Abstract
Background & Aims
Diabetic gastroparesis is associated with changes in interstitial cells of Cajal (ICC), neurons, and smooth muscle cells in both animal models and humans. Macrophages appear to be critical to the development of cellular damage that leads to delayed gastric emptying (GE), but the mechanisms involved are not well understood. Csf1op/op (Op/Op) mice lack biologically active Csf1 (macrophage colony stimulating factor), resulting in the absence of Csf1-dependent tissue macrophages. We used Csf1op/op mice to determine the role of macrophages in the development of delayed GE.
Methods
Animals were injected with streptozotocin to make them diabetic. GE was determined weekly. Immunohistochemistry was used to identify macrophages and ICC networks in the gastric muscular layers. Oxidative stress was measured by serum malondialdehyde (MDA) levels. Quantitative reverse-transcription polymerase chain reaction was used to measure levels of mRNA.
Results
Csf1op/op mice had normal ICC. With onset of diabetes both Csf1op/op and wild-type Csf1+/+ mice developed increased levels of oxidative stress (75.8 ± 9.1 and 41.2 ± 13.6 nmol/mL MDA, respectively). Wild-type Csf1+/+ mice developed delayed GE after the onset of diabetes (4 of 13) whereas no diabetic Csf1op/op mouse developed delayed GE (0 of 15, P = .035). The ICC were disrupted in diabetic wild-type Csf1+/+ mice with delayed GE but remained normal in diabetic Csf1op/op mice.
Conclusions
Cellular injury and development of delayed GE in diabetes requires the presence of muscle layer macrophages. Targeting macrophages may be an effective therapeutic option to prevent cellular damage and development of delayed GE in diabetes.
Keywords: Diabetic Complications, Gastroparesis, Interstitial Cells of Cajal
Abbreviations used in this paper: ANOVA, analysis of variance; Csf1, macrophage colony stimulating factor; GE, gastric emptying; HO1, heme-oxygenase 1; ICC, interstitial cells of Cajal; MDA, malondialdehyde; NDS, normal donkey serum; NOD, nonobese diabetic; NS, not statistically significant; Op, osteopetrosis mutation; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; WT, wild type
Summary.
Diabetic Csf1op/op mice do not develop delayed gastric emptying but wild-type Csf1+/+ mice do. This result reinforces the concept that macrophages are necessary for the development of delayed gastric emptying.
Gastroparesis is a complication of diabetes defined by delayed emptying of stomach contents in the absence of obstruction and is frequently accompanied by early satiety, nausea, vomiting, and pain.1, 2, 3 Recent publications have highlighted the cellular changes associated with gastroparesis in humans4, 5 and in mouse models of the disease.6, 7 These include changes to interstitial cells of Cajal (ICC), extrinsic and enteric nerves and smooth muscle cells.8 The development of delayed gastric emptying (GE) is associated with reduced expression of the receptor tyrosine kinase, Kit, a marker for interstitial cells of Cajal,6 impairment of ICC networks and changes in the electrical slow wave recorded from smooth muscle cells.5, 9
Macrophages originate from monocytes, play a critical role in innate immunity and also participate in adaptive immunity.10, 11 Release of cytokines from polarized macrophages can both increase inflammation as well as play important anti-inflammatory roles.10 Resident macrophages are present in the muscle layers of the gastrointestinal tract.12, 13 They can be polarized in response to changes in the microenvironment. Polarized macrophages in mice may be classified in two main groups: the proinflammatory, classically activated macrophages (M1) and anti-inflammatory, alternatively activated macrophages (M2).11 Polarization results in a change in their phenotype and their physiology, including alterations in the expression of surface proteins and the production of specific cytokines.10, 11
Macrophages appear to play an important role in the development of delayed GE in both diabetic mice and humans. CD206 positive M2 macrophages expressing heme oxygenase 1 (HO1) are critical to the prevention of development of delayed GE in diabetic mice and induction of HO1 in macrophages leads to reversal of the delay in GE.6 In humans the number of CD206-positive M2 macrophages correlates with the number of ICC which in turn correlates with GE.14 The exact role of macrophages in the development of delayed GE during diabetes is still not known. For example, it remains unclear whether it is the presence of a particular subtype (ie, M1 macrophages) versus the absence of a subtype (ie, M2 macrophages) that leads to development of delay in GE.
Mice homozygous for the naturally occurring Csf1op (osteopetrosis, op) gene mutation lack functional macrophage-colony stimulating factor (Csf1, also known as MCSF or Csfm)15 and are deficient in Csf1-dependent macrophages. Because previous studies have shown the absence of macrophages from the muscle layers of the small intestine of Csf1op/op mice,12 they offer the opportunity to understand the role of macrophages in the development of GE in diabetes.
Materials and Methods
Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic.
