Abstract
Myocardial perfusion and coronary vascular resistance are regulated by signalling metabolites released from the local myocardium that act either directly on the vascular smooth muscle cells (VSMC) or indirectly via stimulation of the endothelium. A prominent mechanism of vasodilation is endothelium-derived hyperpolarization (EDH) of the arteriolar smooth muscle, with epoxyeicosatrienoic acids (EETs) and hydrogen peroxide (H2O2) playing important roles in EDH in the coronary microcirculation. In some cases, EETs and H2O2 are released as transferable hyperpolarizing factors (EDHFs) that act directly on the VSMCs. By contrast, EETs and H2O2 can also promote endothelial Ca2+-activated K+ channel activity secondary to the amplification of extracellular Ca2+ influx and Ca2+ mobilization from intracellular stores, respectively. The resulting endothelial hyperpolarization may subsequently conduct to the media via myoendothelial gap junctions, or potentially lead to the release of a chemically-distinct factor(s). Furthermore, in human isolated coronary arterioles dilator signalling involving EETs and H2O2 may be integrated; being either complimentary or inhibitory depending on the stimulus. With an emphasis on the human coronary microcirculation, this review addresses the diverse and integrated mechanisms by which EETs and H2O2 regulate vessel tone, and also examines the hypothesis that myoendothelial microdomain signalling facilitates EDH activity in the human heart.
Keywords: human coronary microcirculation, coronary blood flow, endothelium-derived hyperpolarization, endothelium-derived hyperpolarizing factor, Ca2+-activated K+ channels, epoxyeicosatrienoic acids, hydrogen peroxide, gap junctions, myoendothelial signalling microdomains, transient receptor potential channels, connexins, cytochrome P450 epoxygenases, NADPH oxidase, mitochondrial electron transport chain, nitric oxide, coronary artery disease
Introduction
The intrinsically high vasomotor tone of arterioles (50-200 μm in diameter) under basal conditions confers the majority of coronary vascular resistance to the arterial microcirculation [26, 12]. These vessels are highly responsive to vasodilator stimuli (e.g. mechanical, humoral, neural and metabolic factors), and play a key role in mediating acute and chronic changes in coronary blood flow (up to ~5-fold increase) to match O2 consumption of the myocardium with metabolic demand [83, 12]. This same arteriolar segment of the coronary circulation is also responsible for autoregulation, which maintains coronary blood flow steady in response to fluctuations in mean arterial pressure [91, 12]. Exhaustion of coronary vasodilator reserve, which typically precedes the progressive obstruction of conduit coronary arteries, results in reduced maximal coronary dilation, leading to myocardial ischaemia and potentially infarction should oxygen demand increase, such as during stress [83, 12].
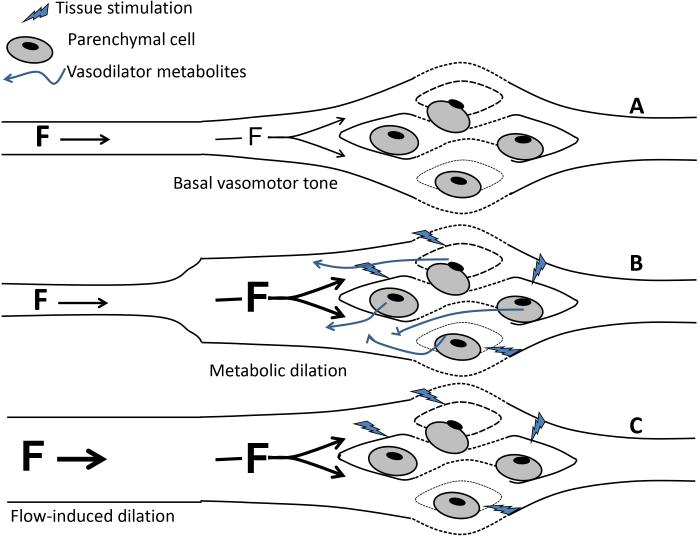
Decreased arteriolar tone initiated by metabolites released from the local cardiomyocytes (e.g. during increased heart rate and ventricular contraction) increases coronary blood flow. This initiating metabolic dilation is then supplemented by endothelium-dependent, FID, which amplifies dilation (and hence flow) in the metabolically active segment of myocardium (Figure 1).
Figure 1.
Physiological benefit of arterial flow-induced dilation (FID; communication between arterioles and upstream feed arteries). (A) Basal arteriolar tone governs tissue perfusion under baseline conditions. (B) When parenchymal cells (e.g. cardiomyocytes) become active they release vasodilator metabolites that reduce resistance in local arterioles, increasing flow (F), since the majority of resistance occurs in this segment of the circulation. With the resulting vasodilation more of the overall organ resistance shifts to upstream arteries. (C) Conservation of mass requires that an increase in flow locally be matched to an equal increase in flow in all upstream feed arteries. This increase in flow evokes endothelium-dependent FID in the upstream vessels, further reducing resistance and increasing coronary blood flow, potentially up to ~5 fold.
Coronary arterioles are also responsive to locally synthesized humoral factors that also induce vasodilation via actions on the endothelium. These include ADP, ATP, adenosine [156], calcitonin gene-related peptide [22], noradrenaline [112], neuropeptide Y [66], serotonin, histamine [135], bradykinin and thrombin; several of which have been employed as experimental agonists of vasodilation of human coronary arterioles (for examples see references [95, 13, 135, 77, 76]). Agonist- or flow-induced vasodilation that is independent of endothelium-derived NO and PGI2 (or prostacyclin) is typically due to EDH of the underlying VSMCs [43]. Collectively, these mechanisms play an important role in optimizing myocardial perfusion and regulating peripheral coronary vascular resistance. In coronary arterioles obtained from patients with CAD, EDH-mediated dilation is potentiated compared to the same tissue obtained from patients without CAD, and these amplified responses offset the loss of NO to maintain virtually full dilator capability [96]. The EC-derived ROS H2O2 and fatty acid epoxides EETs are important regulators of VSMC membrane potential and tone in the human coronary microcirculation, as well as in selected coronary, carotid, cerebral, renal, mesenteric, submucosal and skeletal muscle arteries from various species [17, 126, 43]. In the coronary microcirculation of patients with CAD there are unique interactions between EETs and H2O2 that differ depending on the endothelial stimulus, and thus the role of these two EDH-related signalling molecules forms a major focus of the current review.
The transfer of EC hyperpolarization to the underlying VSMCs via myoendothelial gap junctions is becoming increasingly appreciated as an important general mechanism of EDH. Furthermore, signalling microdomains associated with close contact sites between EC and VSMC membranes is important not only for supporting gap junction presence and function, but may also provide a low resistance conduit for the passage of transferable EDHFs to the VSMCs. Considering that myoendothelial signalling microdomains and gap junctions are becoming increasingly recognized as an important facet of human artery function, this will also form a major focus as an important avenue for future research in the microcirculation of the human heart.
EDH: an overview
The ubiquitous driving force for maximal EDH activity is a stimulated elevation in EC [Ca2+]i that leads to the opening of KCa channels and EC hyperpolarization [29]. Ultimately, this leads to VSMC hyperpolarization, but the mechanisms that link these two events are controversial, and differ depending on the type or size of artery, species, disease state, developmental stage and specific experimental conditions [59, 29]. In some vessels, EDH may be mediated by a transferable EDHF that initiates VSMC hyperpolarization directly following its release from the endothelium [18, 40, 82]. Under specific experimental conditions and in specific tissues the molecular identity of EDHF has been variously proposed as EETs, H2O2, K+ ions, the endocannabinoid anandamide, CNP, nitroxyl, hydrogen sulphide, adenosine and NO [108, 40, 58, 92, 23, 7, 82]. Although anandamide and CNP have subsequently been ruled out as EDHFs [54, 120], it is nevertheless intriguing to consider the physiological benefit of having such a diverse repertoire of transferable factors that could potentially be involved in VSMC hyperpolarization. Common arguments against the significance of a transferable EDHF(s) is: (a) whether it is produced in sufficient amounts by the endothelium to activate its putative VSMC effector target, and (b) a range of KCa channel blockers (including the primary EC localized KCa channels; and and on occasion of SM BKCa) is required to fully prevent EDH activity (also see Section “Endothelial and smooth muscle hyperpolarization: central role of KCa channels”).
