Abstract
Background
Patients receiving adjuvant chemotherapy have reported cognitive impairments, which may last years after completion of treatment. Working memory- and long-term memory-related changes in this population are not well understood. In this study we aimed to demonstrate that cancer-related cognitive impairments are associated with under recruitment brain regions involved in working and recognition memory compared to controls.
Method
Oncology patients (n=15) receiving adjuvant chemotherapy with evidence of cognitive impairment by neuropsychological testing and self-report and age/education group-matched, cognitively normal controls (n=14) underwent functional magnetic resonance imaging (fMRI). During fMRI scanning, participants performed a non-verbal n-back working memory task and a visual recognition task.
Results
On the working memory task, when 1-back and 2-back data were averaged and contrasted with 0-back data, significantly reduced activation was observed in the right dorsolateral prefrontal cortex for oncology patients versus controls. On the recognition task, oncology patients displayed decreased activity of left-middle hippocampus compared to controls. Neuroimaging results were not associated with patient-reported cognition.
Conclusion
Decreased recruitment of brain regions associated with working and recognition memory encoding were observed in the oncology group. These results suggest reduced neural functioning post-chemotherapy and corroborate patient-reported cognitive difficulties following cancer treatment, though a direct association was not observed.
Keywords: fMRI, Cancer-related cognitive impairment (CRCI), working memory, recognition memory, patient-reported outcomes (PRO)
Introduction
Recent studies have shown that cancer patients receiving adjuvant chemotherapy experience cancer-related cognitive impairments (CRCI)1 that can negatively impact a patient’s daily life2, 3 4. CRCI manifests as difficulty in real-life routines such as remembering phone numbers5, or deficits in laboratory tests such as working memory 6 and learning new information (i.e. encoding)7. Neuroimaging studies have revealed decreased activity in the frontal cortex during working memory tasks 8, as well as reduced frontal, temporal, parietal, and cerebellar cortical volume 9–11 in these patients. A recent EEG study has shown that prolonged exposure to chemotherapeutic agents is associated with decreases in hippocampal neurogenesis and theta-band activity, which may lead to deficits in learning and subsequent retrieval 12. Furthermore, animal studies suggest a link between chemotherapy-induced cellular damage in the hippocampus and behavioral impairment in visuospatial memory13.
Results of CRCI studies using neuroimaging 4, 9 neuropsychological tests14, and patient-report15 are not consistent; many brain regions have been implicated, but with high variability from study to study (see recent review by Scherling and Smith4). There is also substantial variability in the degree to which these brain differences are related to neuropsychological test domains or patient-reported cognitive function. In addition, whereas some findings support the association between neuroimaging and patient-reported cognitive function9, 16, 17, systematic review of this evidence indicates no clear association across studies18.
The aim of this study was to demonstrate that patients who underwent adjuvant chemotherapy experience cancer-related cognitive impairments related to under recruitment of brain regions involved in working and recognition memory compared to controls. In this study we utilized all three approaches (i.e., neuroimaging, neuropsychological testing, and patient-reported cognitive function assessments) to evaluate CRCI. Because deficits in working memory and learning can have direct consequences on longer-term memory, such as those involved in recognition, we examined non-verbal working memory and visuospatial recognition memory tasks. Recognition memory is the ability to accurately discriminate a novel stimulus from a previously experienced stimulus19, and it involves the medial temporal lobe (MTL)20. Damage to the MTL has been shown to impact both recognition and working memory21. Working memory involves prefrontal and frontal parietal networks22, 23. Therefore, our primary hypothesis was that brain activation during working memory and recognition tasks would be reduced in oncology patients in the prefrontal and MTL brain regions compared with healthy controls. Our secondary hypothesis was that the reduced activation would be associated with impairments in neuropsychological test performance and patient-reported cognition. Finally, we explored whether there was an association between task behavior, neuropsychological test performance and patient reported cognitive function.
Methods
Procedure
This study was approved by the Institutional Review Board at Northwestern University and the NorthShore University Health System. After physician referral from affiliated outpatient clinics based upon patient report of cognitive difficulty, patients were recruited and consented by research assistants. In total 185 patients were identified as having reported cognitive impairments; of those, 77 where ineligible, 59 refused to participate, and 49 were consented. Healthy controls were recruited through flyers and by word of mouth. Forty-six controls were identified as potentially eligible, 7 were ineligible, 5 refused and 34 were consented. All consented participants, 49 patients and 34 controls, completed neuropsychological tests and patient-reported outcomes (PRO). Participants were then included or excluded from the study based on their neuropsychological performance (see participants section). Controls performing within normal range were selected to group-match the patients on demographics and then underwent the same MRI procedure. In total, 16 patients and 16 controls underwent MRI and were included in the study. Details on the procedures for participant selection, neuropsychological test battery, patient-reported outcome, MRI, behavior, and image processing and statistical analysis are described below.
