Positive |
In addition to screening for PMA, chloroform extracts for a subset of thirty-five cyanobacterial and green algal cultures (28 and 7, respectively) were independently evaluated by the zebrafish assay for teratogenicity, as described above (see 2.2. Zebrafish embryo teratogenicity assay). Of the strains evaluated for teratogenicity, 27 were also evaluated (as described above) for PMA (see Table S1 and S2 of Supplementary Data). A Fisher’s Exact Test and logistic regression were subsequently used to determine a relationship between presence of PMA and teratogenicity (n=27).
3. Results
3.1. Teratogenicity of C. raciborskii extracts and fractions, and purification of 1-4
Chloroform extracts, and initial chromatographic fractions, from C. raciborskii were evaluated for teratogenicity in the zebrafish embryo model. Embryos exposed to crude extracts, and initial fractions, were consistently characterized by developmental dysfunctions such as bent body axis, pooling of blood in the pericardium and delayed development and hatching observed between 4-6 dpf (Fig. 2) which was notably consistent with that previously observed for the strain (Berry et al., 2009), and moreover, specifically similar to the teratogenicity observed for PMA isolated from other cyanobacteria (Jaja-Chimedza et al., 2012a). Furthermore, initial chemical characterization (by LC-MS) of the bioactive fractions, likewise, indicated the presence of PMA as major components in these fractions (see below).
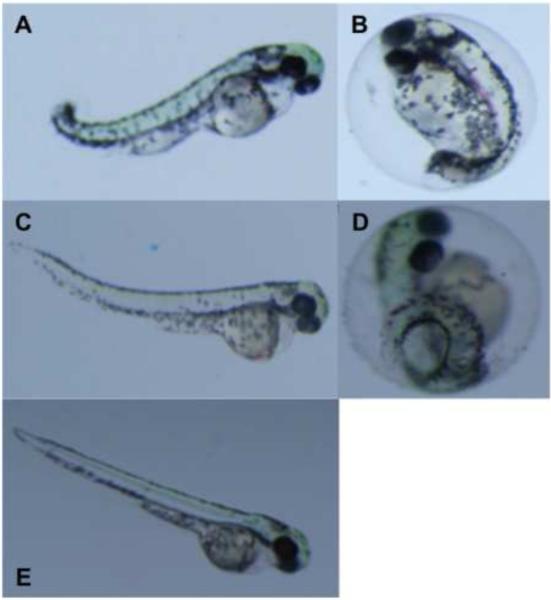
Fig. 2.
Teratogenicity of chemical fractions from C. raciborskii containing a mixture 1-4, in the zebrafish embryo model. Shown, as representatives of development dysfunction, are observed effects in embryos exposed to 50 μg mL−1 of the 100% ethyl acetate fraction including severely bent body axis and edema (A), and delayed hatching (B), at 6dpf. In addition, embryos exposed to a lower concentration, i.e. 20 μg mL−1, of the fraction, likewise, showed less pronounced effects including bent body axis (D) and similarly delayed hatching (E) at 6 dpf. For comparison, 6 dpf control (untreated) embryos are shown (F).
Accordingly, compounds 1-4 were isolated using a two-step method previously developed for isolation of PMA from cyanobacteria (Jaja-Chimedza et al., 2012a). Specifically, HPLC separation (coupled to mass spectrometry) identified an apparent series of six chemically related compounds (Fig. S1 of Supplementary Data). Of these, the four most abundant congeners were purified in sufficient quantities, i.e. 57µg (1), 707µg (2), 768µg (3) and 202µg (4), for subsequent chemical characterization. Initial chemical analysis of the compounds using a low-resolution MS (i.e. HPLC-ESI-MS) indicated nominal molecular masses (of the protonated molecular ions, i.e. [M+H]+) of 563 (1), 621 (2), 679 (3) and 737 (4), in addition to the corresponding [M+Na]+ and [M+K]+ adducts of M+23 and M+40, respectively, for each. Notably, the mass difference of 58 amu observed for each of the sequentially less polar congeners is consistent with PMA (Banker et al., 2000; Jaja-Chimedza et al., 2012a; see below), specifically differing by the number of methoxylated subunits (−CH2CH[OCH3]−).