Distinct from EDHF-mediated vasodilation, in a number of tissues EC hyperpolarization is transmitted into the subintimal VSMCs via coupling of the two cell layers by myoendothelial gap junctions. This hyperpolarization subsequently spreads in a radial manner through the different layers of the vessel media via homocellular VSMC gap junctions [29], and longidtudinally via (primarily) homocellular EC gap junctions [6]. Furthermore, in addition to their EDHF-type actions, EETs and H2O2 may in some tissues promote EC extracellular Ca2+ influx and mobilization from intracellular ER stores, respectively [50, 39]. In these cases EETs and H2O2 act as autocrine signalling intermediaries that amplify and/or prolong EC hyperpolarization, thereby promoting vasodilation in a manner independent of their release from the endothelium. Notably, EETs and H2O2 may also modulate gap junctional communication, and therefore affect the relay of EC hyperpolarization into and along the vessel wall [104, 29, 43].
Endothelial and smooth muscle hyperpolarization: central role of KCa channels
EC hyperpolarization mediated by the opening of KCa channels is recognised as the important initiating step for EDH-mediated vasodilation. ECs express small-, intermediate- and, in some cases (notably, in intact arteries in some models of hypoxia-related disease [68]), large-conductance KCa channels, variably designated as SKCa/KCa2.3/SK3, IKCa/KCa3.1/IK1 and BKCa/KCa1.1/BK1/Slo1-MaxiK, respectively [43]. Notably, while BKCa have been described in isolated ECs of a few vessel types (in hypoxia, or a stressed culture state), native ECs of healthy vessels generally lack the regulatory β subunit that confers full sensitivity to Ca2+. Most vascular BKCa currents are in fact confined to VSMCs [113, 43]. Interestingly, in vessels such as the porcine coronary (conduit) artery, BKCa-mediated VSMC hyperpolarization may be transmitted to the overlying endothelium via low resistance heterocellular coupling ([11, 143]; also see Section “Cxs, gap junctions and myoendothelial signalling microdomains: a brief overview”).
In a number of arteries SKCa and IKCa can be expressed within different subcellular EC compartments, and thus are spatially separated; findings that are thought to confer their specialised roles in endothelium-dependent vasodilation [118, 69, 119, 43]. Indeed, SKCa are suggested to be abundant in caveolae, where they may play a role in the activation of NOS by electrochemically driving Ca2+ entry at these sites. In some arteries, SKCa are also relatively enriched at EC-EC junctions proximal to interendothelial gap junction plaques [118, 1, 32]. By contrast, in several vessels IKCa are preferentially expressed within EC projections [118, 21, 5] that extend through fenestrations/holes in the IEL to establish close apposition with neighbouring subintimal VSMCs. These connections are variably designated as MECCs, or myoendothelial junctions (for reviews see references [119, 43]; also see Sections “Cxs, gap junctions and myoendothelial signalling microdomains: a brief overview” and “Myoendothelial signalling microdomains and gap junctions: a role in the human coronary microcirculation?”).
Pretreatment of vascular preparations with apamin (a selective SKCa blocker) plus charybdotoxin (non-selective blocker of IKCa and BKCa) or the triple combination of apamin plus TRAM (selective blocker of IKCa) plus iberiotoxin (selective blocker of BKCa) consistently abolishes EDH activity in coronary arteries of humans [95, 13, 8, 77], pigs [41, 16, 7, 143], rats [138, 4], guinea pigs [152, 27], dogs [102] and mice [90, 103]; thus highlighting the ubiquitous role of KCa in EDH activity. However, the relative roles of individual KCa subtypes and the concerted activity of multiple subtypes is highly variable between species, coronary vascular region and exact experimental conditions (see Table 1 for details) [99, 37, 54, 105, 152, 95, 101, 57, 27, 41, 16, 7, 8, 10, 138, 143, 4, 77, 9, 47, 82].
Table 1.
Pharmacological diversity of endothelial cell (EC) small- and intermediate- and vascular smooth muscle cell (VSMC) large-conductance Ca2+-activated K+ channels (SKCa, IKCa and BKCa, respectively) in EDH-mediated responses. Administration of apamin (selective blocker of SKCa), TRAM (selectively for IKCa), iberiotoxin (IbTx; selective for BKCa) and charybdotoxin (CbTx; non-selective blocker of IKCa and BKCa), either alone or in various combinations, reveal heterogenous dependency on individual channel subtypes and their concerted activity to a range of endothelial stimuli, including the agonists bradykinin (Bk), acetylcholine (ACh), thrombin (Tmb) and substance P (SP), as well as the haemodynamic stimuli of flow, pulsatile perfusion (PP) and pressure oscillations (P.osc; to induce rhythmic cyclic stretch). EDH is variably studied as EC and VSMC hyperpolarization (EC-hyp and VSMC-hyp, respectively), and EDH-mediated dilations. EC stimuli are generally (but not always) studied following the induction of smooth muscle tone with a variety of agonists, such as U46619 (a thromboxane A2 analogue), prostaglandin F2α (PGF2α), 2,2-aminoehylpyridine (AEP) and endothelin-1, as well as following pressure-induced myogenic tone (P).
| species | coronary vessel | tone | stimulus | attenuated/abolished | unaffected | refs |
|---|---|---|---|---|---|---|
| human | arterioles (CAD) | P+ET-1 | Bk | CbTx>apamin | IbTx | [95, 77] |
| arterioles (non-CAD) | U46619 | Bk | apamin+CbTx>apamin+IbTx | apamin/CbTx/IbTx/apamin+IbTx | [8] | |
| arterioles (CAD) | P+ET-1 | Tmb | CbTx | IbTx | [13] | |
| arterioles (CAD) | P+ET-1 | flow | CbTx/IbTx | nd | [94, 82] | |
| porcine | conduit (EC-hyp) | none | Bk | apamin+TRAM+IbTx>apamin+TRAM | TRAM | [143] |
| none | SP | apamin+TRAM>apamin | IbTx/TRAM | [16, 143] | ||
| conduit (VSMC-hyp) | none | Bk | apamin+TRAM+IbTx>apamin+TRAM | IbTx | [41, 143] | |
| SP | apamin+TRAM | IbTx | ||||
| conduit | P | P.osc | apamin+IbTx | nd | [105] | |
| conduit | U46619 | Bk | apamin+CbTx | apamin+IbTx | [8] | |
| conduit | U46619 | Bk | apamin+TRAM | apamin+IbTx | [9] | |
| arterioles | U46619 | Bk | apamin+CbTx>CbTx=IbTx | apamin | [57] | |
| arterioles | U46619 | Bk | apamin+CbTx>apamin+IbTx>IbTx | apamin/CbTx | [10] | |
| canine | conduit | PGF2α | ACh | CbTx | IbTx/apamin | [99] |
| conduit | P | PP | apamin+CbTx>apamin+IbTx | nd | [102] | |
| arterioles | P | ACh | IbTx | nd | [101] | |
| rat | perfused heart | P | Bk | CbTx | IbTx | [54] |
| small arteries | P | flow | apamin+CbTx | nd | [138, 4] | |
| arterioles | P | ACh | IbTx | nd | [47] | |
| guinea-pig | conduit | PGF2α | ACh | apamin+CbTx | apamin+IbTx | [152] |
| conduit (rel+VSMC-hyp) | AEP | ACh | nd | apamin/IbTx | [37] | |
| conduit | U46619 | ACh | apamin (slight)/CbTx/apamin+CbTx | nd | [27] | |
| arterioles | U46619 | ACh | CbTx/apamin+CbTx | nd | ||
| murine | conduit | PGF2α | ACh | apamin+CbTx | nd | [90] |
| arterioles | P | ACh | apamin+CbTx | nd | [103] |
nd none determined. >denotes preceding reagent combinations as reducing EDH-type responses to a greater extent.
In porcine conduit coronary arteries, EC and VSMC hyperpolarization evoked by substance P is abolished following the combined administration of apamin and TRAM, whereas those evoked by bradykinin are only partially attenuated by this treatment, with residual apamin+TRAM-insensitive responses being abolished by iberiotoxin [41, 16, 143]. Furthermore, in coronary arterioles obtained from patients with CAD either apamin or charybdotoxin attenuate EDH-mediated dilation to bradykinin (indicative of the participation of SKCa and IKCa and/or BKCa) [95, 77, 76], whereas in tissues from patients without CAD apamin plus charybdotoxin fully blocks dilation but apamin plus iberiotoxin is without any effect (indicative of sole mediation by IKCa) [8]. Besides the vessel disease state, a possible explanation for these disparate findings may relate to the mode of induction of vessel tone employed prior to the study of dilator responses (i.e. pressure-induced myogenic tone supplemented by endothelin-1 in tissues from CAD patients [95, 77, 76] and the thromboxane A2 analogue U46619 in non-CAD tissues [8]; see also Table 1). Notably, in cerebral arteries U46619 can attenuate SKCa activity, and in isolated coronary VSMCs U46619 variably attenuates or enhances BKCa activity through interaction with the pore-forming α and regulatory β subunits, respectively (for review see reference [44]).