Participants
Eligibility criteria for patients included solid, non-central nervous system tumor or hematologic malignancy of any stage, physician-rated Eastern Cooperative Oncology Group (ECOG) performance status ≤ 225, and completion of 3 or more cycles of chemotherapy within the previous 6 months. Patients or controls were excluded if they had premorbid cognitive dysfunction, or comorbidities known to be associated with cognitive impairment (such as major depression or generalized anxiety26). Eligibility criteria for patients included impairment in cognitive domains (≥1 standard deviation below the mean on at least three domains, or ≥1.5 standard deviations below the mean on at least one or more domains, consistent with International Cognition and Cancer Task Force recommendations23). Eligibility criteria for controls included normal performance on neuropsychological evaluation.
Neuropsychological Tests
Participants completed the following neuropsychological tests designed to assess memory, executive function, problem solving/reasoning ability, visuospatial/constructional ability, language, and attention: Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Trail making Tests A and B, Stroop Color-Word Test, Test of Nonverbal Intelligence (TONI-III), Wechsler Adult Intelligence Scale (WAIS-III) Similarities, Digit Span, and Letter-Number Sequencing subscales, and MicroCog Assessment of Cognitive Functioning 27 with reaction time.
Patient-Reported Outcome
A battery of PRO and health-related quality of life (HRQL) measures was administered to participants to quantify patient-reported cognitive problems, overall functional status and well-being. These include: Functional Assessment of Cancer Therapy-Cognitive Function Scale version 1 (FACT-Cog v1) 28, FACT-General (FACT-G)29, FACT-Fatigue30, and Hospital Anxiety and Depression Scale (HADS)31. All FACT questionnaires assessed for difficulties experienced within the previous 7 days. FACT-Cog v1 (reliability alpha=0.9632) assesses patient-reported cognitive impairment, impact on quality of life, and the extent to which others notice cognitive deficits 28, with lower scores indicating worse cognitive function. FACT-G assesses physical, social, emotional, and functional well-being, with higher scores indicating better quality of life. FACT-Fatigue assesses fatigue, with higher scores indicating less fatigue. The HADS measures general anxiety and depression, with higher scores indicating higher levels of anxiety and depression. Physician-rated ECOG performance status was also provided for each patient.
MRI, Behavior, and Image Processing
All participants who were included in the study underwent magnetic resonance imaging (MRI) on a Siemens 3T Trio scanner. Functional MRI (fMRI) data were acquired using a susceptibility-weighted echo-planar imaging sequence (TR=2200ms, TE=20ms, flip angle=80°, matrix=64×64, resolution=3.4375×3.4375×3mm3). A T1-weighted MPRAGE image was also acquired (TR=2100ms, TE=4.38ms, flip angle=8, matrix=256×192, resolution=0.859×0.859×1mm3). During fMRI, participants performed non-verbal n-back (0-, 1-, and 2-back) visual working memory and recognition tasks adapted from Ragland et al 33. Stimuli were presented to subjects using Cogent 2000 and Cogent graphics software (http://www.vislab.ucl.ac.uk/cogent_2000.php) running in the Matlab environment (Mathworks, Natick MA).
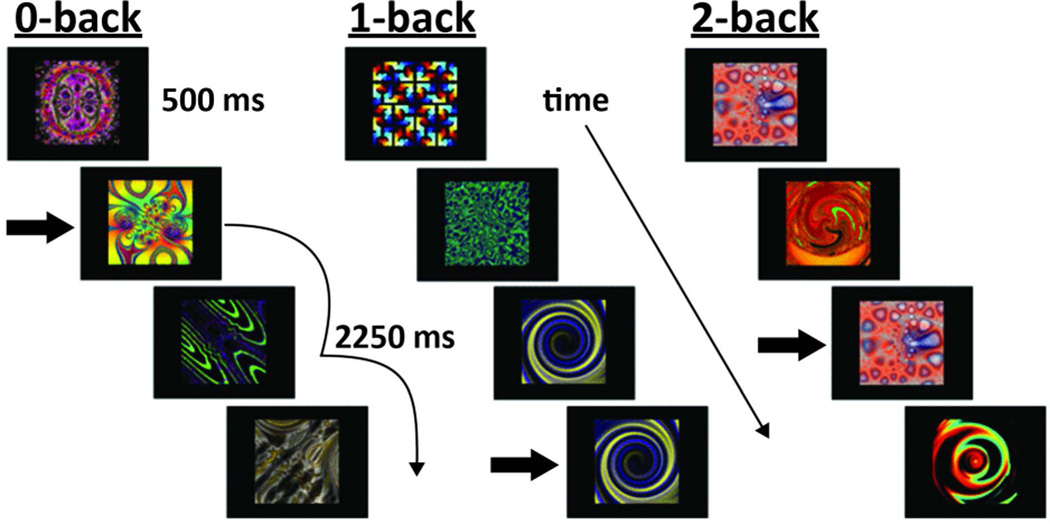
During the n-back task, we employed a blocked design, with each block lasting 33.75sec and repeated 3 times in a pseudo-randomized order. Within each block, 39 fractal images were presented sequentially for 500ms with a 375ms inter-stimulus interval. Participants pressed a button to indicate whether or not the fractal image on the screen was the same as the one shown either 0, 1, or 2 items before (Figure 1). The n-back task served as an indirect encoding phase for the subsequent recognition test. Upon completion of the n-back task, the recognition test consisted of 20 fractal images from the n-back task interleaved with 22 new images (foils). Test fractals were presented one at a time in random order for 500ms. Participants pressed a button to indicate whether or not they had seen a given fractal in the previous n-back task. Recognition accuracy was calculated as overall mean accuracy ([pHits + pCorrectRejections]/2) and recognition sensitivity was computed using d-prime (d′) (pHits - pFalseAlarms) for each of the 0, 1, and 2-back conditions.
