Abstract
Background
Risk factors for therapy-related leukemia (TRL) development, an often lethal late complication of cytotoxic therapy, remain poorly understood and may differ for survivors of different malignancies. Breast cancer (BC) survivors now account for the majority of TRL cases, making study of TRL risk factors in this population a priority.
Methods
Patients with TRL following cytotoxic therapy for a primary BC were identified from The University of Chicago TRL registry. Those with an available germline DNA sample were screened with a comprehensive gene panel covering known inherited BC susceptibility genes. Clinical and TRL characteristics of all subjects and those with identified germline mutations are described.
Results
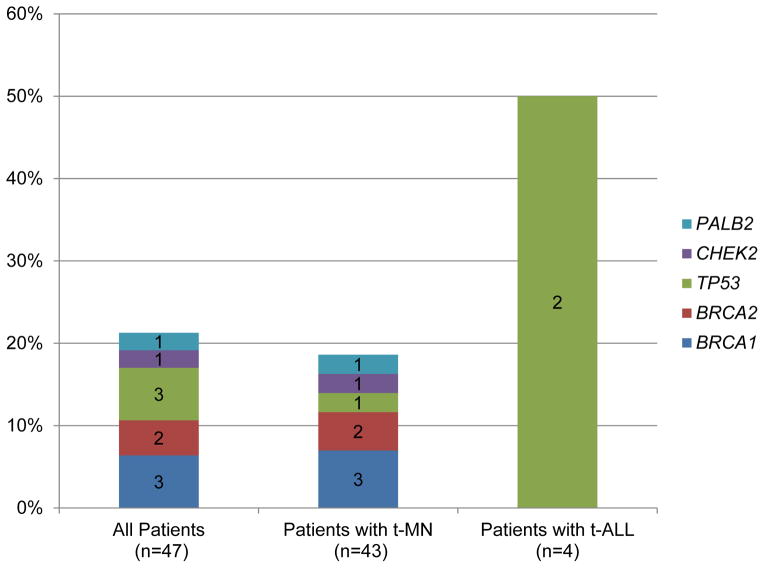
Nineteen (22%) of 88 BC survivors with TRL had an additional primary cancer and 40 (57%) of the 70 with available family history had a close relative with breast, ovarian, or pancreatic cancer. Of the 47 subjects with available DNA, 10 (21%) were found to carry a deleterious inherited mutation in: BRCA1 (n=3, 6%), BRCA2 (n=2, 4%), TP53 (n=3, 6%), CHEK2 (n=1, 2%), and PALB2 (n=1, 2%).
Conclusions
BC survivors with TRL have personal and family histories suggestive of inherited cancer susceptibility and frequently carry germline mutations in BC susceptibility genes. These data support the role of these genes in TRL risk and suggest that long term follow-up studies of women with germline mutations treated for BC and functional studies of the effects of heterozygous mutations in these genes on bone marrow function following cytotoxic exposures are warranted.
Keywords: therapy-related, leukemia, breast cancer, inherited
INTRODUCTION
Therapy-related leukemias (TRL), including therapy-related myeloid neoplasms (t-MN) and therapy-related acute lymphoblastic leukemia (t-ALL), are an often lethal, late complication of prior cytotoxic therapy for survivors of a first cancer.1–4 With increases in cancer survivorship,5 the number of cases of TRL is expected to rise. Thus, efforts to understand and prevent this complication are essential.
At present, TRL are thought to be direct consequences of mutational events induced by prior cytotoxic exposures, but exact mechanisms and risk factors remain unclear. Associations between specific exposures and the phenotype of the TRL that develops support a key role for the exposures in the genesis of TRL. For example, exposure to topoisomerase II inhibitors is associated with TRL characterized by clonal cytogenetic abnormalities involving KMT2A/MLL on chromosome band 11q23 with a short latency of 2–3 years post exposure. In contrast, exposure to alkylating agents or radiation is associated with TRL with abnormalities of chromosomes 5 and/or 7 which more often occur with a 5–7 year latency.6 Further, the incidence of TRL is increased in breast cancer (BC) adjuvant trials using higher chemotherapy dose intensity, concomitant use of radiation, and/or the use of hematopoietic growth factors.7, 8
However, the observation of acute myeloid leukemia (AML) and ALL cases occurring in patients after undergoing surgery only for a primary malignancy1–3, 9 raises the possibility that some TRL may be independent second primary cancers unrelated to prior cytotoxic exposures. Individuals with inherited cancer syndromes, like Li Fraumeni syndrome or dyskeratosis congenita, which predispose affected individuals to both leukemias and solid tumors, could explain some of these cases and present clinically like TRL. Another possibility is that individuals who carry an inherited mutation in a cancer susceptibility gene could be at higher risk for TRL after DNA damaging exposures than other patients.