A further possibility to consider is the absolute level of tension experimentally applied to arterial preparations. As VSMCs depolarize there is a progressive increase in the open probability of BKCa (which are also intrinsically sensitive to changes in voltage). Furthermore, as tension develops, accumulated IP3 can be transferred from the depolarized VSMCs to the overlying ECs via myoendothelial gap junctions. The result is S/IKCa activation and hyperpolarization that serves to moderate myogenic or agonist-induced constriction (designated as “myoendothelial feedback”) [69, 70]. Thus, the level of KCa activity induced following increased VSMC tone could limit further activation of these channels following EC stimulation, and thus their ability to participate in EDH.
Biosynthesis and bioavailability of endothelium-derived EETs and H2O2
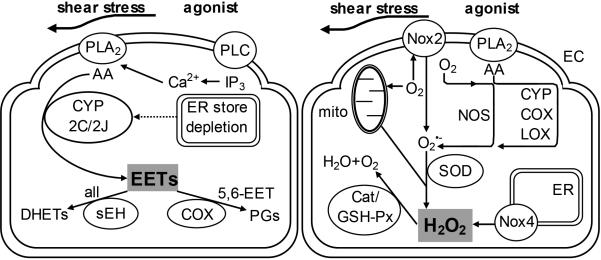
In response to haemodynamic stimuli, such as cyclic stretch and shear stress, and physiological agonists, such as bradykinin and acetylcholine, ECs synthesize a combination of four EET regioisomers (5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET) in varying proportions via CYP epoxygenases (primarily the 2C and 2J isoenzymes) from various lipid substrates that include arachidonic, linoleic, eicosapentaenoic and docosahexaenoic acids [43]. Bioavailability of all regioisomers is primarily determined by sEH, which hydrate EETs to form corresponding DHET regioisomers, whilst that of 5,6-EET is also determined by COX activity (which metabolizes this regioisomer into prostanoids [43]; Figure 2).
Figure 2.
Biosynthesis and bioavailability of endothelial cell (EC)-derived epoxyeicosatrienoic acids (EETs) and hydrogen peroxide (H2O2). The activation of phospholipase A2 (PLA2) following stimulation with shear stress or secondary to inositol 1,4,5-trisphosphate (IP3)-sensitive endoplasmic reticulum (ER) Ca2+ store depletion by agonists leads to synthesis of arachidonic acid (AA), which is metabolized by cytochrome P450 (CYP) 2C or 2J isoenzymes to produce EETs; with soluble epoxide hydrolases (sEH; for all EET regioisomers) and cycolooxygenase (COX; for 5,6-EET only) metabolizing EETs to dyhydroxyeicosanoic acids (DHETs) and prostaglandins (PGs) respectively, thereby influencing EET bioavailability (left panel). Shear stress and agonist stimulation also results in the reduction of molecular O2 to form the reactive oxygen species (ROS) superoxide , as a by-product of metabolism, by a number of sources, including nitric oxide synthases (NOS), CYP, COX, lipooxygenase (LOX) and mitochondria (mito). is then further reduced by superoxide dismutases (SOD) to form H2O2, the bioavailability of which is determined by endogenous antioxidant enzymes, which include catalase (Cat) and glutathione peroxidease (GSH-Px). Nicotinamide adenine dinucleotide phosphate oxidase isoforms (Nox2 and Nox4) synthesize ROS as their sole enzymatic product, with Nox2 producing and Nox4 mainly H2O2 (right panel). PLC, phospholipase C.
Evidence of a role for a selective EET receptor in arteries rests on VSMC BKCa activation by EETs requires guanosine triphosphate (GTP)-dependent adenosine diphosphate (ADP)-ribosylation of Gs proteins [80, 81, 53]; a finding consistent with the ability of EETs to elevate cAMP in ECs [104, 87]. Indeed, photoaffinity labelling techniques have demonstrated the ability of a particular 47 kDa candidate receptor to bind EETs (with the rank order of potency 14,15-EET>11,12-EET>8,9-EET) within the nM range that is typically associated with EDH activity (and hence at concentrations much lower than the μM range in which EETs bind to other eicosanoid receptors). This particular candidate receptor was found to be expressed in the plasmalemma of VSMCs and, to a lesser degree, ECs of bovine coronary arteries ([25]; and for a review on potential EET binding sites in the vasculature see [43]). By contrast, certain regioisomers (5,6-EET, 8,9-EET and, variably, 11,12-EET [within the low-to-mid nM range]; but not 14,15-EET, except at an arguably non-physiological threshold of 1-3 μM) may also be endogenous agonists of TRPV4 channels in ECs and VSMCs, and therefore mediate EDH activity in a manner distinct from the occupation of a selective EET receptor [141, 36, 139, 3].
During the production of EETs, CYP epoxygenases produce ROS, namely , as a by-product of lipid metabolism. In isolated porcine coronary arteries and in patients with CAD, levels of CYP-derived ROS may reach levels that significantly attenuate NO-mediated vasodilation [49]. ECs are also capable of producing ROS from a number of other sources, including several NADPH oxidase (Nox) isoforms, the mitochondrial electron transport chain, uncoupled NOSs, xanthine oxidase, COXs and lipooxygenases ([142, 42]; Figure 2). The majority of these sources reduce molecular O2 to produce , which reacts rapidly with NO to form ONOO−, thus reducing NO bioavailability and associated dilator responses [51]. Indeed, ONOO− may exacerbate ROS formation by oxidizing the NOS cofactor BH4, leading to the uncoupling of NOS and an increase in formation by the oxygenase component of the enzyme [51]. anions are further reduced by SODs (Cu/Zn-SOD [cytosolic], Mn-SOD [mitochondrial] and extracellular SOD) to form H2O2 (Figure 2); an uncharged ROS that has been suggested to be an important signalling mediator for diverse aspects of coronary circulatory homeostasis, including autoregulation [147], reactive hyperaemia [72] and coronary dilation coupled to increased myocardial metabolism such as during tachycardia [71, 148], and may also be involved in cardioprotection during ischaemia-reperfusion [146]. Notably, of the Noxs typically expressed in the endothelium (Nox2, Nox4 and Nox5), Nox4 has been proposed to produce H2O2 directly without intermediary , and is purportedly a vasculoprotective isoform and a potential physiological regulator of vessel tone [97, 109, 123].
Noxs differ with regard to their requirement for cytosolic subunits and in the regulation of their activity. For example, Nox2 (formerly known as the phagocytic oxidase, or gp91phox) resides with the regulatory subunit p22phox, and these collectively form a flavocytochrome b558 heterodimer, with full oxidase activity requiring association of the heterodimer with cytosolic subunits (including p47phox, p67phox and the GTPase Rac1) [19]. Increases in Nox2 activity are linked to receptor occupation by certain agonists (including angiotensin II and bradykinin) as well as by risk factors known to promote endothelial dysfunction and cardiovascular disease, such as diabetes mellitus, hypertension, LDL and arsenic intoxication [137, 34, 76, 136, 38, 42]. By contrast, neither Nox4 nor Nox5 require cytosolic subunits for oxidase activity, with Nox4 being constitutively active and Nox5 activated by Ca2+ [97].
Integrated regulation of coronary vasodilation by EETs and H2O2
A number of studies have collectively highlighted diverse roles of EC-derived EETs and H2O2 in the regulation of VSMC tone in the human coronary microcirculation, with considerable variation being apparent under different experimental conditions and/or disease state. In general, EETs and H2O2 do not share common EC/VSMC effector targets to initiate decreases in vessel tone (an obvious exception being VSMC BKCa [17, 82]), but there is nevertheless accumulating evidence of both complementary and inhibitory interactions between their respective signalling pathways, indicative of integrated EDH signalling.