Figure 1. N-back working memory task.
In the 0-back condition participants respond whenever the indicated figure appeared, regardless of any figures that preceded it. In the 1-back condition participants respond whenever the figure is the same as in the previous trial. Subjects respond in the 2-back condition whenever the figure matches the stimulus that came 2 trials before it. Arrows indicate correct responses for all conditions. Stimuli courtesy of J. Daniel Ragland (University of Pennsylvania).
Preprocessing of fMRI data was performed with AFNI and included: exclusion of the first three volumes in each run, slice-timing and motion correction, co-registration to the participant’s T1-weighted image, spatial normalization to the Talairach-Tournoux template with atlas regions of interest, and resampling to 3.25mm3 resolution. Data were then spatially smoothed with Gaussian kernel to a resolution of 8mm FWHM followed by linear regression with a model hemodynamic response function (HRF) and its temporal derivative for each block and each task. A priori regions of interest (e.g., MTL) were analyzed for the recognition task.
Statistical Analysis
For neuropsychological testing, PRO, and n-back behavioral data, student t-tests and effect sizes (Cohen’s D) were used to compare groups with SPSS 21.0 (SPSS Inc., Chicago, IL). For recognition memory performance, a 2 × 2 mixed repeated-measures ANOVA and post-hoc t-tests were used to test for the main effects of recognition (hits vs. misses), group and recognition×group interaction. Effect sizes for these analyses were calculated as partial η2. For fMRI data, a whole-brain analysis was conducted with mixed-effects meta-analysis (3dMEMA in AFNI) to determine group-level differences, after covarying for FACT-Fatigue and HADS. MEMA uses group-level variation and a precision estimate of the effect of interest from individual participants. For working memory, the n-back data were corrected for multiple comparisons to achieve p<0.05 with individual voxel threshold at p<0.001 and minimum cluster size ≥ 17 voxels. Each of the 1-back and 2-back conditions was first contrasted with 0-back to examine between-group differences, however no significant group differences were found. We therefore averaged the 1-back and 2-back conditions to contrast with 0-back and compared across the groups. For recognition, activity corresponding to the correct “yes” (hits) was contrasted with the correct “no” (correct rejections) and compared between groups.
We tested our secondary hypotheses in brain regions showing significant group differences in any of the above comparisons, by conducting Pearson correlation analyses between regional BOLD activity and neuropsychological test and patient-reported cognitive function. For neuropsychological test comparisons, we used RBANS subtest percentiles and total index scores, Trail Making A and B T-scores, Stroop T-scores, TONI-III percentiles, WAIS-III subtest scaled scores and cumulative percentiles, and MicroCog 1 and 2 reaction time scaled scores. For patient-reported cognitive function, we used the FACT-Cog v1 Perceived Cognitive Impairments subscale score and FACT-Cog v1 total score.
For our exploratory hypotheses we used Pearson correlation analysis to examine associations between task behavior, neuropsychological test performance, and patient-reported cognitive function measures that showed significant group differences. All correlation analyses were performed in patient and control groups separately, and were not corrected for multiple comparisons.
Results
Participant Demographics
Originally 16 oncology patients and 16 healthy controls were included in this study. Three were subsequently removed from analysis because one patient had difficulty understanding directions for the button press and two controls had insufficient trials on the recognition task. A total of 15 oncology patients and 14 controls were included in the final analysis reported here. These subjects showed no group differences in age, education, gender or race (Table 1). The majority of patients (53.3%) had an ECOG performance status of 0, with 27% at 1 and 20% at 2. Diagnoses included breast cancer (73.3%), colorectal cancer (6.7%), Hodgkin’s lymphoma (6.7%), leukemia (6.7%), and myeloma (6.7%).
Table 1.