Animals and in Vivo Experimentation
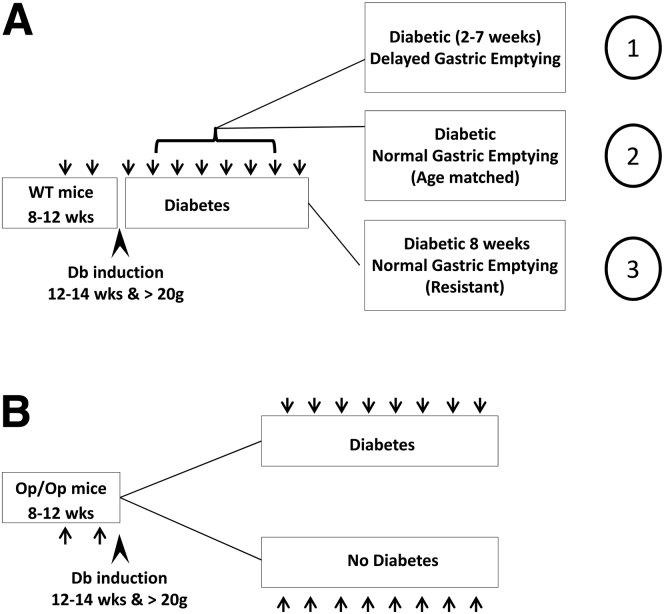
The experimental plan is summarized in Figure 1. Mice heterozygous for a loss of function mutation in the Csf1 gene were obtained from the Jackson Laboratory (Bar Harbor, ME; strain: B6; C3Fe a/a-Csf1op/J, cat. no. 000231). The osteopetrosis (Csf1op) mutation is a single base pair insertion in the coding region of the Csf1 gene that generates a stop codon 21 base pair downstream of the insertion.15 Detection of wild-type (WT) Csf1+/+ and heterozygous animals by Sanger sequencing of polymerase chain reaction (PCR) products was performed by Laragen (Culver City, CA). Homozygous Csf1op/op mice were distinguished from their normal littermates phenotypically at 10–15 days of age by the absence of incisor eruption. Due to the lack of teeth, Csf1op/op pups were started on a liquid diet at 3–4 weeks of age and were weaned from the mother at 5–6 weeks of age. These mice were maintained until they were up to 6 months old by the use of a specialized wet diet (Bio-Serv, Frenchtown, NJ).
Figure 1.
Experimental plan used for the different groups of mice. (A) Starting at 8 weeks old, female Csf1+/+ wild-type (WT) mice were trained, and two baseline gastric emptying (GE) readings (arrows) were obtained before the mice were treated with streptozotocin starting at 12 weeks (arrowhead). Only mice weighing more than 20 g were used. After development of diabetes, when the mice were 12–14 weeks old, GE was measured weekly for up to 8 weeks. Tissues were collected from three groups of Csf1+/+ mice: (1) diabetic mice that develop delayed GE indicated by t1/2 values outside the normal range on 2 consecutive weeks, (2) mice with normal GE matched to the mice with delayed GE by duration of diabetes, and (3) resistant mice that did not develop delayed GE after 8 weeks of diabetes. (B) Sibling Csf1op/op (Op/Op) mice underwent the same regimen. These mice did not develop delayed GE, and they were killed for tissue and blood collection after 8 weeks of diabetes (20–22 weeks of age). Csf1op/op (Op/Op) without diabetes were observed for the same period of time and used as controls (20–22 weeks of age).
WT Csf1+/+ and Csf1op/op mice were treated with a single injection of streptozotocin (160 mg/kg) at 12 weeks of age to make them diabetic. The mice that did not become diabetic after 1 week received up to two further injections of streptozotocin spaced a week apart. Using this regimen, all WT Csf1+/+ mice became diabetic (defined as blood glucose >250 mg/dL). Mice weighing less than 20 g at 12 weeks old were not used in this study. Starting 4 days after induction, blood glucose levels were measured daily. Subtherapeutic insulin (Lantus insulin glargine; Sanofi-Aventis U.S., Bridgewater, NJ) was injected once a day intraperitoneally when the glucose levels were over 500 mg/dL to keep the diabetic mice alive and but to still keep blood glucose levels between 400 and 600 mg/dL in order for complications of diabetes to develop. Oxidative stress was measured by determining malondialdehyde (MDA) levels in the plasma as previously described elsewhere6 using a commercial kit to detect thiobarbituric acid reactive substances (Oxi-Tek; Zeptometrix, Buffalo, NY).
Gastric emptying of solids (cooked egg yolk) was measured after an overnight fast using a 13[C]-octanoic acid breath test as described previously elsewhere.16 Csf1op/op mice required more training than WT Csf1+/+ mice due to their lack of teeth. However, by preparing the egg meal to be a soft consistency, the majority of animals could be trained to eat it within 5 minutes of being offered the meal. Because Csf1op/op mice do not have teeth, they were fed with less scrambled cooked egg yolk (100 mg) than used for the Csf1+/+ mice (200 mg). If the mice did not eat >50% of the egg meal after 3 weeks of training, they were removed from the study. Four baseline values for GE half-time (t1/2) were obtained before the delivery of streptozotocin. After the onset of diabetes, GE was measured weekly.