Because breast cancer (BC) survivors now account for the largest number of TRL cases,2, 10 and the genes responsible for inherited susceptibility to BC are well characterized, patients who develop TRL after BC represent an ideal population in which to examine the role of inherited cancer susceptibility in the etiology of TRL. However, a comprehensive assessment of all currently known moderate to high penetrance BC susceptibility genes in patients with TRL after BC has not been performed. Here we present the clinical and TRL characteristics of 88 well-annotated BC survivors with TRL and the results of a comprehensive screen for inherited mutations in known BC susceptibility genes.
METHODS
Study population
Cases were drawn from The University of Chicago TRL registry, which contains data on all consented patients with a history of cytotoxic exposures for a prior malignant or nonmalignant condition who subsequently developed myelodysplastic syndrome (MDS) or an acute leukemia evaluated at The University of Chicago between 1972 and 2012. Additional clinical data were abstracted by individual chart review. Family histories consisted of physician documentation at initial consultation. Formal pedigrees were available for eight subjects who had prior cancer risk evaluation. This study was approved by The University of Chicago Institutional Review Board in accordance with the Declaration of Helsinki.
Definitions
Latency was defined as time from first cytotoxic exposure to the first bone marrow examination diagnostic of a TRL. Mechanism of action of chemotherapeutic agents was categorized, as previously defined.4 Cytogenetic abnormalities were detailed according to the International System for Human Cytogenetic Nomenclature.11
Tissue sources
Constitutional DNA sources included EBV-transformed lymphoblastoid cell lines (LBLs) generated at the time of complete remission (CR), buccal swabs, peripheral blood (PB) or bone marrow (BM) at the time of CR, and cultured skin fibroblasts. A leukemia sample was used if it was the only sample available with sufficient DNA.
BC susceptibility gene sequencing
BROCA targeted genomic capture and next generation sequencing (NGS) was performed as previously described (Supplementary Table 1).12 Single nucleotide variants (SNVs), small insertions and deletions (indels), and large genomic rearrangements (LGR) were identified as previously described.12, 13 Deleterious mutations, defined as nonsense and frameshift mutations, LGR, and missense mutations with experimental evidence supporting their deleterious nature, were validated by independent PCR amplification and Sanger sequencing or by real-time PCR using TaqMan probes (Life Technologies). Variants were only considered germline if they were confirmed in a constitutional DNA source.
Acquired mutation sequencing
Oncoplex targeted genomic capture and NGS was performed as previously described (Supplementary Table 2).14 All variants with data supporting a role in leukemia were validated by independent PCR amplification and Sanger sequencing. Constitutional DNAs were used to confirm the somatic nature of identified variants when available.
Statistical methods
Kaplan-Meier curves were used to calculate overall survival (OS). Stata version 12.1 was used for all analyses (StataCorp; College Station, TX).
RESULTS
Clinical characteristics of BC survivors who developed TRL
In total, 88 female BC survivors were identified (Table 1). The median age at primary BC diagnosis was 52 years (range, 23–83). Nineteen women (22%) had an additional primary cancer diagnosis. A family cancer history was available for 70 subjects (80%), among whom 40 (57%) reported at least one first- or second-degree relative with breast, ovarian, or pancreatic cancer. Among those for whom prior cytotoxic exposure data were available (n=86; 98%), chemotherapy was a component of the exposures for 67 patients (78%). All but one received a multiagent regimen. Regimens incorporating both doxorubicin and cyclophosphamide were most common (n=37; 56%). Radiation exposure was reported for 68 patients (79%). Four patients (5%) had undergone a prior autologous stem cell transplant and 11 patients (13%) had received myeloid growth factors.
Table 1.