Demographic information for oncology and control groups.
| Oncology Patients (N=15) |
Controls (N=14) |
P Value | |
|---|---|---|---|
| Gender (Male/Female) | 86.7% female | 92.9% female | 0.58 |
| Race (Caucasian/AA) | 86.7% White, 13.3% Black |
78.6% White, 21.4% Black |
0.56 |
| Age: mean (SD) | 50.7 (± 7.5) years | 53.0 (±7.2) years | 0.42 |
| Education: mean (SD) | 15.6 (±2.3) years | 16.8 (±2.2) years | 0.16 |
| Handedness (L/R) | 1/14 | 1/13 | 0.93 |
| Physician-rated ECOG performance status |
53.3% 0 26.7% 1 20.0% 2 |
N/A | -- |
| Cancer diagnosis | 73.3% breast 6.7% colo-rectal 6.7% Hodgkin’s lymphoma 6.7% leukemia 6.7% myeloma |
N/A | -- |
| Extent of disease | 20.0% No evidence of disease 26.7% local 13.3% regional 20.0 % metastatic 20.0 % N/A |
N/A | -- |
Neuropsychological Tests
As expected (see eligibility criteria), patients scored significantly lower than controls in most of the cognitive domains, indicating impairment (Table 2). The groups did not differ on Trails A, B, Stroop Interference, TONI-III, and Digits Backwards.
Table 2.
Neuropsychological test scores for oncology patients and controls.
| Mean ± SD | Oncology patients |
Controls | P Value | Cohen’s D |
|---|---|---|---|---|
| RBANS Percentile | ||||
| Immediate memory | 39.5±32.7 | 67.9±14.1 | 0.006 | 1.111 |
| Delayed memory | 30.0±27.2 | 55.8±17.6 | 0.005 | 1.112 |
| Visuospatial/Constructional | 17.7±23.7 | 57.8±23.8 | <0.001 | 1.684 |
| Language | 31.7±20.6 | 56.0±20.0 | 0.003 | 1.196 |
| Attention | 44.1±28.6 | 80.6±21.2 | 0.001 | 1.445 |
| Total Index | 24.3±19.9 | 69.1±19.6 | <0.001 | 2.262 |
| Trails A T-score | 48.2±7.0 | 53.6±7.8 | 0.062 | 0.723 |
| Trails B T-score | 48.9±11.2 | 55.2±10.6 | 0.133 | 0.576 |
| Stroop Interference T-score | 48.2±6.5 | 52.2±5.1 | 0.076 | 0.686 |
| TONI-III Percentile | 52.0±30.0 | 55.0±25.4 | 0.769 | 0.112 |
| WAIS-III | ||||
| Letter-Number age ss | 9.9±3.3 | 12.6±2.6 | 0.018 | 0.933 |
| Similarities age ss | 10.2±3.0 | 12.8±2.3 | 0.014 | 0.973 |
| Digit Span age ss | 9.87±3.4 | 13.08±2.3 | 0.009 | 1.062 |
| Digits forward cumulative percentile | 58.8±35.1 | 31.0±19.4 | 0.015 | 0.961 |
| Digits backward cumulative percentile | 62.0±33.3 | 48.6±34.5 | 0.304 | 0.398 |
| MicroCog Total Reaction Time ss | ||||
| Timer 1 | 10.5±2.4 | 12.1±1.9 | 0.031 | 0.747 |
| Timer 2 | 9.2±3.7 | 11.7±1.9 | 0.031 | 0.848 |
Patient-Reported Outcomes
On FACT-Cog v1, patients reported significantly more perceived cognitive impairments, a greater impact of cognition on quality of life, and more comments from others regarding their cognition (Table 3). FACT-Cog v1 total scores indicated lower overall cognitive functioning compared to controls. Regarding HRQL, on FACT-G patients reported decreased physical and functional well-being, but comparable social and emotional well-being. Compared to controls, patients indicated greater fatigue on the FACT-Fatigue and reported higher levels of anxiety and depression on the HADS.
Table 3.
Patient-reported outcomes measures for oncology patients and controls.
| Mean ± SD | Oncology Patients |
Controls | P Value | Cohen’s D |
|---|---|---|---|---|
| FACT-Cog v1 | ||||
| Perceived Cognitive Impairments (0–80) | 38.44±16.11 | 67.85±8.36 | <0.001 | 2.267 |
| Impact on QOL (0–16) | 11.00±4.78 | 14.93±1.94 | 0.009 | 1.063 |
| Comments from Others (0–16) | 11 .91±3.47 | 15.43±1.14 | 0.002 | 1.342 |
| FACT-Cog total (0–108) | 80.17±14.15 | 91.76±9.82 | 0.017 | 0.945 |
| FACT-G | ||||
| Physical Wellbeing (0–28) | 18.40±7.60 | 25.92±2.47 | 0.002 | 1.311 |
| Social Wellbeing (0–28) | 22.97±2.86 | 22.38±4.13 | 0.655 | 0.168 |
| Emotional Wellbeing (0–24) | 20.07±3.41 | 20.46±2.98 | 0.741 | 0.124 |
| Functional Wellbeing (0–28) | 18.73±6.51 | 23.00±4.05 | 0.045 | 0.781 |
| FACT-Fatigue (0–52) | 33.00±15.03 | 46.21±5.91 | 0.005 | 1.142 |
| HADS | ||||
| Anxiety (0–20) | 6.40±4.24 | 3.71±2.46 | 0.049 | 0.768 |
| Depression (0–20) | 4.07±4.10 | 1.21±1.48 | 0.021 | 0.913 |
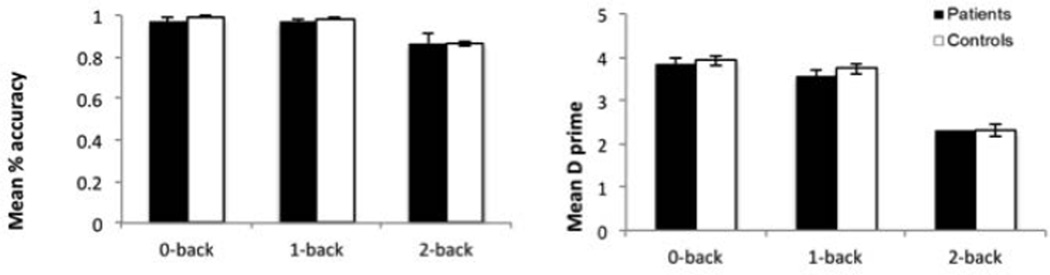
N-back Behavior
Patients did not differ significantly from controls on working memory behavioral measures (Figure 2, Table 4). However, moderately reduced effect sizes were observed between groups in d′ and accuracy for 0-back.