Dissection of Tissue
The mice were killed with an overdose of carbon dioxide. The stomach was cut along the lesser curvature, and the mucosal layer was removed by sharp dissection from the smooth muscle layer of the body and antrum for experiments.
Immunohistochemistry
The antibodies used were a rat monoclonal raised against the mouse F4/80 epitope conjugated to Alexa Fluor 488 (cat. no. MF48020; Life Technologies, Grand Island, NY), and a rat monoclonal raised against mouse Kit, ACK2 (cat. no. 14-1172-85; eBioscience, San Diego, CA). The immunohistochemical procedures used for each cell type are summarized herein.
Macrophages
Macrophages were identified using a modification of a previously published fluorescence immunolabeling technique.7 Freshly dissected tissues were incubated in F4/80 antibody Alexa Fluor 488 conjugate diluted to 0.4 μg/mL with Ca2+-containing HEPES-buffered physiologic salt solution containing 135 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 10 mM glucose, and 10 mM HEPES, adjusted to pH 7.4 with Tris and incubated at 37°C for 1 hour in the dark. The tissue was then fixed in 4% paraformaldehyde in 0.1 M phosphate buffer. After the tissue was rinsed in 0.1 M phosphate-buffered saline (PBS) then water, the nuclei in the tissue were stained by incubating the tissue for 30 minutes at 4°C with 0.3 μM 4′6-diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes, Eugene, OR) in water.
Interstitial Cells of Cajal
After fixation by immersion in cold acetone for 15 minutes, ICC were detected by use of an anti-Kit antibody (ACK2). The tissue was washed three times in 0.1 M PBS then blocked in 10% normal donkey serum (NDS) in PBS and 0.3% Triton overnight at 4°C. The Kit antibody (1.7 μg/mL) was applied by incubation in 5% NDS/PBS/0.3% Triton for 2 days at 4°C. After rinsing in PBS, donkey anti-rat IgG conjugated to fluorescein (3 μg/mL; Chemicon, Temecula, CA) was added in 2.5% NDS/PBS/0.3% Triton and incubated overnight at 4°C in the dark. After rinsing in PBS then water, nuclei in the tissue were stained by incubating the tissue for 30 minutes at 4°C with 0.3 μM 4′6-diamidino-2-phenylindole dihydrochloride (DAPI) in water.
Confocal Microscopy for Kit-Positive Interstitial Cells of Cajal and Assessment and Quantification of Kit Immunoreactivity
Labeled tissues were examined with a laser scanning confocal microscope using a 20× (NA 0.95) XLUMPlanFl objective (Olympus Japan) in Fluoview (Olympus Japan) using the optimal confocal aperture to give a resolution of 0.994 × 0.994 × 1.13 μm (X × Y × Z). Stacks of confocal images across the full thickness of the muscularis propria were collected from three nondiabetic Csf1op/op mice (20–22 weeks old), three diabetic Csf1op/op mice (20–22 weeks old), three nondiabetic Csf1+/+ mice (20–22 weeks old), and three diabetic Csf1+/+ with normal GE and 3 diabetic Csf1+/+ with delayed GE mice (2, 3, and 4.6 weeks after onset of diabetes). Six images from the gastric body and three from the antrum were taken for each animal. For quantification of the labeling, all of the confocal image stacks were flattened into projections using the FV10-ASW Viewer (Olympus). The flattened images were renumbered in random order and then assessed by scoring the integrity of the ICC on a 10-cm visual analogue scale for network density. Scoring was done in random order by two independent investigators blind to the image and tissue source.
Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction
RNA extracted from gastric muscularis propria of Csf1op/op and wild-type Csf1+/+ mice were used for this real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR). RNA extraction was done with RecoverAll (Ambion, Austin, TX) according to the manufacturer’s instructions. The SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) was used to generate cDNA from extracted RNA and qRT-PCR was performed on cDNA using commercial primer sets and RT2SYBR Green/ROX qRT-PCR master mix according to the manufacturer's instructions (SABiosciences, Frederick, MD). The data were normalized to the expression of actin, a housekeeping gene, by transforming the difference in threshold cycle (ΔCt) to the ratio of the gene of interest to the housekeeping gene (2−ΔCt),17 and expressed as the mean ± standard error of the mean (SEM) for six animals of each group.
Statistical Analysis
The statistical methods used were unpaired t tests and one-way analysis of variance (ANOVA) with post-test adjustments for multiple comparisons. P < .05 was considered statistically significant. All authors had access to the study data and reviewed and approved the final manuscript.