Clinical characteristics, prior cytotoxic exposures, and therapy-related leukemia characteristics of 88 breast cancer survivors
| Number (%) | ||
|---|---|---|
|
| ||
| Age at diagnosis of breast cancer | ||
| ≤35 | 9 (10) | |
| 36–45 | 14 (16) | |
| 46–55 | 29 (33) | |
| ≥56 | 34 (39) | |
| Unknown | 2 (2) | |
|
| ||
| Race/Ethnicity | ||
| Caucasian (non-AJ) | 65 (74) | |
| Caucasian (AJ) | 3 (3) | |
| African American | 5 (6) | |
| Other/Unknown | 15 (17) | |
|
| ||
| Additional cancer diagnoses (n=19)* | ||
| Second primary breast cancer | 7 (8) | |
| Ovarian cancer | 3 (3) | |
| Other | 12 (14) | |
|
| ||
| Family history of cancer in a first or second degree relative (n=70) | ||
| Breast cancer | 33 (47) | |
| Breast, ovarian, or pancreatic cancer | 40 (57) | |
|
| ||
| Prior therapy | ||
| Chemotherapy + Radiation | 49 (56)# | |
| Chemotherapy only | 18 (20)# | |
| Radiation only | 19 (22) | |
| Unknown | 2 (2) | |
|
| ||
| Chemotherapy class exposures | ||
| Topoisomerase II inhibitor | 40 (45) | |
| Alkylating agent | 58 (66) | |
| Unknown | 8 (9) | |
|
| ||
| Type of therapy-related leukemia | ||
| t-MN | 81 (92) | |
| t-ALL | 7 (8) | |
|
| ||
| Latency; median in months (IQR) ** | 58 (28–105) | |
| Cytogenetics*** | ||
| Normal karyotype | 7 (8) | |
| Abnormal karyotype | 77(88) | |
| Abnormalities of chromosome 5 and/or 7 | 43 (49) | |
| Recurring balanced translocations | 29 (33) | |
| Other clonal abnormality | 7 (8) | |
| Unknown | 4 (5) | |
|
| ||
| Overall survival; median in months (IQR) | ||
| From breast cancer diagnosis**** | 102 (60–173) | |
| From therapy-related leukemia diagnosis | 13 (5–22) | |
Includes three women with multiple primary tumors; other cancers include: uterine (n=2), melanoma (n=2), lung (n=2), non-Hodgkin lymphoma (n=2), osteosarcoma (n=1), bladder (n=1), cervical (n=1), multiple myeloma (n=1)
Specific agents were unknown for n=4 in the chemotherapy + radiation group and n=2 in the chemotherapy only group
Latency was unknown for 3 patients
2 patients had abnormalities of both chromosomes 5 and/or 7 and a recurring balanced translocation (t(15;17) and t(9;22))
Overall survival was unknown for 3 patients
Abbreviations: AJ=Ashkenazi Jewish; t-MN=therapy-related myeloid neoplasm; t-ALL=therapy-related acute lymphoblastic leukemia; IQR=interquartile range
TRL characteristics in BC survivors
Most BC patients developed t-MN (n=81; 92%), but 7 cases (8%) of t-ALL were also observed (Table 1). The median latency from first cytotoxic exposure to TRL diagnosis among the 86 patients for whom latency was available was 58 months (interquartile range (IQR), 28–105 months). Clonal cytogenetic abnormalities were observed in 77 of the 84 subjects (92%) with an available karyotype. Among these, abnormalities of chromosomes 5 and/or 7 and recurring balanced translocations were both common, occurring in 51% (n=43) and 35% (n=29) of patients, respectively. Rearrangements involving KMT2A/MLL on chromosome band 11q23 were the most common (n=11 of 84; 13%), followed by t(15;17) (n=6; 7%), and those involving 21q22 (n=5; 6%) (Supplementary Table 3). Over one quarter of the observed recurring balanced translocations were t(9;11)(p22;q23) (n=8 of 29; 28%). OS after TRL diagnosis was poor (median 13 months; IQR, 5–22).
Inherited mutation detection and distribution
BROCA targeted capture and NGS of the 47 subjects with DNAs available resulted in >500-fold median coverage with 97% and 99.5% of bases covered at least 50- and 10-fold, respectively. The clinical characteristics of sequenced subjects did not differ from the 41 without available DNAs (Supplementary Table 4). Overall, 10 BC survivors (21%) who developed TRL carried a deleterious inherited mutation, distributed among BRCA1 (n=3, 6%), TP53 (n=3, 6%), BRCA2 (n=2, 4%), CHEK2 (n=1, 2%), and PALB2 (n=1, 2%) (Figure 1). By TRL subtype, 8 of 43 patients (19%) with t-MN had an inherited mutation, distributed among all 5 of these genes. Of t-ALL cases, 2 of 4 (50%) had a mutation, both in TP53.