Figure 2. Between-groups behavioral results for the n-back task.
Left panel demonstrates mean accuracy and right panel demonstrates mean d-prime. While there were no statistically significant differences between groups for each n-back condition, the differences for the 0- and 1-back conditions showed moderate effects sizes.
Table 4.
N-back working memory behavior results for oncology patients and controls. Results show 0-back and combined 1- and 2-back mean accuracy (hits + correct rejections/2), reaction time, sensitivity (d″), hits, misses, false alarms, and correct rejections for oncology patients and controls. Effect sizes are displayed for between-group comparisons.
| 0 back | 1- 2-back combined | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean % accuracy (SD) |
Mean RT (ms) (SD) |
Sensitivity d′ (SD) |
Hits (SD) |
Misses (SD) |
False Alarms (SD) |
Correct Rejections (SD) |
Mean % accuracy (SD) |
Mean RT (ms) (SD) |
Sensitivity d′ (SD) |
Hits (SD) |
Misses (SD) |
False Alarms (SD) |
Correct Rejections (SD) |
|
| Oncology | 97.18 (6.2) | 638.46 (245.1) | 3.72 (0.7) | 0.98 (0.1) | 0.02 (0.1) | 0.01 (0.0) | 0.99 (0.0) | 92.22 (0.07) | 838.95 (247.2) | 2.87 (0.9) | 0.83 (0.1) | 0.17 (0.1) | 0.03 (0.0) | 0.97 (0.0) |
| Controls | 99.02 (1.5) | 612.80 (180.8) | 3.91 (0.2) | 0.99 (0.0) | 0.01 (0.0) | 0.01 (0.0) | 0.99 (0.0) | 92.70 (0.07) | 802.60 (294.8) | 3.01 (0.8) | 0.87 (0.1) | 0.13 (0.1) | 0.04 (0.0) | 0.96 (0.0) |
| Effect size (Cohen’s d) | 0.48 | 0.12 | 0.4 | 0.13 | 0.13 | 0.23 | 0.23 | 0.07 | 0.13 | 0.17 | 0.38 | 0.38 | 0.42 | 0.42 |
N-back fMRI
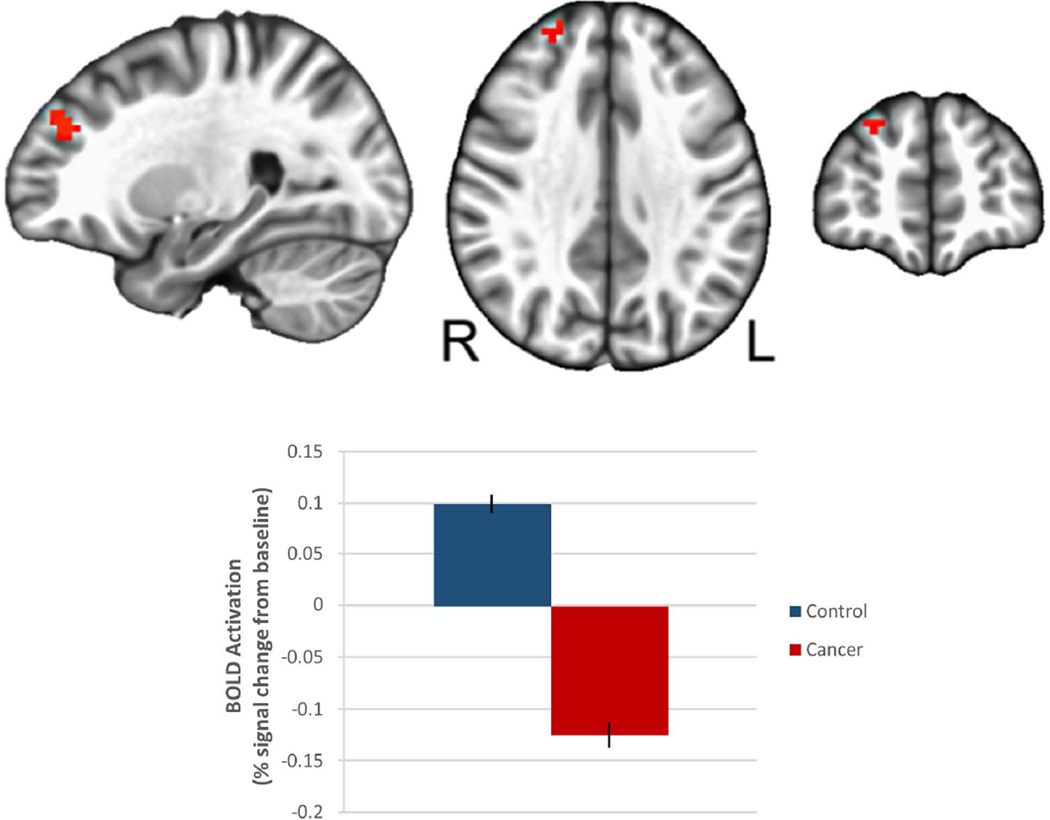