Evidence proposed to confer a role for EETs and H2O2 as transferable EDHFs (Figure 3) has been obtained from serial perfusion (or cascade) bioassays, in which effluent perfused serially (or collected) from an agonist- or flow-stimulated endothelium-intact “donor” artery evokes hyperpolarization and dilation of a downstream (or isolated) endothelium-denuded “detector” preparation [18, 58, 67, 77, 82].
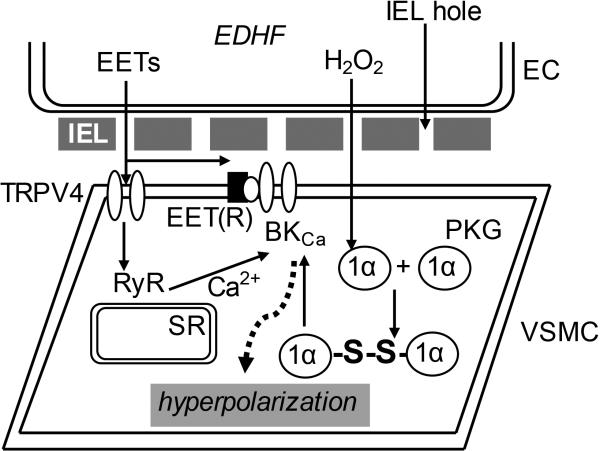
Figure 3.
Endothelium-derived hyperpolarizing factor (EDHF)-type actions of epoxyeicosatrienoic acids (EETs) and hydrogen peroxide (H2O2) in the coronary circulation. Endothelium-derived EETs activate vascular smooth muscle cell (VSMC) large conductance Ca2+-activated K+ channels (BKCa) potentially via two different mechanisms; through coupling via the putative EET receptor [EET(R)], or by activating VSMC transient receptor potential vanilloid type 4 (TRPV4) channels to induce localized Ca2+ influx that activated sarcoplasmic reticulum (SR) ryanodine receptors (RyR) to trigger spatially discrete, non-propagating Ca2+ sparks that in turn activate BKCa. BKCa activation by H2O2 occurs via a distinct mechanism, whereby the ROS triggers dimerization of VSMC protein kinase G (PKG) 1α monomers to form PKG heterodimers via disulphide bridge (-S-S-) formation at cysteine42. This residue acts as a redox sensor that, when activated, increases the affinity of PKG for BKCa. The low resistance diffusion of EETs and H2O2 from EC to VSMC is potentially facilitated by holes in the internal elastic lamina (IEL) separating the two cell types.
Complementary roles of EETs and H2O2 in flow-induced dilation of human coronary arterioles
In coronary arterioles of CAD patients FID is attenuated by the H2O2 degrading enzyme catalase [94, 82]. Accordingly, analyses carried out in serial perfusion bioassays demonstrate that: (a) when flow proceeds from donor to detector both vessels dilate, but in the reverse direction only the donor vessel dilates; (b) detector dilation to perfusate originating from the donor is prevented by PEG-Cat applied to either the detector vessel directly or through the donor lumen; (c) compared to untreated donor effluents, dilation of detector vessels is attenuated when these are incubated with donor effluents that have previously been passed through catalase-bound bead columns; and (d) donor vessel effluent evokes PEG-Cat- and iberiotoxin-sensitive VSMC K+ currents in cell-attached patches [82]. In these same vessels H2O2-mediated activation of VSMC BKCa occurs through a cGMP-independent, PKG-1α-mediated mechanism that involves dimerization of the kinase [153] (Figure 3), presumably through oxidation and disulphide bridge formation via the cysteine42 redox sensor [15, 106]. By contrast, H2O2 exerts direct inhibitory actions on BKCa (through interactions with cysteine 911 within the Ca2+ bowl region of the α subunit) when applied selectively to the cytosolic side of the channel [133, 88]. The involvement of H2O2 in EDHF activity may therefore rest on potentially competing phosphorylation/oxidation actions on BKCa.
Even though compelling evidence supports H2O2 as a flow-induced EDHF in the human coronary microcirculation [82], it should be noted that FID of these arterioles is attenuated by miconazole [96] (an epoxygenase inhibitor and EET antagonist [25]) to a similar extent as seen with preparations incubated with catalase (~60-70%) [96, 94]. Notably, in contrast to the same tissue stimulated with bradykinin, EETs are not detected in the downstream effluent of endothelium-intact human coronary arterioles exposed to flow (DX Zhang and DD Gutterman, unpublished observations; also see Section “Comparison of bradykinin- and flow-induced dilation of human coronary arterioles”). While it has been observed that miconazole at 3 or 10 μM can directly inhibit KCa currents, this was not observed at the 1 μM concentration that was found to reduce FID in human coronary arterioles [62, 96]. It is therefore more likely that the inhibitory effect of miconzole against FID actually reflects reduced EET synthesis and/or action, rather than inhibition of KCa. Collectively, these findings indicate that EETs facilitate EDH activity in an autocrine manner, with H2O2 subsequently serving as the transferable mediator of vasodilation ([33, 43]; Figure 4). In line with this proposal, FID in this tissue is also dependent on the activation of EC TRPV4 channels [14], for which most EETs (with the exception of 14,15-EET) are potent endogenous agonists [141, 36, 89]. Indeed, EETs have been demonstrated as an essential intermediary involved in flow-induced EC TRPV4 activity and dilation of the murine carotid artery [87]. It is therefore plausible that in the human coronary microcirculation EETs are signalling transducers of shear stress, essential for TRPV4 activation and the synthesis and/or release of dilator H2O2. The mechanism linking potential EET-TRPV4 activity with mitochondria-derived H2O2 synthesis/release from the endothelium of these vessels as yet remains unexplored (also see Section “Comparison of bradykinin- and flow-induced dilation of human coronary arterioles”).
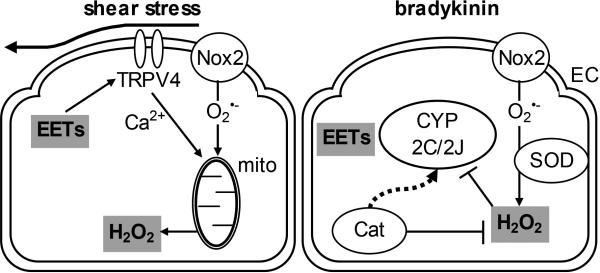
Figure 4.
Signalling interactions between epoxyeicosatrienoic acids (EETs) and hydrogen peroxide (H2O2) in endothelial cells (EC) of the human coronary microcirculation. During shear stress stimulation the production of EETs by cytochrome P450 isoforms (CYP 2C and/or 2J) leads to activation of transient receptor potential vanilloid type 4 (TRPV4) channels to induce extracellular Ca2+ influx. TRPV4-dependent Ca2+ influx plus superoxide formation by Nox2 are required to trigger H2O2 release from mitochondria, with dilator responses attenuated to similar degrees by the H2O2 degrading enzyme catalase (Cat) or inhibition of epoxygenase/antagonism of EETs (left panel). By contrast, stimulation with bradykinin triggers Nox2-dependent formation, and H2O2 formed following the dismutation of directly inhibits EET synthesis by CYP 2C and 2J; with EET synthesis and action being de-restricted in the continuous presence of Cat (right panel).
The idea that epoxyeicosanoids play a critical role in transducing shear stress via TRPV4 is, however, challenged by the observation that increased TRPV4 activity that follows the addition of arachidonic acid may not be adequately explained by the accumulation of EETs. Indeed, arachidonic acid is a significantly more potent inducer of TRPV4 activity than EETs in human coronary ECs [154], while, notably, DHETs are generally even less potent [78, 77, 76]. Notably, this effect of arachidonic acid depends on membrane hyperpolarization [154]; a finding that contrasts with the corresponding ability of EETs, which promote EC hyperpolarization secondarily to extracellular Ca2+ influx [35]. Indeed, the effects of selective SKCa and IKCa blockers (e.g. apamin and TRAM) against flow-induced EC TRPV4 activity in the human coronary microcirculation remain to be determined; evidence that could potentially clarify which of arachidonic acid or EETs (or both) underpin the mechanotransduction of shear stress. Indeed, none of the four EET regioisomers within the purported physiological nM range alter [Ca2+]i in human coronary ECs when applied exogenously [30, 154]. It is thus unknown whether or not EETs: (a) share a common TRPV4 binding site with arachidonic acid (putatively an arachidonate recognition sequence located near the intracellular N-terminus [100]), and thus whether (b) EETs and arachidonic acid play distinct but cooperative roles in initiating and sustaining TRPV4 activity with changes in membrane potential.