Figure 1.
Inherited mutations in breast cancer susceptibility genes among 47 subjects with therapy-related leukemia
Observed patterns among those with specific inherited mutations included (Table 2): 1) those with germline TP53 mutations were the only patients with an inherited mutation to develop t-ALL (2 of 3 (67%) vs 0 of 7 (0%) patients with inherited mutations in other genes), and all 3 developed TRL with complex karyotypes; and 2) those with a BRCA1 or BRCA2 mutation had an especially long latency to TRL development (median 133 vs 53 months in those without an inherited mutation), and most developed TRL featuring a normal karyotype (n=2 of 5, 40%) or a single karyotypic abnormality (n=2 of 5, 40%). Six of the 10 patients (60%) with an inherited mutation had a family history of cancer, 2 (20%) did not, and for the remaining 2 (20%), the family history was unknown (Table 3).
Table 2.
Clinical and cytogenetic characteristics by germline mutation status among 47 sequenced subjects*
| No mutation (n=37)** | BRCA1 or BRCA2 (n=5) | TP53 (n=3) | PALB2 (n=1) | CHEK2 (n=1) | |
|---|---|---|---|---|---|
| Age at primary diagnosis (years; range) | 53 (31–79) | 50 (33–53) | 23 (23–24) | 51 | 42 |
|
| |||||
| Latency (median in months; range) | 53 (11–792) | 133 (30–408) | 48 (30–81) | 90 | 21 |
|
| |||||
| Therapy-related leukemia type | |||||
| t-MN | 35 (95) | 5 (100) | 1 (33) | 1 (100) | 1 (100) |
| t-ALL | 2 (5) | 0 | 2 (67) | 0 | 0 |
|
| |||||
| Cytogenetics (n; %) | |||||
| Normal karyotype | 4 (11) | 2 (40) | 0 | 0 | 0 |
| Clonal abnormality | 30 (81) | 3 (60) | 3 (100) | 1 (100) | 1 (100)^ |
| Balanced translocations# | 14 (38) | 1 (20) | 1 (33) | 0 | 1 (100)^ |
| Chr 5 and/or 7 abn***# | 15 (41) | 2 (40) | 1 (33) | 1 (100) | 0 |
| Complex*** | 11 (30) | 1 (20) | 3 (100) | 1 (100) | 0 |
| Unknown | 3 (8) | 0 | 0 | 0 | 0 |
|
| |||||
| Survival from TRL diagnosis (median in months; IQR) | 13 (7–27) | 14 | 29 | 14 | 52 |
Tissue sources used for sequencing included: LBLs (n=24), buccal swabs (n=8), PB or BM in remission (n=6), skin fibroblasts (n=1), and PB or BM samples with leukemia (n= 8).
1 patient’s age at diagnosis and latency were unknown.
Patient had FISH studies only.
2 subjects had both a balanced translocation (t(15;17) and t(9;22)) and an abnormality of chromosome 5 and/or 7.
Complex karyotype as defined in ref 33. 11 of 15 subjects with chromosome 5 and/or 7 abnormalities with no inherited mutation had a complex karyotype as well as 1 of 2 subjects with BRCA1/BRCA2, 1 subject with a TP53, and the 1 subject with a PALB2 mutation.
Abbreviations: IQR=interquartile range; abn=abnormalities; TRL=therapy-related leukemia; t-MN=therapy-related myeloid neoplasm; t-ALL=therapy-related acute lymphoblastic leukemia; chr=chromosome
Table 3.