Oncology patients demonstrated significantly reduced activation compared to controls in the right dorsolateral prefrontal cortex (DLPFC, Brodmann Area 9/10) when the 1-back and 2-back data were averaged and contrasted with the 0-back data (Figure 3, Table 5). These differences remained after covarying for fatigue, anxiety and depression, which can negatively affect working memory performance36, 37 and were greater in patients compared with controls (Table 3).
Figure 3. Between-groups functional differences in the right dorsolateral prefrontal cortex for the n-back working memory task.
Oncology patients show significantly decreased BOLD activation in right dorsolateral prefrontal cortex as compared with controls.
Table 5.
Oncology patients demonstrated significantly reduced activation compared to controls in the right dorsolateral prefrontal cortex (DLPFC, Brodmann Area 9/10) when the 1-back and 2-back data were averaged and contrasted with the 0-back data.
| Region With Peak Activation |
Hem | “BA” | Center of Mass Coordinates |
Peak T Value | Cluster Size (in mm3) |
Cluster Size (in voxels) |
||
|---|---|---|---|---|---|---|---|---|
| L/R | A/P | S/I | ||||||
| Control subjects > cancer subjects | ||||||||
| Dorsolateral prefrontal cortex |
R | 9,10 | −25 | −47 | 31 | 3.86 | 618 | 18 |
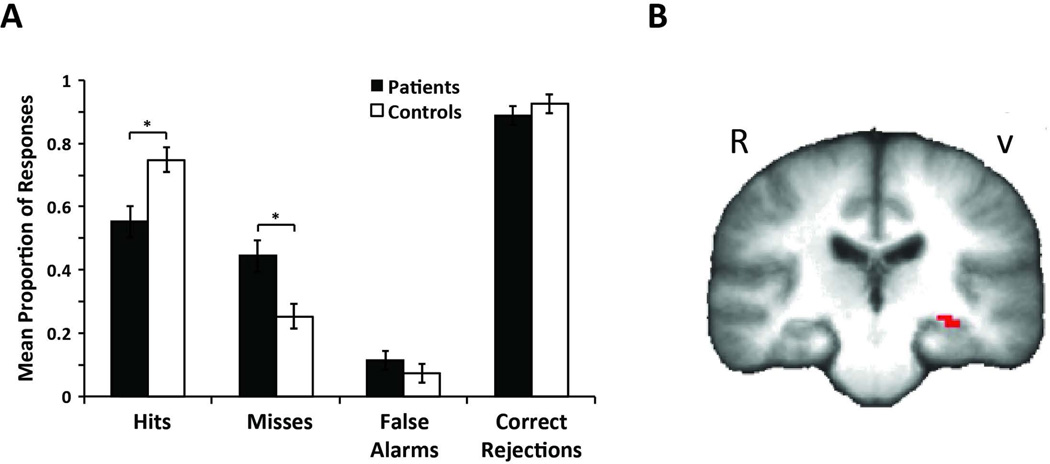
Recognition Behavior
Behavioral results from the recognition task are displayed in Table 6 and Figure 4A. We found a significant main effect of recognition response [F(1,24)=16.48, MSE=0.07, p<0.001, pη2=0.41] and a significant recognition response×group interaction [F(1,24)=6.76, MSE=0.072, p<0.02, pη2=0.22]. A Post hoc t-test revealed that this interaction was driven by a significant reduction in the proportion of correct hit responses given by patients (55%) compared to controls (75%). We also observed a trend for higher false alarm rates in patients (11%) compared to controls (7%). Overall mean recognition accuracy was statistically poorer for patients compared to controls.
Table 6.