Comparison of bradykinin- and flow-induced vasodilation in human coronary artrerioles
In contrast to FID, bradykinin-evoked, catalase-sensitive EDH-type dilation of human coronary arterioles is unaffected by iberiotoxin, the selective CYP 2C inhibitor sulfaphenazole or the EET antagonist 14,15-EEZE, with inhibitory effects of these agents being unmasked only in preparations incubated continuously with catalase [77]. Together with the demonstration that EETs do not undergo direct oxidative modification, these findings have led to the proposal that bradykinin-evoked H2O2 accumulation directly inhibits epoxygenases and suppresses EET generation ([77]; Figure 4). This inhibitory interaction contrasts with the apparently interdependent facilitator and mediator roles of EETs and H2O2, respectively, in FID (see Section “Complimentary roles of EETs and H2O2”). In addition, while H2O2 appears to fulfil the criteria of a transferable EDHF in dilator responses to both flow and bradykinin, the VSMC effector target in each case is different; being BKCa for the former and an as yet unidentified iberiotoxin-insensitive hyperpolarizing target for the latter [77, 82]. These differences are puzzling, but, speculatively, may relate to: (a) the different principal sources of H2O2 generation (i.e. mitochondrial electron transport chain for flow [85, 84, 14] and Nox2 for bradykinin [76]); (b) differing absolute concentrations of H2O2 generated intracellularly; (c) [related to (a) and (b)], the existence of discrete ROS signalling microdomains specific to each stimulus and spatially confined to cell regions where only the respective effector target may be activated; (d) a potential physiological role for arachidonic acid itself in FID, which would, in theory, negate the need for CYP activity (N.B. The efficacy of more selective epoxygenase inhibitors, such as sulfaphenazole, against FID in this vascular bed have not yet been tested); or (e) disparate experimental methodology. It should be noted, however, that flow-induced mitochondrial H2O2 accumulation and dilation in fact depends on Nox2 activity (BT Larsen, DX Zhang and DD Gutterman, unpublished observations), whereas the Nox2-dependent elevation in H2O2 in response to bradykinin is not associated with alterations in mitochondrial ROS generation [76]. Speculatively, the differential effects of bradykinin- and flow-evoked Nox2 activity on the generation and release of mitochondrial ROS may rest on: (a) synergistic activation by Nox2 and TRPV4-dependent Ca2+ influx; (b) spatially discrete and differentially-activated pools of Nox2; or (c) differing absolute levels of Nox2 derived ROS; i.e. theoretically sub-threshold with bradykinin.
As noted above, the iberiotoxin-insensitive EC/VSMC hyperpolarizing effector target(s) of H2O2 in bradykinin-induced dilation is currently unknown. In select arteries under specific experimental states, H2O2 activates other classes of VSMC K+ channel(s) to evoke dilation, such as KATP and KV, as has been variably demonstrated for exogenous H2O2 (for examples of each effector target see references [74, 111]). While serial perfusion bioassays appear to confirm EDHF-type activity for bradykinin-induced H2O2 in human coronary arterioles [77], it is currently unknown whether the only dilator activity of this ROS involves its release from the endothelium. Furthermore, residual catalase-insensitive bradykinin-evoked dilation is abolished by sulfaphenazole, iberiotoxin or 14,15-EEZE, and in cascade bioassays this residual response is clearly transferable from donor EC to detector VSMC. Collectively, these findings indicate that in the continuous presence of catalase CYP 2C9-derived EETs (given that CYP 2J2 and 2C9 are the main epoxyegnases in human coronary arterioles, and residual dilation is prevented by selective 2C inhibition) retain partial dilator activity by serving as transferabletransferable EDHFs [77]. Compared to FID (see Section “Complimentary roles of EETs and H2O2 in flow-induced dilation of human coronary arterioles”), the differential EDHF vs autocrine activity of EETs may rest on the accumulation of different regioisomers (e.g. 14,15-EET does not appear to be a physiological agonist of TRPV4 [141]), or, as suggested for H2O2 above, the absolute level of EET accumulation. Indeed, EETs are not detected in the luminal perfusate originating from EC-intact donor vessels stimulated by flow [33], and previous attempts to identify the specific regioisomer(s) released in response to bradykinin could not be reproduced in a consistent manner [77], even though 11,12-EET is clearly produced in the highest amount in arterioles exposed to arachidonic acid [154].
The surprising apparent ability of arachidonic acid itself to act as a direct agonist of TRPV4 in human coronary arterioles [154] is puzzling, given that previous studies appear to have ruled out this possibility [141, 140, 139, 87]. Notably, while these latter studies analysed the murine (m)TRPV4 in heterologous expression systems, the differences observed cannot be due to homologue (i.e. human vs murine)-specific ligand gating because human (h)TRPV4 expressed in HeLa cells can be activated by exogenous 5,6-EET at 100 nM [48]; a similar concentration previously found to activate mTRPV4 [141], and more importantly far lower than the micromolar range necessary in human coronary ECs [154]. Furthermore, in HEK293 cells expressing mTRPV4 5,6-EET, but not arachidonic acid, in the nanomolar range activates TRPV4 in a membrane delimited fashion [141]. Correspondingly, manipulation of epoxygenase levels modulates TRPV4-dependent Ca2+ influx in response to shear stress and hypotonic cell swelling in a manner consistent with the obligatory conversion of arachidonic acid to EETs [139, 87]. Though purely speculative, one possibility for the disparate findings in human coronary ECs may be a disease-related alteration in the sensitivity of TRPV4 to activating stimuli (i.e. in tissue obtained from CAD patients). Even so, a current difficulty in interpreting the significance of these findings with exogenous arachidonic acid is that the increase in IP3 associated with the response to physiological agonists (following the occupation of PLC-coupled receptors) and flow (which is typically accompanied by the efflux of purinergic receptor agonists, i.e. ATP; see references [151, 150, 149]) is known to dramatically and synergistically increase the senstivity of TRPV4 to EETs (to 1 nM 5,6-EET, for example) by evoking a direct physical association between the IP3 receptor and a specific region of TRPV4 that at least in part overlaps its Ca2+-calmodulin binding site [48].
Potential EC actions of H2O2 in the human coronary microcirculation
Distinct from a transferable EDHF-type action of H2O2, it was noted that in rabbit iliac (conduit) arteries either selective elevations in endothelium-generated H2O2, applied H2O2 (threshold of between 10 and 30 μM) or that generated in oxygenated physiological buffer following the addition of ascorbic acid or BH4 to the myograph chamber amplifies EDH-type relaxation to acetylcholine and the SERCA inhibitor CPA in a manner that is prevented by putative gap junction block with Cx-mimetic peptides [39, 56, 38] (see Section “Myoendothelial signalling microdomains and gap junctions: a role in the human coronary microcirculation?”). Simultaneous analyses of ER and cytosolic Ca2+ led to the proposal that H2O2 promotes the mobilization of Ca2+ from ER stores, possibly through oxidation of the IP3 receptor, leading to enhanced EC KCa activity and potentiated vessel relaxation [39]. Intriguingly, relaxant responses to exogenous H2O2 in this vessel are endothelium-independent, but in the continuous presence of a low (essentially non-relaxant) concentration of CPA H2O2 evokes an additional EDH-type component of relaxation that prevented by the combination of apamin plus TRAM [39]. As such, observations in human coronary arterioles that dilator responses to exogenous H2O2 are endothelium-independent [94] do not rule out a role for enhanced endothelial Ca2+ mobilization as a candidate mechanism. Indeed, a similar synergy is evident in cultured human aortic ECs pre-treated with 100 μM H2O2, which potentiates [Ca2+]i elevation evoked by histamine or following the inhibition of SERCA with thapsigargin, whereas H2O2 applied alone does not affect [Ca2+]i [86]. By contrast, in ECs isolated from bovine aorta and human umbilical vein increases in [Ca2+]i are evoked by H2O2 alone [155, 86], and therefore the effects of H2O2 on EC Ca2+ may be tissue- or condition (isolated cell) specific.