Detailed clinical characteristics of the 10 breast cancer survivors who carry inherited BC susceptibility gene mutations and developed therapy-related leukemia
| Patient ID |
Gene | Mutation | Age at Breast Cancer Dx |
Chemo /XRT |
Other tumors |
Family cancer history |
TRL type |
TRL latency (mo) |
TRL OS (mo) |
Sample used for sequencing |
Karyotype at TRL Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UPIN12 | BRCA1 | dup ex13 | 33 | Y/Y | Ovary; 2nd Breast | Breast; Prostate | t-MN | 408 | 3 | BM leukemia; confirmed in buccal DNA | 45,XX,add(4)(q23),del(5)(q13.3q33),del(6)(p21.3p23),−7,+8, −16[20] |
| UPIN19 | BRCA1 | del ex13–15 | 53 | Y/Y | 2nd Breast; NHL | Breast; Ovary; Colon; Melanoma; Uterine | t-MN | 133 | 59# | Skin fibroblasts | 46,XX[30] |
| UPIN81 | BRCA1 | 187delAG | 50 | Y/Y | Thyroid: Lymphoma; Head & Neck | t-MN | 30 | 6# | PB in CR | 46,XX[30] | |
| UPIN49 | BRCA2 | 5301insA | 48 | Y/N | Unknown | t-MN | 216 | 14 | LBL | 46,XX,t(3;21)(q26.2;q22.3)[30]/46,XX[1] | |
| UPIN52 | BRCA2 | 8138del5 | 51 | Y/Y | Unknown | t-MN | 63 | 7 | LBL | 45,XX,−7[27]/46,XX[3] | |
| UPIN04 | TP53 | E339X | 24 | Y/N | Leukemia; Brain; Lung | t-ALL | 30 | 66 | LBL; confirmed in BM in CR | 96,XXXX,+X,+1,+2,−4,−5,+6,−9,+10,+12,+14,−15,−16,−17,+18,+21,+22[7]/ 97,idem,+mar[10]/46,XX[11]/Two related non-clonal abnormal cells | |
| UPIN60 | TP53 | G245S | 23 | Y/Y | Sarcoma in XRT field | Sarcoma | t-MN | 81 | 29 | LBL | 46,XX,der(15)del(15)(q23q24)t(15;17)(q2 4.1;q21.1),der(17)t(15;17)(q24.1;q21.1)[2 4]/48,XX,idem,+8,+8[12]/46,XX[4] |
| UPIN09 | TP53 | I232T | 23 | Y/Y | None | t-ALL | 48 | 28 | BM in CR | 54,XX,+X,+4,+6,+14,+17,+18,+21,+21[3]/46,XX[23] | |
| UPIN53 | PALB2 | Y1183X | 51 | N/Y | Breast | t-MN | 90 | 14 | LBL | 45,XX,−2,der(3)t(2;?;3)(p11.2;?;p11.1), der(5)del(5)(q15q33.3)t(5;12)(q33.3;q22), del(7)(q11.2q36),+8,add(9)(q34),der(12)t (5;12)(q33.3;q22),der(12)t(3;12)(p11.2;p1 3),−18[15]/46,idem,+mar[3]/46,XX[2] | |
| UPIN70 | CHEK2 | 1100delC | 42 | Y/Y | None | t-MN | 21 | 52 | Buccal | FISH only: +9,inv(16),+21 |
Latency from primary cancer diagnosis for this subject;
Still in follow-up.
Abbreviations: Dx=diagnosis; TRL=therapy-related leukemia; OS=overall survival; mo=months; NHL=non-Hodgkin lymphoma; XRT=radiation therapy; t-MN=therapy-related myeloid neoplasm; t-ALL=therapy-related acute lymphoblastic leukemia; BM=bone marrow; PB=peripheral blood; LBL=lymphoblastoid cell line; CR=complete remission; Y=yes; N=no
Additional informative cases
We identified three additional patients who did not fit our original study population who had previously identified germline BRCA1 mutations. We include them here for descriptive purposes (Supplementary Table V): 1) one patient who developed chronic myeloid leukemia (CML) following BC treated with surgery only; 2) one patient who developed CML 33 months prior to a diagnosis of BC; and 3) one ovarian cancer survivor who developed a t-MN with a t(9;11) after cytotoxic chemotherapy.