3.2. Chemical characterization of 1-4
All four compounds (1-4) obtained from the purification were white amorphous solids. HRMS data for all four compounds were obtained, i.e. [M+H]+ 563.4488 (1), 621.4900 (2), 679.5312 (3), and 737.5733 (4), and suggested molecular formulae of the molecules as C31H62O8 (1), C34H68O9 (2), C37H74O10 (3) and C40H80O11 (4), respectively, which were further confirmed by NMR analyses (see Supplemental Information). Fragmentation of the molecular ion of each of the compounds showed sequential losses of 32 amu, corresponding to the loss of CH3OH, as generally indicative of a polymethoxylated structure, and specifically correlating with the number of proposed methoxy groups associated with each compound. Likewise, as discussed above, MS data also showed that the masses of 1-4 differed sequentially by 58 amu, corresponding to the number of methoxylated, i.e. −CH2CH(OCH3)−, subunits, and similarly supported the identity of these molecules as PMA. Moreover, these molecular formulae, and fragmentation patterns, are consistent with those observed for PMA previously isolated from other cyanobacteria (Mynderse and Moore, 1979; Mori et al., 1991a and 1991b; Banker et al., 2000; Jaja-Chimedza et al., 2012a).
The NMR data for 1-4, including chemical shifts, and associated splitting patterns, as well as coupling constants and hetero- and homonuclear correlations, were, indeed, similar to those observed for the PMA previously isolated from the other strains of cyanobacteria (Mynderse and Moore, 1979; Mori et al., 1991a and 1991b; Banker et al., 2000; Jaja-Chimedza et al., 2012a). Accordingly, NMR and MS data enabled, by comparison to the published data, the identification of 1-4 as previously characterized PMA. Specifically, 1-4 were thus identified, respectively, as 4,6,8,10,12,14,16,18-octamethoxy-1-tricosene, 4,6,8,10,12,14,16,18,20- nonamethoxy-1-pentacosene, 4,6,8,10,12,14,16,18,20,22-decamethoxy-1-heptacosene and 4,6,8,10,12,14,16,18,20,22,24-undecamethoxy-1-nonacosene previously isolated from Tolypothrix sp. (Mynderse and Moore et al., 1979), Scytonema spp. (Mori et al., 1991a and 1991b) and more recently A. ovalisporum (Banker et al., 2000; Jaja-Chimedza et al., 2012a). Further details of the chemical characterization, and particularly NMR, are given in the Supplementary Data.
3.3. Screening of cyanobacterial and green algal cultures for PMA
Following the identification of 1-4 as teratogenic metabolites from C. raciborskii, a culture collection of freshwater cyanobacteria and green algae was screened for presence of 1-4, as the most common variants observed among cyanobacteria (see Discussion, below), in order to assess taxonomic distribution of these metabolites. Of the 97 isolates, extracts from cultures of 10 were confirmed to contain one or more of these previously described PMA (Table 1). Specifically, PMA were detected in the 40% ethyl acetate SPE fraction (see 2.6 Screening of cyanobacterial and green algal cultures for PMA) of isolates including, as expected, the previously characterized reference strains (i.e. C. raciborskii AQS, A. ovalisporum) used as positive controls. In addition to C. raciborskii AQS and A. ovalisporum APH (previously shown to produce PMA), cyanobacterial isolates identified to produce PMA were Anabaena 66-2 (2), Microcystis 81-11 (2), Nostoc 23-2 (1-4), and Pseudanabaena 108-1 (1-2). Interestingly, of the 10 algal isolates identified as apparent producers of PMA, taxonomically divergent production was observed for 4 strains of eukaryotic green algae. Green algae isolates from which PMA were identified specifically included Pediastrum 104-6 (1), and Scenedesmus 3-4 (1-4), 79-1 (1-2) and 80-15 (1-2).
Table 1.