Notably, there is also evidence of diametrically opposing effects of H2O2 on VSMC tone between arteries. For example, exogenous H2O2 (1-300 μM) causes mainly constriction in rat proximal (conduit) coronary arteries, but marked relaxation in distal arteries [121]. Such diversity is also apparent in human vessels, such as when comparing the coronary arterioles with intestinal submucosal arterioles; the latter demonstrates constriction to H2O2 in the absence of an intact endothelium [61]. Furthermore, the ability of H2O2 to enhance ER Ca2+ store depletion is more profound in ECs obtained from small (e.g. murine mesenteric) compared to large (e.g. murine aorta) sized vessels [131]. Of interest, application of exogenous H2O2 at concentrations of up to 100 μM may reflect physiological concentrations of the ROS thought to accumulate intracellularly (1-15 μM), because only 1-15% of applied H2O2 is thought to be biologically available within the cytosol [122].
Whilst in some circumstances H2O2 itself is suggested to act as a transferable EDHF in the human coronary microcirculation [77, 82], it is currently unknown whether this is the sole dilator role of this ROS within this particular vascular bed. Further studies are therefore required to clarify the potential autocrine signalling role of endothelium-derived H2O2 in the microvasculature of the human heart. In addition, it is currently unknown whether myoendothelial gap junctions play a role in the transfer of EC hyperpolarization radially to the vascular media to mediate human coronary microvascular dilation.
Cxs, gap junctions and myoendothelial signalling microdomains: a brief overview
Gap junctional communication underlies a number of key aspects of vasomotor control, which include the transfer of metabolites and signalling molecules/second messengers (<1 kDa; including IP3 and cAMP) between neighbouring cells, as well as providing a conduit for the passage of current [43]. Specifically, hyperpolarization initiated in the endothelium can be transmitted radially into the subintimal VSMCs via myoendothelial gap junctions, and can continue in a radial manner through the different layers of the media via homocellular smooth muscle gap junctions. EC hyperpolarization can also spread longitudinally along the length of the vessel through low-resistance interendothelial gap junction plaques, which in general are significantly larger and more numerous than those observed at points of heterocellular close contact (represented in Figure 5) and between neighbouring VSMCs [43], where such junctions are small. In an integrated fashion, such electrical continuity allows locally initiated hyperpolarization and dilation to encompass upstream (i.e. feed arteries) and downstream vascular regions to coordinate changes in blood flow over relatively large distances [31, 6, 43]. Heteroecellular communication via gap junctions in most cases is bidirectional, thereby allowing increased vessel tone to be tightly regulated via myoendothelial feedback (described previously in Section “Endothelial and smooth muscle hyperpolarization: central role of KCa channels”; and for a review see reference [69]).
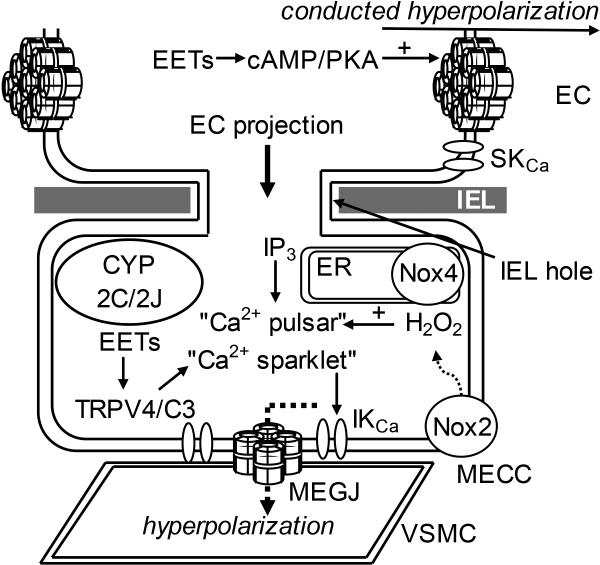
Figure 5.
Potential future avenues for research into the hypothesized roles of myoendothelial microdomain and gap junctional signalling in EDH-mediated vasomotor regulation in the human coronary microcirculation. It remains to be determined whether myoendothelial gap junctions (MEGJ) play a permissive role in the relay of endothelial cell (EC) hyperpolarization to the underlying vascular smooth muscle cells (VSMC) to evoke dilation, and whether trans-internal elastic lamina (IEL) EC projections facilitate myoendothelial close contacts (MECC), and whether these are sites of putative signalling microdomains, as demonstrated in selected arteries In a number of vascular beds, the mobilization of discrete pools of inositol 1,4,5-trisphosphate (IP3)-sensitive endoplasmic reticulum (ER) Ca2+ stores and influx through transient receptor potential vanilloid type 4 (TRPV4) and canonical type 3 channels (TRPC3; both of which are activated by specific epoxyeicosatrienoic acid [EET] regioisomers) associated specifically with these sites (designated as “Ca2+ pulsars” and “Ca2+ sparklets”, respectively), leads to the opening of Ca2+-activated K+ channels (KCa) to initiate an EC hyperpolarization that is conducted to the VSMCs via MEGJs. It also remains to be determined whether EC projections are preferentially enriched with cytochrome P450 2C/2J, Nox2/4 and superoxide dismutase (SOD) to facilitate localized EETs and H2O2 synthesis and action at these sites. Although suggested from culture studies, it is also currently unknown whether EETs modulate MEGJ-related intercellular communication; given their ability to increase interendothelial coupling through a cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA)-dependent mechanism. SKCa, small conductance Ca2+-activated K+ channel.
Vascular gap junctions are composed of two opposing hemichannels/connexons, each consisting of six Cx subunits, which join to form a pore between adjacent cells [59, 29]. Intercellular communication is enhanced by the aggregation of individual gap junction channels into plaques, which can be visualised by electron microscopy at points of homo- and heterocellular contact [116, 59]. Vascular cells variably express Cxs37, 40, 43, and 45 (classified according to their approximate molecular weight in kDa), the expression and distribution of which vary between vessel types and different regions within single vascular beds, as well as in development and disease [117, 24, 29]. Indeed, gap junctions can be composed of hemichannels that contain only a single Cx subtype (homotypic), but multiple Cxs of specific subtypes often co-localise at EC or VSMC borders or at discrete MECCs; consistent with the formation of hemichannels that are either each constructed from a different Cx subtype (heterotypic) or from a mixture of subtypes (heteromeric) [29, 43].
Historically, several studies conducted during the late 1950s and 60s identified MECCs and, without direct investigation into their possible function, suggested that such sites may be important for the regulation of vascular tone [98, 46, 110]. However, it was not until 1982 that pentalaminar structures consistent with the ultrastructure of gap junction plaques were identified at these sites [129]. Kristek and Gerova (1992) demonstrated the presence of MECCs in rabbit and canine coronary arteries, and postulated that, in addition to being sites of metabolite transfer, MECCs may also facilitate the passage of current between neighbouring ECs and subintimal VSMCs ([73]; for more comprehensive historical perspectives see additional reviews [65, 119, 43]).
These hypotheses have ultimately inspired a modern and rapidly growing field examining the roles of these sites in vascular function. It has been demonstrated that the expression of Cxs at MECCs (of which Cxs 37, 40, and 43, but not as yet Cx45, have been demonstrated in varying compositions between different arteries, disease states and during development) has been shown to increase with decreasing vessel size; findings that have been suggested to generally confer an increased role for EDH in vasodilation in smaller over larger sized vessels [43]. It should be noted, however, that IEL hole density is not necessarily an indicator of the presence of trans-IEL EC/VSMC projections, and, in turn, the presence of projections and MECCs are not necessarily a clear indicator of the presence of myoendothelial gap junction plaques, with the latter being present at a highly variable proportion (0-50%) of IEL holes depending on the artery, species and disease state [115, 119, 43]. In human saphenous veins MECCs are present on projections arising mainly from ECs, and number around 10 per cell, but their morphology is not always compatible with the formation of gap junction plaques [132]. Thus, whilst IEL holes and the associated cell projections and MECCs are required for myoendothelial gap junctional communication (see Figure 5), there is significant diversity in their relative ability to transmit heterocellular signals (for reviews see references [119, 43]). IEL holes lacking cell projections (or, indeed, MECCs lacking gap junctions) have been suggested to provide a low-resistance pathway for the heterocellular diffusion of vasoactive signalling molecules; given that a non-fenestrated IEL represents a potentially significant diffusion barrier, even to relatively low mass molecules [114].
Myoendothelial signalling microdomains and gap junctions: a role in the human coronary microcirculation?