Recognition behavior results for oncology patients and controls
| Old Items | New Items | ||||||
|---|---|---|---|---|---|---|---|
| Mean Accuracy (SD) |
Mean RT (ms) (SD) |
Sensitivity d′ (SD) |
Hits (SD) |
Misses (SD) |
False Alarms (SD) |
Correct Rejections (SD) |
|
| Oncology | 0.72** (0.09) | 1227.95 (214.3) | 1.99 (1.2) | 0.55* (0.2) | 0.44* (0.2) | 0.11 (0.1) | 0.89 (0.1) |
| Controls | 0.82 (0.08) | 1336.33 (728.2) | 3.26 (1.7) | 0.75 (0.1) | 0.25 (0.2) | 0.07 (0.1) | 0.92 (0.1) |
| Effect size (Cohen’s d) | 1.19 | 0.20 | .86 | 1.26 | 0.95 | 0.40 | 0.30 |
Displayed are estimates of mean recognition accuracy ([pHits + pCorrectRejections]/2), reaction time, recognition sensitivity (d′= pHits - pFalseAlarms) and proportions of hits, misses, false alarms, and correct rejections for oncology patients and controls. Effect sizes are displayed for between-group comparisons.
p<0.05
p<0.01
Figure 4. Between-group behavioral and functional differences in recognition task.
A. Proportion of hits, misses, false alarms and correct rejections for the visual recognition task. B. Controls show increased activation when contrasting hits to correct rejections in the middle hippocampus. Regions of increased activation not displayed also include left lateral parietal cortex, left fusiform gyrus, and left lateral occipital cortex. Conversely, oncology patients showed decreased activation than controls in the left hippocampus.
Recognition fMRI
The initial fMRI recognition analysis did not reveal any significant regional differences between groups after correcting for multiple comparisons. Based on a priori hypotheses that the MTL may be especially susceptible to chemotherapy neurotoxicity, we performed an exploratory analysis in this region. We found that a small, 5-voxel cluster of decreased activation in patients in the left-middle hippocampus (p=0.01 voxel-wise uncorrected, Figure 4B).
Correlations Between Task Behavior, Brain Activation, Neuropsychological Performance and Patient-Reported Outcomes Measures
The n-back d′ was normalized to that of the 0-back condition, which was used as pseudo fixation for both groups. For 1-back, d′ performance in patients was positively correlated with WAIS Letter-Number sequencing (r=0.52, p=0.048) and WAIS Similarities (r=0.55, p=0.025). No correlations were observed for 2-back. For the recognition memory task, positive correlations were observed in patients between hits and RBANS attention scores (r=0.69, p=0.006), hits and Trial Making B T-scores (r=0.68, p=0.007), hits and MicroCog time 1 and time 2 standard scores (time 1 r=0.63, p=0.015; time 2 r=0.55, p=0.044). All correlations were uncorrected for multiple comparisons. No correlations were observed in controls between task behavior and any neuropsychological tests. Within DLPFC, where significant group differences were found on the n-back task, no significant correlations were observed between DLPFC BOLD activity and any neuropsychological test or PRO measure for either group.
For controls, MicroCog time 1 was positively correlated with functional well-being and FACT-G total, and negatively correlated with HADS depression. RBANS attention was positively correlated with FACT-Cog comments from others. For patients, numerous correlations were observed between PRO and neuropsychological measures; a detailed table can be found in Supplementary Table 1. For example, slower reaction times on the Microcog tests were associated with more negative comments from others regarding their cognition. Surprisingly, physical and functional well-being were negatively associated with performance on RBANS total index score and the WAIS Letter-Number scaled score. Similarly, FACT-G total scores were negatively correlated with several measures of memory and working memory; and a higher level of self-reported depression symptoms was correlated with better performance on working memory tasks. None of the correlations survived multiple comparison correction.
Discussion
The primary finding of this study was that oncology patients showed reduced activation in the DLPFC during a working memory task, as compared with controls. This research extends and helps clarify a growing literature that links working memory deficits in oncology patients to aberrant activity in frontal brain regions16, 17. Our results remained unchanged after covarying for fatigue, depression and anxiety, suggesting that oncology patients did not recruit the right DLPFC sufficiently to perform this task. Another finding was that oncology patients exhibited hypoactivation during visual recognition in the left hippocampus, a brain region involved in encoding and retrieval in episodic memory and in the maintenance of learned information. These functional deficits are consistent with findings in oncology patients showing reduced gray matter in frontal and MTL regions 9. Similarly, studies in human and animal models have linked a pattern of white matter degradation in fronto-temporal circuits to chemotherapy-related cognitive decline39.
Secondarily, we hypothesized that patients would differ from controls on behavioral correlates of working memory and visuospatial recognition memory. For the working memory task, moderate effect sizes indicated that oncology patients performed worse than controls as task difficulty increased from 0- to 2-back (Table 4). Patients showed more missed items in recognition performance, suggesting that oncology patients may have a specific problem encoding and maintaining initially acquired information. This is plausible considering that the participants viewed the fractals without knowing that they would later perform a recognition task.