Somatic mutations in TRL after BC
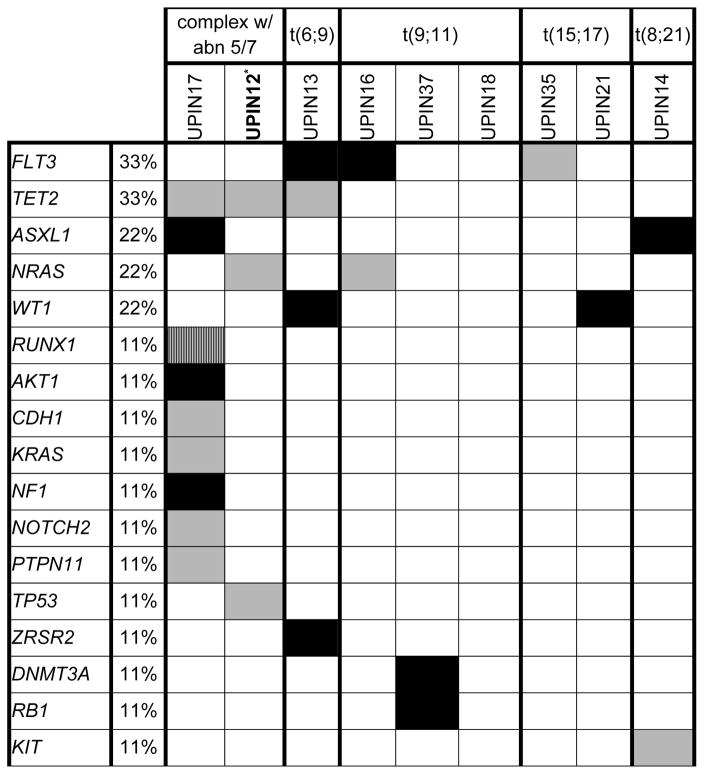
In order to identify somatic mutations that contribute to TRL post BC, we sequenced leukemia samples available from 9 subjects using Oncoplex. Somatic mutations were identified in 8 of the 9 subjects (Table 4). These mutations were distributed among 17 genes (Supplementary Table VI). The median number of somatic mutations per sample was 2 (range, 0 to 9). FLT3 andTET2 were the genes most commonly mutated, with each mutated in 3 of 9 (33%) subjects. Mutations in ASXL1, NRAS, and WT1 were observed in 2 of 9 (22%) subjects. Combinations seen in de novo AML including a KIT exon 17 mutation in a t(8;21) t-MN and a FLT3 mutation in a t(15;17) t-MN were identified. The leukemia sample from UPIN12, who developed a t-MN with a complex karyotype in the setting of a germline BRCA1 mutation, had somatic mutations in TET2, NRAS, and TP53.
Table 4.
Somatic mutations in 9 therapy-related leukemia cases after breast cancer
UPIN 12 carries an inherited BRCA1 mutation.
Black=frameshift, small insertions/deletions, or nonsense mutations; Striped=splice site mutation; Gray=missense mutation
Abbreviations: abn 5/7=abnormalities of chromosome 5 and/or 7; t=translocation
DISCUSSION
Through a comprehensive screen of inherited BC susceptibility genes, we found that one in five of the BC survivors with TRL in our series carry a deleterious inherited mutation. These mutations are distributed among five genes, all with key roles in DNA repair and/or DNA damage sensing pathways. In addition, many of the well-annotated BC survivors with TRL in our series have a personal history of additional malignancies and/or a family history of cancer in close relatives, suggesting a cancer-prone population. Our data support a role for inherited cancer susceptibility in TRL post-BC.
TRLs have typically been considered a direct and stochastic consequence of cytotoxic therapies. However, investigations have provided evidence in support of the role of underlying cancer susceptibility, particularly among BC survivors. Using SEER data, Martin et al demonstrated that young women with BC had the highest risk of t-MN development (RR 4.14) and that the age-dependent risk of TRL among these young women mirrored the risk of developing a second BC or an ovarian cancer, suggesting a shared underlying genetic risk factor.15 Two other small series also add support. In the first, sequencing of BRCA1, BRCA2, TP53, and CHEK2 1100delC identified deleterious germline mutations in 3 of 14 unselected BC patients with TRL (21%).16 In the second, sequencing of BRCA1 and BRCA2 in 13 women with TRL after early onset BC identified germline BRCA2 mutations among two (15%).17 Our data add to the spectrum of genes involved and confirm the high yield of genetic testing in this population. Our findings support a recommendation for genetic testing for all women with TRL post-BC to allow primary prevention in at risk close relatives and those who survive their TRL.
All of the BC susceptibility genes with mutations identified in this series function to sense or repair DNA damage and most are closely tied to leukemia risk. PALB2 and BRCA2, key components of the Fanconi anemia (FA) DNA repair pathway, cause FA, an inherited bone marrow failure syndrome featuring an 800-fold increased risk of MDS/AML, when mutations in both alleles are inherited.18, 19 Reduced expression of BRCA1, a gene also involved in the FA pathway, has been demonstrated in t-MN cases,20 and an increased risk of leukemia has been reported in an epidemiologic study in relatives of BRCA1 mutation carriers.21 Inherited mutations in TP53 cause Li Fraumeni syndrome in which 3–5% of the tumors that develop are leukemias.22, 23 TP53 is also somatically mutated in 2% of de novo AML24 and 11–38% of t-MN.25, 26 Data for CHEK2 involvement in leukemia are limited, but leukemias have been reported in kindreds with inherited CHEK2 mutations.27
Observations from our study provide additional evidence that some cases of TRL are more likely independent secondary primary cancers, whereas others are more clearly linked to the cytotoxic exposures. UPIN 49, for example, carries an inherited BRCA2 mutation and developed a t-MN with a t(3;21) 18 years after treatment for BC. This timeframe is well beyond the expected 2–3 years for t-MN with translocations involving 21q22,6 suggesting a possible independent event. Our previous report of two cases of acute promyelocytic leukemia in women with BC treated with surgery only with BRCA2 mutations28 and the two cases of CML occurring either prior to BC or after BC treated with surgery alone in BRCA1 mutation carriers reported here also support this idea and suggest that inherited heterozygous mutations in BRCA1 and BRCA2 may contribute to leukemia risk.