The mechanisms that underlie the structural remodelling of ECs to form trans-IEL projections and MECCs have on the whole remained elusive; but a role for PAI-1 activity has been alluded to from in vitro and in vivo studies of EC-VSMC co-cultures and intact murine coronary arterioles [64]. PAI-1 mRNA may in fact be locally translated at MECCs through a novel mRNA protein binding complex, thereby promoting PAI-1 enrichment within EC projections rather than at the main cell body [63]. Circulating PAI-1 has also been proposed to accumulate at specific sites in the endothelium to regulate MECC formation, as alluded to from heart transplants from PAI-1-deficient to wild-type mice, following which the normally absent MECCs in the coronary circulation of the transplanted heart are re-established within 5 days post-surgery (i.e. following exposure of the PAI-1-deficient coronary vasculature to the circulation of wild-type animals) [64].
PAI-1 is a biomarker for a number of vascular disease states, including diabetes and obesity, and, correspondingly, in coronary arterioles of mice maintained on a high fat diet the number of MECCs is increased [64]. These findings may relate to observations in saphenous arteries of obese rats, in which there are morphological changes in the head of EC projections favouring greater surface area contact with the subjacent subintimal VSMCs. This is associated an elevation in the expression of IKCa localized at IEL holes and increased incidence of myoendothelial gap junctions (~12-fold higher than that in lean rats), which together serve to potentiate EDH-mediated dilation and offset the loss of NO that is typically associated with this cardiovascular risk factor [20]. It should be noted, however, that the role of PAI-1 in MECC formation in human arteries has not yet been verified. Furthermore, studies investigating the presence and role of MECCs and associated EDH signalling microdomains in the human coronary microcirculation are lacking, as are investigations that are commonly employed to infer the presence and role of myoendothelial gap junction-related communication in EDH, including: (a) the radial spread of endothelium-loaded tracer dyes [60]; (b) simultaneous recordings of EC and subintimal VSMC membrane potential, with observed similarities at rest and following EC stimulation (per the temporal and waveform characteristics) generally being indicative of strong electrical continuity between the two cell layers, as demonstrated in porcine conduit coronary arteries [11, 143]; (c) the efficacy of putative blockers of gap junctional communication, such as Cx-mimetic peptides (see below), against (a) and (b), and also EDH-mediated dilation [60, 59, 29]; and (d) detailed ultrastructural analyses of human coronary resistance arteries. Indeed, the role of gap junctions, and specifically those constructed from Cx43, in subcutaneous resistance arteries from pregnant women is inferred by the efficacy of a Cx43-specific Cx-mimetic peptide against EDH-type relaxation [75], with the presence of myoendothelial gap junctions at MECCs of these vessels having been supported by electron microscopy analyses [75].
The specificity of Cx-mimetic peptides is suggested to be due to their homology with Cx-specific variations in the first and second extracellular loops (the so-called ‘Gap26’ and ‘Gap27’ domains, respectively) of these proteins [29]. Indeed, interpretation of the findings in coronary arterioles obtained from non-CAD patients that 18α-glycyrrhetinic acid and carbenoxolone, as further putative gap junction blockers, fail to attenuate EDH-type responses to bradykinin [8] is difficult given that these agents are associated variably with elevated Cx expression, NO synthesis, modulation of EC membrane potential and non-specific KV and voltage-gated Ca2+ channel block [27, 134, 59]. Notably, non-junctional effects of these agents are apparent in human arteries, such as subcutaneous arterioles in which 18β-glycyrrhetinic acid and carbenoxolone attenuate acetylcholine-evoked EC hyperpolarization via non-specific block of voltage-dependent Ca2+ and KV channels [27]. As such, studies analysing the efficacy of more specific gap junction blockers against dilator responses of the human coronary microcirculation are required.
The presence of MECCs and the role of microdomain signalling are apparent only in a limited number of human arteries. In human mesenteric arteries, for example, trans-IEL projections arising from both ECs and VSMCs are associated with an IKCa-Cx37 microdomain that underpins dilator responses to bradykinin (with vasopressin preconstriction; [21]); findings that may relate to observations in coronary arterioles from non-CAD patients, in which EDH-type responses to the same agonist are mediated entirely by IKCa ([8]; see Table 1). In human renal arterioles projections arising from mainly ECs form MECCs that separate the two cell types by ~10-15 nm [128]. In the context of the thesis of this review, MECCs have been demonstrated in human coronary arterioles at pre-capillary sphincters [125], but their role in regulating resistance in the coronary microcirculation (and also how these alter in CAD) is not currently known and warrants further investigation (see Figure 5). Notably, in the pulmonary circulation of patients with hypertension there is IEL thickening and a decrease in the number of IEL holes [2].
The idea that MECCs and associated trans-IEL cell projections serve as important spatially discrete signalling microdomains that amplify the biological role of EDH is supported by the demonstration that these are also sites of enriched expression of several key EDH signalling components. In addition to gap junction Cxs and IKCa (and potentially SKCa in specific tissues and states) these include TRP channels (of which TRPA1, TRPC3 and TRPV4 have been demonstrated at such sites in specific arteries), the Na+/K+-ATPase on the closely apposed VSMC membrane [5, 119, 124, 127, 107, 43], and Kir on VSMCs or ECs, or both, depending on the vessel and state [43]. Novel findings indicate that caveolin-1 may also be enriched in EC projections of specific vessels, but, surprisingly, not necessarily (or always) within membrane structures (caveolae) most commonly associated with this regulatory protein (SL Sandow, unpublished observations). Together with the proposal that these sites also contain their own discrete pools of IP3-sensitive ER Ca2+ stores [69, 119, 43], the functional assembly of myoendothelial signalling microdomains is indicative of highly specialised and potentially specific function (Figure 5). Variations in the composition of ion channels, Cxs and organelle content, as well as the specific morphology of projections and MECCs, are thought to confer significant plasticity in the mechanisms of vessel function [43]. The mobilization of ER Ca2+ and the influx of extracellular Ca2+ at these sites is thus spatially restricted and does not usually propagate as a Ca2+ wave that encompasses the main cell body, with such discrete IP3-mediated Ca2+ release events (designated Ca2+ “pulsars”) and TRP-mediated extracellular Ca2+ influx (designated Ca2+ “sparklets”) being central to the activation of spatially associated KCa channels and hyperpolarization that is subsequently transmitted via juxtaposed myoendothelial gap junctions ([79, 5, 127]; Figure 5). The proposed specialised role of such microdomains in EDH-mediated dilation is alluded to by observations that the activation of as little as three or four TRPV4 channels per EC is sufficient to evoke maximal EDH-type dilation [127].
Conclusions and future directions
This review highlights the diverse and integrated signalling roles of EETs and H2O2 in the coronary circulation, and examines the implications for further research into regulation of the human coronary microcirculation in health and disease. Specialised myoendothelial microdomain signalling complexes are becoming increasingly appreciated as important contributors to the general mechanism of EDH-mediated vasodilation and coordinated function. Considering the potential for artery-specific differences in ion channel and Cx composition, ER content and specific MECC morphology, associated differences in EDH signalling between arteries, species, disease and development renders pharmaceutical targeting of global in vivo EDH activity difficult. However, such variations may also provide specific targets for modulating EC function in an artery-specific manner.
The presence and role of EC and/or VSMC projections, MECCs and associated microdomain signalling in the human coronary microcirculation is unknown. Indeed, the efficacy of Cx-specific inhibitors of gap junctional communication against EDH-mediated dilation, as has been reported in numerous arteries of animal models, has not yet been undertaken in the resistance arteries of the human heart. A priority for future research should therefore be establishing the relevant ultrastructural features of the human coronary microcirculation, and subsequently whether these are associated with myoendothelial signalling microdomains relevant to EDH activity.
Despite evidence linking KCa, several TRP channel subtypes, ER Ca2+ stores, Cxs and gap junctions (in varying compositions) as being preferentially enriched within discrete myoendothelial microdomain sites in numerous arteries, the association with other potentially key EDH signalling components, such as CYP 2C and 2J, Nox2, Nox4 and SOD, has not yet been investigated. Indeed, the recent suggestion that endothelial NOS and haemoglobin α, which together form an NO synthesizing and signal regulation complex [130], may be enriched at these sites has served only to heighten interest in myoendothelial microdomain signalling. The potential enrichment of Nox4 in the membrane of discrete pools of ER, coupled with the purported ability of this Nox to produce H2O2 directly, could also be an important future avenue for research. Indeed, EC-specific overexpression of Nox4 potentiates acetylcholine- and histamine-evoked relaxation in the murine coronary circulation and lowers blood pressure via mechanisms involving H2O2. Notably, there are currently no selective Nox4 inhibitors to assist in establishing the physiological role of this oxidase [28]. Furthermore, determining whether there are actually distinct EC ER pools and how these may be altered in different functional states, including development and disease, is an area for further work.