Results
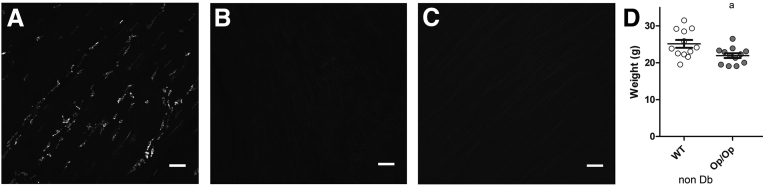
The gastric muscularis propria of WT Csf1+/+ mice was densely populated with macrophages as detected by F4/80 immunoreactivity (Figure 2A). These cells had a characteristic morphology with several flattened pseudopodia that branched from the main axis of the cell. However, no F4/80-positive macrophages were found in the muscle layers from the stomach of Csf1op/op mice before or after the development of diabetes (see Figure 2B and C). Before the development of diabetes, the Csf1op/op mice were smaller in size when compared with the WT Csf1+/+ mice (see Figure 2D; WT 25.11 ± 3.72, Op/Op 21.92 ± 2.23 g; P < .05, unpaired t test).
Figure 2.
Csf1op/op mice lack macrophages in the gastric muscularis propria. (A) Resident macrophages in the muscularis propria of wild-type (WT) Csf1+/+ mice. Whole-mount preparations from the murine stomach were labeled with the F4/80 antibody. Csf1op/op mice lack macrophages in the gastric muscularis propria when (B) not diabetic and (C) 8 weeks after the development of diabetes. Scale bar: 50 μm for each image. Age of mice: 20–22 weeks. (D) Weight of WT Csf1+/+ and Csf1op/op (Op/Op) mice before the development of diabetes. The circles represent individual animals. Csf1+/+ (WT) were heavier than Csf1op/op (Op/Op) at the beginning of the experiment. Data are represented as mean ± standard error of the mean. aP < .05, unpaired t test. Age of mice: 12–14 weeks.
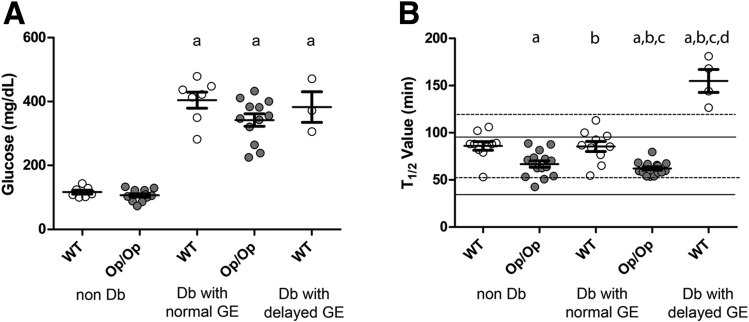
The blood glucose values in nondiabetic mice did not differ between the groups (Figure 3A; WT 117 ± 16, Op/Op 106 ± 18 mg/dL; P = not statistically significant [NS], one-way ANOVA with Tukey’s post-test). After treatment with streptozotocin to induce diabetes, the diabetic mice had similar elevated nonfasting blood glucose levels regardless of whether they were WT Csf1+/+ or Csf1op/op (see Figure 3A; WT 410.89 ± 62.79, Op/Op 342.15 ± 67.94; P = NS, one-way ANOVA with Tukey’s post-test). Both groups of mice received similar levels of insulin to maintain blood glucose <600 mg/dL after the onset of diabetes (WT 3.98 ± 0.57, Op/Op 5.77 ± 1.55 unit/week; P = NS, t test).
Figure 3.
Glucose levels and gastric emptying status in wild-type (WT) Csf1+/+and Csf1op/op(Op/Op) mice before and after induction of diabetes (Db). Data are represented as mean ± standard error of the mean (SEM) of the grouped data (P < .05, one-way ANOVA with Tukey’s post test). The circles represent individual animals. (A) As expected, glucose levels were statistically significantly higher in diabetic versus nondiabetic mice, P < .05, n = 5 mice/group. (B) Gastric emptying values in the same five groups of mice. The normal ranges for GE times (2.5th to 97.5th percentiles) in nondiabetic mice are shown for WT mice as dotted lines and for Op/Op mice as solid lines. Data are represented as mean ± SEM of the grouped data (P < .001, one-way ANOVA), and circles represent individual animals. Statistical significance (P < .05, Tukey’s post test) is indicated by (a) versus nondiabetic WT, (b) versus nondiabetic Op/Op, (c) versus diabetic WT with normal emptying, and (d) versus diabetic Op/Op. Age of mice: 12–14 weeks at the induction of diabetes, 20–22 weeks for diabetic mice with normal GE, 16–22 weeks for diabetic WT mice with delayed GE.