Isolates of cyanobacteria and green algae for which PMAs 1-4 were identified by LC-MS screening. See Table S1 of Supplementary Data for list of all isolates screened. Indicated with “X” for each isolate is the PMA identified.
| PMA | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Cyanobacteria | |||||
| C. raciborskii AQSa | X | X | X | X | |
| A. ovalisporum APHb | X | X | X | X | |
| Anabaena 66-2 | X | ||||
| Microcystis 81-11 | X | ||||
| Nostoc 23-2 | X | X | X | X | |
| Pseudanabaena 108-1 | X | X | |||
| Green Algae | |||||
| Pediastrum 104-6 | X | ||||
| Scenedesmus 3-4 | X | X | X | X | |
| Scenedesmus 79-1 | X | X | |||
| Scenedesmus 80-15 | X | X |
Present study.
Previously characterized in Jaja-Chimedza et al., 2012a
To assess the contribution of PMA to the teratogenicity of cyanobacterial and green algal cultures, a subset of isolates (see Table S2 of Supplementary Data) was independently evaluated using the zebrafish embryo teratogenicity assay. Of the 10 isolates (out of 35 tested) that were teratogenic, 8 were also screened for PMA, and of these 6 were, in fact, also positive for one or more variant (Table 1). Conversely, of the 10 isolates (out of 97) that were positive for presence of PMA (Table 1), seven were also evaluated in the bioassay, and of these 6 were teratogenic. Comparison of teratogenicity and PMA screening data (Fisher’s Exact Test) confirmed a statistically significant (p=0.001) relationship between the presence of the compound and biological activity with an odds ratio of 54 in logistic regression analyses.
4. Discussion
In the present study, the zebrafish embryo was used, as a model for vertebrate development, to identify, isolate and subsequently characterize previously reported (Berry et al., 2009) teratogenic metabolites from C. raciborskii, and specifically identified a series of PMA variants (1-4). These same variants (n = 8, 9, and 10, see Fig. 1) were, in fact, first isolated based on their unusual structure from field collections of a terrestrial tolytoxin-producing cyanobacterium, Tolypothrix (Mynderse & Moore, 1979). More than a decade later, these and several additional variants, were isolated from another tolytoxin-producing cyanobacterium, Scytonema burmanicum, as well as S. mirabile (Mori et al., 1991a) and S. ocellatum (Mori et al., 1991b). Another decade later, similar PMA variants (n = 9, 10, and 11) were identified in a cultured CYN-producing strain of A. ovalisporum, isolated from Lake Kinneret, Israel; initially these PMA variants were isolated based solely on their structure, and showed no indications of toxicity in cell-based assays (Banker et al., 2000). Most recently, the zebrafish embryo model was used for bioassay-guided fraction enabling the isolation of six PMA congeners from A. ovalisporum, including a novel variant (n =7), and moreover, demonstrating that these compounds are, indeed, bioactive (Jaja-Chimedza et al., 2012a), and specifically teratogenic. Notably, in addition to their identification from cyanobacteria, putatively biotransformed metabolites of PMA have been identified in a marine sponge, and attributed to cyanobacterial sources (Rao and Faulkner, 2002). Adding to this growing knowledge of these compounds in the environment, therefore, the present study is the first identification of PMA as teratogenic metabolites from C. raciborskii as widespread freshwater cyanobacterial HAB species. And, as such, supports a possible contribution of the PMA to the otherwise recognized toxigenicity (i.e. CYN- and STX-production) of this species.