In contrast, TRL with a t(9;11) seen in 10% of our patients and in three other series of BC patients suggest that BC survivors are uniquely predisposed to TRL with this abnormality. Chandra et al reported that 62% of the t-MN cases with t(9;11) at their institution were in the setting of a prior BC.29 T(9;11) was identified among 11% (3 of 36) t-MN cases in a recent series of BC survivors9 and was overrepresented among t-MN cases (11%, 20 of 182) vs de novo AML (1%, 35 of 2381) in a study in which BC survivors accounted for 37% of t-MN cases.2 Further study of the non-homologous end joining repair mechanism implicated in the t(9;11) translocation in BC survivors with TRL is warranted.
Finally, we observed seven cases of t-ALL among our 88 BC survivors (8%) with TRL. We identified deleterious mutations, both occurring in TP53, among two of four cases (50%) studied. Both of these mutation carriers developed BC prior to age 30, a clinical phenotype that in and of itself warrants genetic testing. However, inherited mutations in BRCA1 and BRCA2 would be expected to account for the majority of mutations identified in early onset BC cases with TP53 mutations expected in only about 4% of those with BC under age 30.30 Our data suggest that when BC is followed by t-ALL, the likelihood of a TP53 mutation is higher.
Our study has limitations. First, this is a small series, which limits our ability to assess for differences among different mutation carrier groups. Second, it is unknown how the proportion of mutation carriers identified in our study population compares to a similar population of BC patients who did not develop TRL. It took several decades to obtain the number of cases presented here, making it difficult to ascertain a control group of similarly treated BC patients with similar length of follow-up who did not develop TRL with which to compare our group. In addition, studies of BRCA1 and BRCA2 among unselected BC patients suggest that about 5% carry a deleterious mutation,31, 32 but comprehensive panel-based genetic testing as used here has not yet been applied to a large, population-based group of BC patients. Thus, the true frequency of mutations in all of the genes studied here among a general population of BC patients is unknown and deserves further study.
In conclusion, we have demonstrated that one in five BC survivors who develop TRL carry an inherited mutation in a BC susceptibility gene. The mutations involve five genes, which all function to maintain DNA integrity, suggesting a role for these pathways in leukemia risk in the setting of cytotoxic exposures or for some, regardless of exposures. Our data suggest that a long-term prospective trial following similarly treated women with breast cancer for whom germline mutation status is known for the development of TRL and that functional testing of the role of these genes in bone marrow dysfunction following cytotoxic exposures are warranted.
Supplementary Material
Acknowledgments
Funding: NIH P01CA40046 (MML, RAL), NCI K12CA139160 (JEC), Cancer Research Foundation (JEC, MML, LAG, RAL), & The University of Chicago Comprehensive Cancer Center Support Grant P30CA14599.
The authors gratefully acknowledge the patients who participated in this study.
Footnotes
Financial Disclosures: The authors have no relevant disclosures.
AUTHORSHIP
JEC, CCP, TW, M-CK, RAL, and OO designed research; JEC, RM, KC, BN, MKL, HW, MB, CCP, TW, and M-CK performed research; JEC, RM, KC, MKL, MMC, DH, LAG, MML, CCP, TW, M-CK, and RAL analyzed data; JEC, MML, RAL, and OO wrote the paper. All authors edited and approved the final manuscript.