Normal GE values for Csf1op/op mice had not been previously reported, so we determined the normal ranges for GE times in Csf1op/op and WT Csf1+/+ mice in separate cohorts. The normal range was defined as between the 2.5th and 97.5th percentiles (see Figure 3B; WT dashed lines, 53–119 minutes, median 80 minutes, n = 9; Csf1op/op solid lines, 34–96 minutes, median 63, n = 15). In the nondiabetic mice in this study, the t1/2 values (mean ± SEM) for GE were statistically significantly lower in Csf1op/op mice compared with WT (see Figure 3B; WT 86 ± 4.5 minutes, n = 10; Op/Op 66.77 ± 5.37 minutes, n = 15; P < .05, one-way ANOVA with Tukey’s post-test). Despite severe diabetes, in Csf1op/op mice the GE values remained in the normal range for up to 8 weeks after induction of diabetes and were not statistically significantly different from the values obtained before diabetes (see Figure 3B, op/op nondiabetic 66.77 ± 3.42; op/op diabetic: 62.12 ± 1.82 minutes, P = NS, one-way ANOVA with Tukey’s post-test). In distinct contrast, 4 out of 13 WT mice developed delayed GE at a mean of 6.25 ± 2.5 weeks after induction of diabetes as indicated by readings outside the normal range (dashed lines) for WT nondiabetic mice (see Figure 3B). This was statistically significantly different from the observation that no Csf1op/op mice had delayed GE over the same period (P = .03, Fisher’s exact test), similar to the percentage of diabetic, nonobese diabetic (NOD) mice that developed delayed GE in our previous studies.6 Diabetic WT Csf1+/+ mice with delayed GE had longer t1/2 values than the nondelayed diabetic WT Csf1+/+, nondiabetic WT Csf1+/+, and both diabetic and nondiabetic Csf1op/op mice (see Figure 3B, WT diabetic with delayed GE; 154.9 ± 12.17 minutes; P < .05, one-way ANOVA with Tukey’s post-test). These data are consistent with an absence of macrophages resulting in resistance to development of delayed GE in the cohort studied.
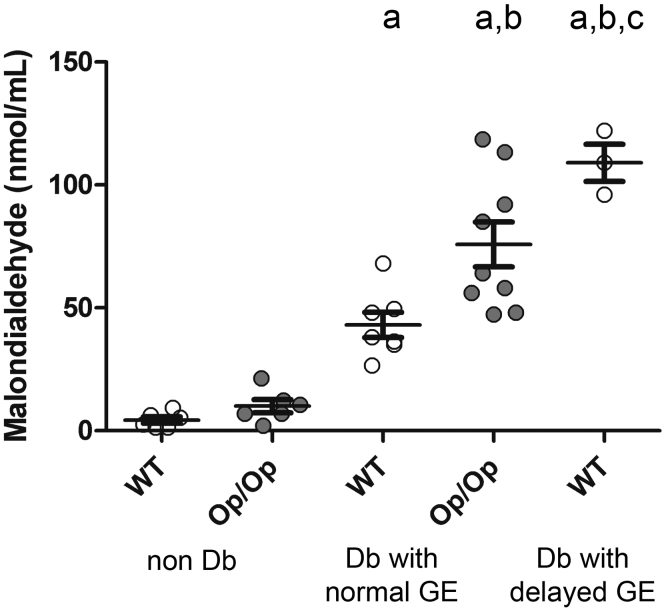
Previous work using NOD mice showed that oxidative stress is elevated in diabetic NOD mice and further elevated in diabetic NOD mice with delayed GE.6 MDA levels greater than 80 nmol/mL were invariably associated with delayed GE.6 In nondiabetic mice the levels of oxidative stress as measured by plasma MDA levels (mean ± SEM) were not different between Csf1op/op mice and WT Csf1+/+ littermates (Figure 4; WT nondiabetic 4.34 ± 1.30, Op/Op nondiabetic 10.03 ± 5.11 nmol/mL; n = 5, P = NS, one-way ANOVA with Tukey’s post-test). After the onset of diabetes MDA levels were statistically significantly elevated in the Csf1op/op compared with the Csf1+/+ littermates (see Figure 4; Op/Op diabetic with normal GE 75.8 ± 9.1 nmol/mL, WT diabetic with normal GE 41.2 ± 13.6; n = 5, P < .05, one-way ANOVA with Tukey’s post-test). As previously reported for NOD mice, development of delayed GE in WT, Csf1+/+ was accompanied by increased levels of MDA (see Figure 4; WT diabetic with delayed GE 102.5 ± 9.19 nmol/mL; n = 5, P < .05, one-way ANOVA with Tukey’s post-test). Despite diabetic Csf1op/op mice all having normal GE, the MDA levels were as high as those found in diabetic WT Csf1+/+ mice with delayed GE (see Figure 4; n = 5, P = NS, one-way ANOVA with Tukey’s post-test).