As discussed above, PMA have, in fact, been previously isolated from several cyanobacterial genera, notably all within the Nostocales, including Tolypothrix (Mynderse and Moore, 1979), Scytonema (Mori et al., 1991a and 1991b) and, most recently, Aphanzomenon (Jaja-Chimedza et al., 2012). In order to further evaluate the phylogenetic distribution of the PMA among cyanobacteria, a culture collection of freshwater cyanobacteria and green-algae was screened, specifically by directed LC-MS analysis, for known PMA variants. Of the 97 algal strains screened, extracts from 10 of the cultures were found to contain PMA, and notably included both cyanobacterial and green algal isolates. The majority of the cyanobacteria (4 out of 6 genera) found in the present study, including C. raciborskii, belong to the order Nostocales (Table 1). However, two isolates, namely Pseudoanabaena 108-1 (Oscillatoriales) and Microcystis 81-11 (Chroococcales), are members of phylogenetically distinct orders. Moreover, the identification of PMA from eukaryotic green algae clearly suggests that these metabolites are not, in fact, phylogenetically restricted to the cyanobacteria. The identification of PMA in taxonomically disparate isolates of cyanobacteria and green algae, combined with established teratogenicity of these metabolites (Jaja-Chimedza et al., 2012a), therefore, suggests that these metabolites warrant consideration with respect to their possible role in the toxicity of phylogenetically diverse freshwater HAB species.
Alongside evaluation of PMA, teratogenicity of an independent sample of cyanobacterial and green algal cultures was evaluated. Notably, a significant relationship between presence of PMA and teratogenicity was observed. Indeed, 6 of 7 isolates positive for one or more PMA, and also evaluated by bioassay, were teratogenic. The only exception was a single green algal isolate (Pediastrum 104-6). This isolate was, however, found to produce only one apparent PMA variant. Moreover, the PMA concentrations were not evaluated for the strains, rather only presence or absence, and quantitative differences may explain this discrepancy. Likewise, of the isolates that were teratogenic, and also screened for PMA (8 of 10), six were both active and PMA-positive. This, generally speaking, suggests that other presence of other lipophilic bioactive metabolites in these isolates. Most notable perhaps is the teratogenicity of a C. raciborskii isolate (121-1) which was not found to produce PMA (see Table S2 of Supplementary Data) as does C. raciborskii AQS, but was teratogenic; in this case, recent studies have, in fact, characterized the contribution of additional lipophilic metabolites (Jaja- Chimedza et al., forthcoming). That said, the presence of PMA in the cyanobacterial and green algal culture collection does, indeed, explain the majority of the teratogenicity observed among these isolates, and likely contributes, there, significantly to the potential developmental toxicity of these species/strains.
5. Conclusions
The present study utilized the zebrafish embryo as a toxicological model to purify, and subsequently to characterize chemically, a homologous series (1-4) of toxic PMA congeners from C. raciborskii as a widely recognized toxigenic cyanobacterial HAB species. These teratogenic compounds may contribute – along with recognized toxins (e.g. CYN, STX) - to the previously observed toxicity of this species (Saker et al., 2003; Nogueira et al., 2006; Berry et al., 2009; Acs et al., 2013; Jonas et al., 2014). Moreover, prior isolation of PMA from diverse genera of cyanobacteria, and the identification of additional cyanobacterial and green algal cultures in the present study, more generally suggests that these metabolites are produced by a taxonomically diverse range of microalgae, and may, therefore, broadly contribute to the toxicity of freshwater algal blooms. The significant correlation between presence of PMA and teratogenicity among freshwater algal (i.e. cyanobacterial, green algal) cultures suggests that these compounds contribute significantly to the toxicity of these isolates. As such, further investigation of these metabolites in relation to blooms and eutrophication of freshwater systems, particularly with respect to possible disruptive effects of algal blooms on ecosystems (e.g. impacts on aquatic vertebrate populations), and perhaps human health impacts, is warranted.
Supplementary Material
Highlights.