Finally, we explored associations between task behavior, neuropsychological test performance and patient reported cognitive function. Due to our criteria on neuropsychological performance, oncology patients showed impairments in executive functioning, manipulation, attention, and working memory. We observed positive correlations between recognition hit rates and RBANS attention scores, Trail Making B and Microcog time 1 and time 2 in patients. The effects of chemotherapy may be driving the deficits in both behavior and neuropsychological performance, resulting in this patient-specific correlation. However chemotherapy-related impairments, including cancer-related symptom burden, may also contribute to these deficits and will need to be examined in future studies. A growing number of studies reported associations between patient-reported cognitive function and activation or connectivity, even in the absence of impaired performance on neuropsychological tests10, 16, 42. However in the current study, neuroimaging correlates were not significantly associated with PRO measures, including patient-reported cognitive impairments, fatigue, depression, and anxiety. This may be due to limitations in study design outlined below.
Limitations of the study include a relatively small sample size, which likely contributed to the lack of relationship between patient-reported cognitive function and brain activation. Also, we did not assess for pre-existing difficulties, or account for insomnia, sleep/wake deficits or alterations in sleep quality, which has been shown to commonly affect cancer patients43, 44. The oncology group was not homogenous in terms of cancer type and gender, and included a large proportion of female breast cancer patients, which may have resulted in differences in activation and behavior. Furthermore, our paradigm combined encoding of each new image into working memory, reorganization, and inhibition, which made the task particularly difficult as the load increased. Future research should explicitly address differences between encoding and retrieval phases of memory tasks, and attempt to separate the effects of chemotherapy from cancer by assessing patients before and after chemotherapy, as well as comorbid symptoms such as fatigue, sleep disturbances, and emotional distress. We are currently conducting a more comprehensive study of cognition, brain activation and brain circuitry in CRCI to address many of these limitations.
In summary, we showed that CRCI was accompanied by under-recruitment of DLPFC and hippocampal regions. Follow-up fMRI experiments should examine specific brain circuitries to examine the neural mechanisms, including potential compensatory mechanisms underlying CRCI.
Supplementary Material
Acknowledgement
This work was supported by NIH grants 1 R01 NR014182-01, and T32-NS047987, an unrestricted educational grant from Ortho Biotech, and The Lynn Sage Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Lynn Sage Cancer Research Foundation.
This experiment was realized using Cogent 2000 developed by the Cogent 2000 team at the FIL and the ICN and Cogent Graphics developed by John Romaya at the LON at the Wellcome Department of Imaging Neuroscience.
Dr. Wagner consulted with Gilead Inc within the past 2 years, interpreting PRO data.
Footnotes
No other financial disclosures
References
- 1.Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat Support Care. 2007;5:273–280. doi: 10.1017/s1478951507000442. [DOI] [PubMed] [Google Scholar]
- 3.de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. 2012;33:2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherling CS, Smith A. Opening up the Window into “Chemobrain”: A Neuroimaging Review. Sensors. 2013;13:3169–3203. doi: 10.3390/s130303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3:223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 7.de Ruiter MB, Reneman L, Boogerd W, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30:2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 10.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- 11.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nokia MS, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci. 2012;36:3521–3530. doi: 10.1111/ejn.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behavioural Brain Research. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 15.Wagner LI, Berg SR, Gandhi M, et al. The development of a Functional Assessment of Cancer Therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18) Supportive Care in Cancer. 2013;21:1033–1041. doi: 10.1007/s00520-012-1623-4. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38:926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- 20.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- 22.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 23.D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 24.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 26.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69:1122–1130. doi: 10.4088/jcp.v69n0712. [DOI] [PubMed] [Google Scholar]
- 27.Elwood RW. MicroCog: Assessment of cognitive functioning. Neuropsychology Review. 2001;11:89–100. doi: 10.1023/a:1016671201211. [DOI] [PubMed] [Google Scholar]
- 28.Wagner LI, Sweet JJ, Butt Z, Lai JS, Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. The Journal of Supportive Oncology. 2009;7:W32–W39. [Google Scholar]
- 29.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 30.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Lai JS, Butt Z, Wagner L, et al. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage. 2009;37:982–995. doi: 10.1016/j.jpainsymman.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragland JD, Turetsky BI, Gur RC, et al. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- 34.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 35.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 36.Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. J Affect Disord. 2006;90:149–161. doi: 10.1016/j.jad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SK, Lange G, DeLuca J, Korn LR, Natelson B. The effects of fatigue on neuropsychological performance in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol. 1997;4:145–153. doi: 10.1207/s15324826an0403_1. [DOI] [PubMed] [Google Scholar]
- 38.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- 39.Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behavioural Brain Research. 2012;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 41.van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 42.Kesler SR, Wefel JS, Hosseini SM, Cheung M, Watson CL, Hoeft F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci U S A. 2013;110:11600–11605. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- 44.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.