References
- 1.Abdulwahab A, Sykes J, Kamel-Reid S, Chang H, Brandwein JM. Therapy-related acute lymphoblastic leukemia is more frequent than previously recognized and has a poor prognosis. Cancer. 2012;118(16):3962–7. doi: 10.1002/cncr.26735. [DOI] [PubMed] [Google Scholar]
- 2.Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–45. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 3.Shivakumar R, Tan W, Wilding GE, Wang ES, Wetzler M. Biologic features and treatment outcome of secondary acute lymphoblastic leukemia--a review of 101 cases. Ann Oncol. 2008;19(9):1634–8. doi: 10.1093/annonc/mdn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 5.Society AC. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 6.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–29. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25(3):292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 8.Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21(7):1195–204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 9.Wolff AC, Blackford AL, Visvanathan K, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33(4):340–8. doi: 10.1200/JCO.2013.54.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121(15):2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer LG, M-JJ, Schmid M. ISCN 2013. 1. Basel/CH: S Karger Ag; 2012. [Google Scholar]
- 12.Walsh T, Lee MK, Casadei S, et al. Detection of inherited mutations for breast and ovarian cancer using genomic capture and massively parallel sequencing. Proc Natl Acad Sci U S A. 2010;107(28):12629–33. doi: 10.1073/pnas.1007983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nord AS, Lee M, King MC, Walsh T. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genomics. 2011;12:184. doi: 10.1186/1471-2164-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56–67. doi: 10.1016/j.jmoldx.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MG, Welch JS, Luo J, Ellis MJ, Graubert TA, Walter MJ. Therapy related acute myeloid leukemia in breast cancer survivors, a population-based study. Breast Cancer Res Treat. 2009;118(3):593–8. doi: 10.1007/s10549-009-0376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz E, Valentin A, Ulz P, et al. Germline mutations in the DNA damage response genes BRCA1, BRCA2, BARD1 and TP53 in patients with therapy related myeloid neoplasms. J Med Genet. 2012;49(7):422–8. doi: 10.1136/jmedgenet-2011-100674. [DOI] [PubMed] [Google Scholar]
- 17.Martin MGSJ, Deych E, et al. BRCA1 and BRCA2 nucleotide variants in young women with therapy-related acute myeloid leukemia. Blood. 2009;(114) ASH Annual Meeting Abstracts. Abstract 1102. [Google Scholar]
- 18.D’Andrea AD. Susceptibility pathways in Fanconi’s anemia and breast cancer. N Engl J Med. 2010;362(20):1909–19. doi: 10.1056/NEJMra0809889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93(4):511–7. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 20.Scardocci A, Guidi F, D’Alo F, et al. Reduced BRCA1 expression due to promoter hypermethylation in therapy-related acute myeloid leukaemia. Br J Cancer. 2006;95(8):1108–13. doi: 10.1038/sj.bjc.6603392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68(3):700–10. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birch JM, Alston RD, McNally RJ, et al. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20(34):4621–8. doi: 10.1038/sj.onc.1204621. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27(8):1250–6. doi: 10.1200/JCO.2008.16.6959. [DOI] [PubMed] [Google Scholar]
- 24.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Yehuda D, Krichevsky S, Caspi O, et al. Microsatellite instability and p53 mutations in therapy-related leukemia suggest mutator phenotype. Blood. 1996;88(11):4296–303. [PubMed] [Google Scholar]
- 26.Shih AH, Chung SS, Dolezal EK, et al. Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia. Haematologica. 2013;98(6):908–12. doi: 10.3324/haematol.2012.076729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson D, Seal S, Schutte M, et al. A multicenter study of cancer incidence in CHEK2 1100delC mutation carriers. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2542–5. doi: 10.1158/1055-9965.EPI-06-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MJ, Li L, Wiernik PH, Olopade OI. BRCA2 mutation and the risk of hematologic malignancy. Leuk Lymphoma. 2006;47(4):765–7. [PubMed] [Google Scholar]
- 29.Chandra P, Luthra R, Zuo Z, et al. Acute myeloid leukemia with t(9;11)(p21–22;q23): common properties of dysregulated ras pathway signaling and genomic progression characterize de novo and therapy-related cases. Am J Clin Pathol. 2010;133(5):686–93. doi: 10.1309/AJCPGII1TT4NYOGI. [DOI] [PubMed] [Google Scholar]
- 30.Lalloo F, Varley J, Ellis D, et al. Prediction of pathogenic mutations in patients with early-onset breast cancer by family history. Lancet. 2003;361(9363):1101–2. doi: 10.1016/S0140-6736(03)12856-5. [DOI] [PubMed] [Google Scholar]
- 31.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869–76. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 32.Malone KE, Daling JR, Doody DR, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66(16):8297–308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 33.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.