Teratogenic polymethoxy-1-alkenes (PMAs) were isolated from C. raciborskii
PMAs were found in several taxonomically diverse cyanobacteria and green algae
Teratogenic PMAs contribute significantly to the toxicity of screened algal cultures
Teratogenic PMAs likely contribute toxicity of algal blooms
Acknowledgments
Support for this research was provided, in part, by ARCH (ES11181) and R21 (ES014 037) grants from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH), and an Oceans and Human Health Initiative grant from the National Oceanic and Atmospheric Administration (NA09NOS4730071). Cultures of C. raciborskii AQS investigated in the study were kindly provided by Dr. Martin Saker and Cristiana Moreira (Centro de Investigacão Marinhae Ambiental, University of Porto, Portugal). The authors would like to thank Dr. Li Liu and Dr. Kathleen Rein (FIU) for assistance with interpretation of inversegated decoupled 13C-NMR studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acs A, Kovacs WW, Csepregi JZ, Töro N, Kiss G, Gyori J, Vehovszky A, Kovats N, Farkas A. The ecotoxicological evaluation of Cylindrospermopsis raciborskiifrom Lake Balaton (Hungary) employing a battery of bioassays and chemical screening. Toxicon. 2013;70:98–106. doi: 10.1016/j.toxicon.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Banker R, Teltsch B, Sukenik A, Carmeli S. 7-epicylindrospermopsin , a toxic minor metabolite of the cyanobacterium Aphanizomenon ovalisporumfrom Lake Kinneret, Israel. J. Nat. Prod. 2000;63:387–389. doi: 10.1021/np990498m. [DOI] [PubMed] [Google Scholar]
- Berry JP, Gantar M, Gibbs PDL, Schmale MC. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;145:61–72. doi: 10.1016/j.cbpc.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JP, Gibbs PDL, Schmale MC, Saker ML. Toxicity of cylindrospermopsin, and other apparent metabolites from Cylindrospermopsis raciborskiiand Aphanizomenon ovalisporum, to the zebrafish (Danio rerio) embryo. Toxicon. 2009;53:289–299. doi: 10.1016/j.toxicon.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne DlL, Hemscheidt TK, Moore RE, Runnegar MT. Biosynthesis of cylindrospermopsin. J. Org. Chem. 2000;65:152–156. doi: 10.1021/jo991257m. [DOI] [PubMed] [Google Scholar]
- Chorus I, Bartram J. Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Monitoring and Management E. & F. N. Spon Press; London: 1999. [Google Scholar]
- El Ghazali I, Saqrane S, Carvalho AP, Ouahid Y, Oudra B, Del Campo FF, Vasconcelos V. Compensatory growth induced in zebrafish larvae after pre-exposure to a Microcystis aeruginosanatural bloom extract containing microcystins. Int. J. Mol. Sci. 2009;10:133–146. doi: 10.3390/ijms10010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantar M, Berry JP, Thomas S, Wang M, Perez R, Rein KS. Allelopathic activity among cyanobacteria and microalgae from Florida freshwater habitats. FEMS Microbiol. Ecol. 2008;64:55–64. doi: 10.1111/j.1574-6941.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja-Chimedza A, Gantar M, Gibbs PD, Schmale MC, Berry JP. Polymethoxy-1- alkenes from Aphanizomenon ovalisporum inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Mar. Drugs. 2012a;10:2322–2336. doi: 10.3390/md10102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja-Chimedza A, Gantar M, Mayer GD, Gibbs PD, Berry JP. Effects of cyanobacterial lipopolysaccharides from Microcystison glutathione-based detoxification pathways in the zebrafish (Danio rerio) embryo. Toxins. 2012b;4:390–404. doi: 10.3390/toxins4060390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A, Buranova V, Scholz S, Fetter E, Novakova K, Kohoutek J, Hilscherova K. Retinoid-like activity and teratogenic effects of cyanobacterial exudates. Aquatic Toxicol. 2014;155:283–290. doi: 10.1016/j.aquatox.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Lefebvre KA, Trainer VL, Scholz NL. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquatic Toxicol. 