Figure 4.
Malondialdehyde levels were significantly higher in diabetic Csf1op/op(Op/Op) mice compared with diabetic wild-type (WT) Csf1+/+mice with normal gastric emptying (GE). Data are expressed as mean ± standard error of the mean (SEM) (n = 5 groups of mice). Malondialdehyde levels were significantly higher in WT with delayed GE (one-way analysis of variance with Tukey’s post test). Data are represented as mean ± SEM of the grouped data, and circles represent individual animals. Significance (P < .05) is indicated by (a) versus nondiabetic WT, (b) versus nondiabetic Op/Op, and (c) versus diabetic WT with normal GE (n = 5). Age of mice: 20–22 weeks.
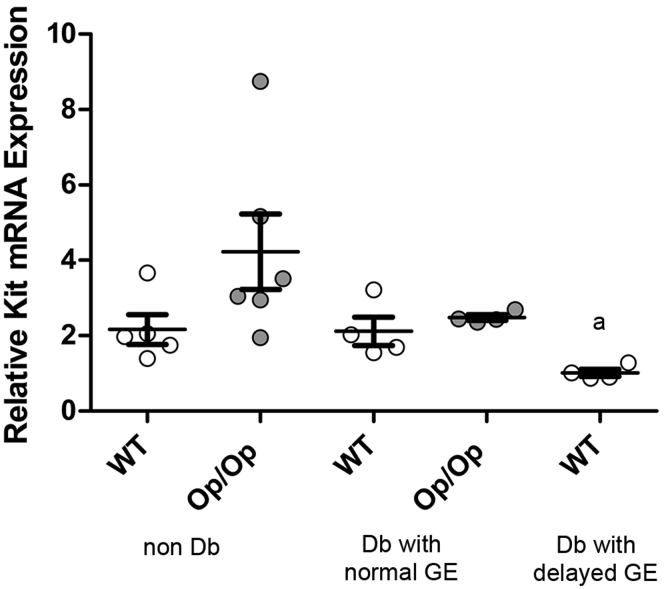
Reduced Kit expression and impairment of ICC networks in gastric muscularis propria are associated with development of delayed GE and impairment of ICC networks in diabetic mice.6 We measured the Kit mRNA levels by qRT-PCR in gastric muscularis propria (Figure 5). Although there was statistically significant variation in the levels of Kit mRNA from the tissues, there was no statistically significant difference in the Kit mRNA levels between Csf1op/op and WT mice with normal GE whether diabetic or not diabetic (see Figure 5; WT nondiabetic 2.16 ± 0.87, Op/Op nondiabetic 4.23 ± 2.45 fold change over actin; n = 5, P = NS, one-way ANOVA with Tukey post-test). Diabetic WT Csf1+/+ mice with delayed GE had statistically significantly lower expression of Kit mRNA compared with diabetic WT Csf1+/+ mice with normal GE (P = .03, t test), but this was not statistically significant for multiple comparisons (see Figure 5; WT diabetic 2.12 ± 0.76; WT diabetic with delayed GE 1.01 ± 0.19, fold change; n = 5, P = NS, one-way ANOVA with Tukey post-test).
Figure 5.
Csf1op/op(Op/Op) and wild type (WT) Csf1+/+mice express similar levels of Kit mRNA. Relative Kit expression was statistically significantly lower in diabetic WT Csf1+/+ mice with delayed gastric emptying (GE) compared with diabetic WT Csf1+/+ mice with normal GE (aP = .03, t test), but there were no statistically significant differences when correcting for multiple comparisons (one-way analysis of variance, P = NS). Data are mean ± standard error of the mean; n = 5 mice. Age of mice: 20–22 weeks.