2004;66:159–170. doi: 10.1016/j.aquatox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Liu L, Bearden DW, Rodriguez JC, Rein KS. Biosynthesis of Athmu, a 1111- hydroxy-β-amino acid of pahayokolide A-B. Tetrahedron Lett. 2012;53:6758–6760. doi: 10.1016/j.tetlet.2012.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihali TK, Kellmann R, Muenchhoff J, Barrow KD, Neilan BA. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl. Environ. Microbiol. 2008;74:716–722. doi: 10.1128/AEM.01988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Kohchi Y, Suzuki M, Carmeli S, Moore RE, Patterson GM. Isotactic polymethoxy-1-alkenes from blue-green algae, synthesis and absolute stereochemistry. J. Org. Chem. 1991a;56:631–637. [Google Scholar]
- Mori Y, Kohchi Y, Noguchi H, Suzuki M, Carmeli S, Moore RE, Patterson GML. Isotactic polymethoxy-1-alkenes from the terrestrial blue-green alga Scytonema ocellatum, structure and synthesis. Tetrahedron. 1991b;47:4889–4904. [Google Scholar]
- Mynderse JS, Moore RE. Isototactic polymethoxy-1-alkenes from the blue-green alga Tolypothrix conglutinate var. Chlorata. Phytochemistry. 1979;18:1181–1183. [Google Scholar]
- Nogueira IC, Lobo-da-Cunha A, Vasconcelos VM. Effects of Cylindrospermopsis raciborskiiand Aphanizomenon ovalisporum ingestion on Daphnia magnamidgut and associated diverticula epithelium. Aquatic Toxicol. 2006;80:194–203. doi: 10.1016/j.aquatox.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Oberemm A, Fastner J, Steinberg CEW. Effects of microcystin-LR and cyanobacterial crude extracts on embryolarval development of zebrafish (Danio rerio) Water Res. 1997;31:2918–2921. [Google Scholar]
- Oberemm A, Becker J, Codd GA, Steinberg C. Effects of cyanobacterial toxins and aqueous crude extracts of cyanobacteria on the development of fish and amphibians. Environ. Toxicol. 1999;14:77–88. [Google Scholar]
- Papendorf O, König GM, Wright AD, Chorus I, Oberemm A. Mueggelone, a novel inhibitor of fish development from the fresh water cyanobacterium Aphanizomenon ovalisporum. J. Nat. Prod. 1997;60:1298–1300. doi: 10.1021/np970231s. [DOI] [PubMed] [Google Scholar]
- Rao M, Faulkner DJ. Isotactic polymethoxydienes from the Philipines sponge Myriastra clavosa. J. Nat. Prod. 2002;65:1201–1203. doi: 10.1021/np020040b. [DOI] [PubMed] [Google Scholar]
- Rogers ED, Henry TB, Twiner MJ, Couffon JS, McPherson JT, Boyer GL, Sayler GS, Wilhelm SW. Global gene expression profiling in larval zebrafish exposed to microcystin-LR and Microcystis reveals endocrine disrupting effects of cyanobacteria. Environ. Sci. Technol. 2011;45:1962–1969. doi: 10.1021/es103538b. [DOI] [PubMed] [Google Scholar]
- Rzymski P, Poniedzialek B. In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers. Water Res. 2014;66C:320–337. doi: 10.1016/j.watres.2014.08.029. [DOI] [PubMed] [Google Scholar]
- Saker ML, Eaglesham GK. The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon. 1999;37:1065–1077. doi: 10.1016/s0041-0101(98)00240-2. [DOI] [PubMed] [Google Scholar]
- Saker ML, Nogueira ICG, Vasconcelos VM, Neilan BA, Eaglesham GK, Pereira P. First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters. Ecotoxicol. Environ. Saf. 2003;55:243–250. doi: 10.1016/s0147-6513(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Snyder R, Gibbs P, Palacios A, Abiy L, Dickey R, Lopez J, Rein K. Polyketide synthase genes from marine dinoflagellates. Mar. Biotech. 2003;5:1–12. doi: 10.1007/s10126-002-0077-y. [DOI] [PubMed] [Google Scholar]
- Staunton J, Weissman KJ. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- Walton K, Gantar M, Gibbs PDL, Schmale MC, Berry JP. Indole alkaloids from Fischerella inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Toxins. 2014;6:3568–3581. doi: 10.3390/toxins6123568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AD, Papendorf O, König GM, Oberemm A. Effects of cyanobacterium Fischerella ambigua isolates and cell free culture media on zebrafish (Danio rerio) embryo development. Chemosphere. 2006;65:604–608. doi: 10.1016/j.chemosphere.2006.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.