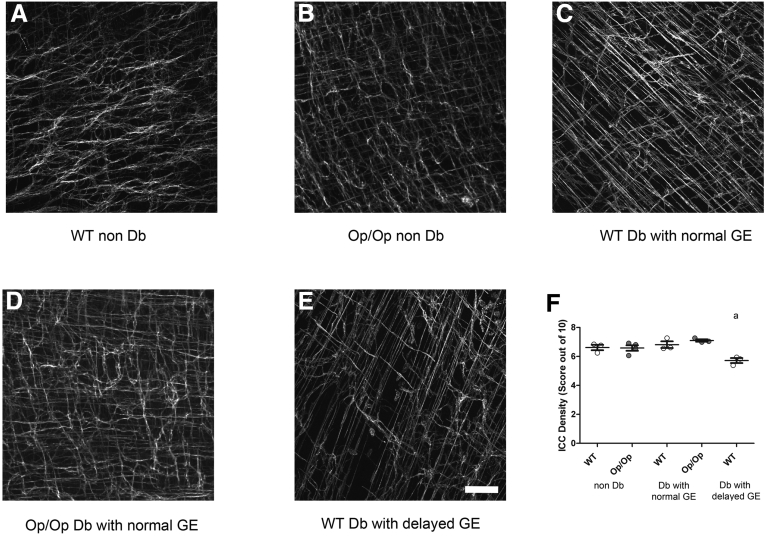
Damage to ICC networks is a common observation in mice and humans with delayed GE.5, 6, 9 To determine whether diabetic Csf1op/op mice had disrupted ICC networks even though GE was normal, we immunolabeled tissues for Kit and examined the ICC networks. There was no difference in density of Kit-positive ICC between gastric muscularis propria tissues from nondiabetic Csf1op/op mice (Figure 6B) and tissues from Csf1op/op mice that had been diabetic for more than 8 weeks (see Figure 6D), as scored by two independent, blinded investigators (see Figure 6F; Op/Op nondiabetic 6.06 ± 0.37, Op/Op diabetic 6.98 ± 0.14 density; n = 5, P = NS, one-way ANOVA with Dunnett’s post-test). No differences were also observed in ICC density between the nondiabetic Csf1op/op mice (see Figure 6B) and the nondiabetic WT (see Figure 6A) Csf1+/+ (see Figure 6F; WT nondiabetic 6.61 ± 0.32; n = 5, P = NS, one-way ANOVA with Dunnett’s post-test). However diabetic Csf1+/+ mice that developed delayed GE (see Figure 6E) had reduced ICC density when compared with the diabetic Csf1+/+ with normal GE (see Figure 6D and F; WT diabetic with delayed GE 5.71 ± 0.17, WT diabetic with normal GE 6.81 ± 0.04 density; n = 5, P < .005, one-way ANOVA with Dunnett’s post-test).
Figure 6.
Interstitial cells of Cajal (ICC) networks were not affected by diabetes in Csf1op/opmice while the development of delayed gastric emptying (GE) in diabetic Csf1+/+was associated with reduced density of ICC. Images of Kit immunoreactivity in the muscularis propria of the distal corpus of the mouse stomach in nondiabetic Csf1+/+ (A) wild type (WT) and Csf1op/op (B) Op/Op; in diabetic Csf1+/+ (C) and Csf1op/op (D) with normal GE; and in diabetic Csf1+/+ with delayed GE (E) (age-matched/representative of n = 3). Scale bar: 100 μm for each image. Whiskers show the mean ± standard deviation of the scores from biological replicates (n = 3 mice; P < .05, one-way analysis of variance with Dunnett’s post test). Each circle represents the overall score from nine regions in each individual animal. Age of mice: 20–22 weeks for diabetic and nondiabetic Csf1op/op mice; 20–22 weeks for WT control; diabetic Csf1+/+ (WT) mice with delayed or normal GE (14–20 weeks old).
Discussion
Our main and surprising finding was that diabetic Csf1op/op mice do not develop delayed GE while their WT siblings do. This suggests that macrophages are required for the development of delayed GE in diabetes. We performed this study because we previously had reported that HO1-expressing, CD206-positive, alternatively activated macrophages protected diabetic NOD mice from development of delayed GE7 and that numbers of CD206-positive macrophages correlate with ICC numbers in humans with diabetic gastroparesis.14 We thus hypothesized that depletion of macrophages—including, of course, M2 macrophages—would lead to higher rates of delayed GE in diabetic mice. Our findings support an alternative hypothesis that although M2 macrophages can protect gastric cell types from injury, macrophages—likely M1 macrophages releasing injurious proinflammatory cytokines—are required to damage these key cell types, including ICC.
The protection of the diabetic Csf1op/op mice against developing delayed GE occurred despite levels of oxidative stress, elevated blood glucose, and dosing with insulin that were not different from the WT siblings used in this study. We also found that changes associated with delayed GE in diabetic mice6 and humans,5, 14 namely, disruption of ICC networks and reduced expression of Kit, were not found in the long-term diabetic Csf1op/op mice. Thus, it appears that mechanisms that result in development of delayed GE in diabetic mice on the time scale we studied are mediated through a population of macrophages that are dependent on Csf1 signaling. However this requires confirmation by testing the effect of populating the gastric muscularis propria of diabetic Csf1op/op mice with macrophages.
We used Csf1op/op mice as we could not produce long-term depletion of macrophages in diabetic mice either by using clodronate liposomes as described by Van Rooijen et al18 or by treating with diphtheria toxin in mice that specifically express the diphtheria toxin receptor on CD11b-positive macrophages.19 We found that clodronate liposomes did not cause a sustained loss of macrophages, which was required for our experiments, and the mice treated with diphtheria toxin all died within 4 weeks (data not shown).
The Csf1op/op mouse does not express macrophages in the lamina propria or the muscularis propria of the small intestine,11, 20 and we found that it does not express gastric macrophages even when made diabetic. Csf1op/op mice have significant developmental problems that include failure to develop teeth, skeletal abnormalities, and lower levels of blood monocytes. Moreover, as elsewhere reported, they are smaller in size when compared with age-matched WT littermates. Despite this, with careful handling, the mice survived the induction of diabetes and survived to the end of the 8 weeks of diabetes.
The Csf1op/op mice had low levels of plasma MDA comparable with the WT Csf1+/+ littermates before induction of diabetes and had significantly higher levels after the onset of diabetes. This is an observation that has not been previously reported. Our study indicates that high oxidative stress as indicated by oxidation of lipids and elevated MDA levels is not sufficient to cause cellular injury without macrophages.
A recent study21 has highlighted a role of resident macrophages in regulating intestinal contractility through an interaction with the enteric nervous system and the intestinal microbiome. It therefore appears that both resident and activated macrophages have previously unknown roles in modulating gastrointestinal motor function separate from their known role in immunity. WT Csf1+/+ mice behaved as observed in other studies on NOD, C57Bl6, and BALB/c mice (unpublished data)6, 16 in that diabetic gastroparesis was associated with higher levels of oxidative stress and that decreased Kit expression is found in the diabetic mice that develop delayed GE. The time course for development of delayed GE (6.25 weeks after onset of diabetes) was similar to that found in NOD mice (5.5 weeks).6 Thus, it appears that the streptozotocin-induced diabetic mouse model is useful for studying mechanisms that might underlie diabetic gastroparesis. Csf1op/op mice did have slightly but significantly faster GE than WT siblings before and after induction of diabetes due to the inability of these animals to eat the full 0.2 g meal. However, a consequence of the faster GE in Csf1op/op mice was that the upper threshold for normal GE was lower in mice after induction of diabetes compared with WT mice but even that lower threshold was not crossed with onset of diabetes. Thus, Csf1op/op mice represent a strain of mice that can be exploited to further investigate the role of macrophages in diabetes-induced tissue injury.
Our data strongly suggest that development of delayed GE in diabetes requires the presence of muscle-layer macrophages and that known mechanisms of injury in diabetes appear to converge on macrophages. In particular, M2 macrophages can protect gastric cells from injury, and M1 macrophages are required to damage these key cells, including ICC. In fact, despite the absence of M2 macrophages, the absence of injurious M1 cells during diabetes in op/op mice results in no changes in ICC and therefore in the absence of delay. Targeting macrophages may therefore be an effective therapeutic option to prevent cellular damage and the development of delayed GE in diabetes.
Acknowledgments
The authors thank Kristy Zodrow, Katie Miller, and Gary Stoltz for their excellent assistance with this work.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by National Institutes of Health grant NIH P01 DK 68055.
References
- 1.Farrell F.J., Keeffe E.B. Diabetic gastroparesis. Dig Dis. 1995;13:291–300. doi: 10.1159/000171509. [DOI] [PubMed] [Google Scholar]
- 2.Vittal H., Farrugia G., Gomez G. Mechanisms of disease: the pathological basis of gastroparesis—a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M., Grover M., Farrugia G. What are the important subsets of gastroparesis? Neurogastroenterol Motil. 2012;24:597–603. doi: 10.1111/j.1365-2982.2012.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grover M., Bernard C.E., Pasricha P.J. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531–539. doi: 10.1111/j.1365-2982.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover M., Farrugia G., Lurken M.S. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi K.M., Gibbons S.J., Nguyen T.V. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;35:2055–2064. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi K.M., Kashyap P.C., Dutta N. CD206-positive M2-macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faussone-Pellegrini M.S., Grover M., Pasricha P.J., NIDDK Gastroparesis Clinical Research Consortium (GpCRC) Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrugia G., Choi K.M., Sha L. Il-10 reverses delayed gastric emptying, slow wave abnormalities and smooth muscle membrane potential gradient changes in diabetic nod/shiltj mice. Neurogastroenterol Motil. 2012;24:154. [Google Scholar]
- 10.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 11.Murray P.J., Allen J.E., Biswas S.K. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikkelsen H.B., Thuneberg L. Op/op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res. 1999;295:485–493. doi: 10.1007/s004410051254. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki H., Kawai T., Shuttleworth C.W. Isolation and characterization of resident macrophages from the smooth muscle layers of murine small intestine. Neurogastroenterol Motil. 2004;16:39–51. doi: 10.1046/j.1365-2982.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernard C.E., Gibbons S.J., Mann I.S. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. 2014;26:1275–1284. doi: 10.1111/nmo.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H., Hayashi S., Kunisada T. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 16.Choi K.M., Zhu J., Stoltz G.J. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:1039–1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 17.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Van Rooijen N., Sanders A., Berg T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 19.Saxena V., Ondr J.K., Magnusen A.F. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 20.Cecchini M.G., Dominguez M.G., Mocci S. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 21.Muller P.A., Koscsó B., Rajani G.M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]