From a broader perspective, it remains to be determined whether dilator H2O2 activity is aetiological to the onset of vascular disease, and thus whether this oxidant is a potentially pathological participant in EDH; compared to elevated dilator H2O2 synthesis associated with, for example, prolonged exercise training [144, 145] or acute H2O2-mediated metabolic coronary vasodilation [71]. Furthermore, an ongoing limitation in understanding the physiological role of EETs and therefore establishing potential drug targets is the lack of formal identification of its putative receptor, and potentially the existence of multiple receptor subtypes with varying distributions in the vascular system. The potential of such diversity is apparent when considering the highly heterogeneous responses of different arteries to individual EET regioisomers applied exogenously (see reference [43] for review).
Further complications in elucidating the mechanisms of EDH in the human coronary microcirculation are: (a) the sparsity of data available for healthy tissue, and therefore determining how such mechanisms alter over the course of specific disease states, such as hypertension, obesity and diabetes; and (b) elucidating potential regional differences in coronary vasomotor regulation. Indeed, related to (b), arterioles obtained from human atrial and ventricular tissue respond very differently to acetylcholine; with profound vasoconstriction evoked in the former and dilation in the latter [93]. Nevertheless, observations that H2O2 mediation of vasodilation in coronary microvascular tissue from CAD patients can be reverted back to NO-mediated vasodilation by inhibiting ceramide formation [52] provide and intriguing avenue for further research and, potentially, for novel drug development in the treatment of cardiac ischaemia and atherosclerosis.
In consideration of the ongoing interest in sEH inhibitors as a potential means of pharmaceutically improving vasodilator and anti-inflammatory EET bioavailability, future research should also focus on: (a) elucidating the physiological role of parent molecule arachidonic acid in FID in the context of human disease [154]; and (b) ascertaining potential alternative routes of EET metabolism, because under physiological conditions 5,6-EET is unstable and, via its δ-lactone (which sEH cannot hydrolyse), may be a substrate for paraoxonase-1 [45] (N.B. paraoxonase-1-deficient mice are hypotensive due to increased 5,6-EET bioavailability [55]). Elucidation of the physiological role of these pathways could leverage the identification of intriguing alternative drug targets relevant to human vascular disease. An improved understanding of the regulatory mechanisms of the human coronary circulation could also assist with the development of novel cardioplegic solutions to improve patient outcomes following coronary artery bypass surgery.
Perspectives.
Stimulated increases in blood flow in the coronary microcirculation of patients with CAD is mediated by complex, diverse and integrated activity of EETs and H2O2, which replace NO-mediated responses normally present in the same tissue from healthy individuals. Several intriguing areas for future research include: (a) the molecular mechanisms that drive the switch in dilator molecule activity during CAD; (b) the significance and role of myoendothelial signalling microdomains and gap junctional communication in the regulation of the human coronary microcirculation, and their alteration in disease; and (c) elucidating the physiological dilator activity of non-metabolized arachidonic acid, as well as alternative (non-sEH/non-COX) routes for EET degradation, e.g. paraoxonase-1. A better understanding of the regulatory mechanisms of the microcirculation of the human heart could uncover interesting new drug targets for correcting altered coronary blood flow in specific disease states, and also potentially improve patient outcomes following coronary artery bypass surgery.
Acknowledgments
none
Funding
This work was supported by the Brain Foundation, the Diabetes Australia Research Trust, the National Health and Medical Research Council of Australia (NHMRC) (Project Grant APP1048885, to Shaun L. Sandow) and the National Heart, Lung and Blood Institute (NHLBI) (Project Grant HL113612, to David D. Gutterman).
Abbreviations
- 14,15-EEZE
14,15-epoxyeicosa-5(Z)-enoic acid
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- BH4
tetrahydrobiopterin
- BKCa
large conductance Ca2+-activated K+ channel
- CAD
coronary artery disease
- cAMP
cyclic adenosine monophosphate
- Cat
catalase
- cGMP
cyclic guanosine monophosphate
- CNP
C-type natriuretic peptide
- COX
cyclooxygenase
- CPA
cyclopiazonic acid
- Cx
connexin
- CYP
cytochrome P450
- DHET
dihydroxyeicosatrienoic acid
- EC
endothelial cell
- EDH
endothelium-derived hyperpolarization
- EDHF
endothelium-derived hyperpolarizing factor
- EET
epoxyeicosatrienoic acid
- ER
endoplasmic reticulum
- FID
flow-induced dilation
- GTP
guanosine triphosphate
- H2O2
hydrogen peroxide
- HEK293
human embryonic kidney cells
- IEL
internal elastic lamina
- IKCa
intermediate conductance Ca2+-activated K+ channel
- IP3
inositol 1,4,5-trisphosphate
- KATP
ATP-activated K+ channel
- KCa
Ca2+-activated K+ channel
- Kir
inwardly rectifying K+ channel
- KV
voltage-gated K+ channel
- LDL
low density lipoprotein
- MECC
myoendothelial close contact site
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nox
nicotinamide adenine dinucleotide phosphate oxidase
superoxide anions
- ONOO−
peroxynitrite
- PAI-1
plasminogen activator inhibitor-1
- PEG-Cat
polyethylene glycol catalase
- PGI2
prostaglandin I2 (prostacyclin)
- PKA
protein kinase A
- PKG
protein kinase G
- PLC
phospholipase C
- ROS
reactive oxygen species
- sEH
soluble epoxide hydrolase
- SERCA
sarcoendoplasmic reticulum Ca2+-ATPase
- SKCa
small conductance Ca2+-activated K+ channel
- SOD
superoxide dismutase
- TRPA1
transient receptor potential ankyrin type 1 channel
- TRPC3
transient receptor potential canonical type 3 channel
- TRPV4
transient receptor potential vanilloid type 4 channel
- VSMC
vascular smooth muscle cell
References
- 1.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacol. 2007;151(3):332–340. doi: 10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiello VD, Gutierrez PS, Chaves MJ, Lopes AA, Higuchi ML, Ramires JA. Morphology of the internal elastic lamina in arteries from pulmonary hypertensive patients: a confocal laser microscopy study. Mod Pathol. 2003;16(5):411–416. doi: 10.1097/01.MP.0000067685.57858.D7. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99(9):988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzawi M, Austin C. The effects of endothelial factor inhibition on the time course of responses of isolated rat coronary arteries to intraluminal flow. J Vasc Res. 2007;44(3):223–233. doi: 10.1159/000100421. [DOI] [PubMed] [Google Scholar]
- 5.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci U S A. 2012;109(44):18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 2011;202(3):271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batenburg WW, de Vries R, Saxena PR, Danser AH. L-S-nitrosothiols: endothelium-derived hyperpolarizing factors in porcine coronary arteries? J Hypertens. 2004;22(10):1927–1936. doi: 10.1097/00004872-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Batenburg WW, Garrelds IM, van Kats JP, Saxena PR, Danser AH. Mediators of bradykinin-induced vasorelaxation in human coronary microarteries. Hypertension. 2004;43(2):488–492. doi: 10.1161/01.HYP.0000110904.95771.26. [DOI] [PubMed] [Google Scholar]
- 9.Batenburg WW, Kappers MH, Eikmann MJ, Ramzan SN, de Vries R, Danser AH. Light-induced vs. bradykinin-induced relaxation of coronary arteries: do S-nitrosothiols act as endothelium-derived hyperpolarizing factors? J Hypertens. 2009;27(8):1631–1640. doi: 10.1097/HJH.0b013e32832bff54. [DOI] [PubMed] [Google Scholar]
- 10.Batenburg WW, Popp R, Fleming I, de Vries R, Garrelds IM, Saxena PR, Danser AH. Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF? Br J Pharmacol. 2004;142(1):125–135. doi: 10.1038/sj.bjp.0705747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beny J. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. Pflugers Arch. 1997;433(3):364–367. doi: 10.1007/s004240050289. [DOI] [PubMed] [Google Scholar]
- 12.Beyer AM, Gutterman DD. Regulation of the human coronary microcirculation. J Mol Cell Cardiol. 2012;52(4):814–821. doi: 10.1016/j.yjmcc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosnjak JJ, Terata K, Miura H, Sato A, Nicolosi AC, McDonald M, Manthei SA, Saito T, Hatoum OA, Gutterman DD. Mechanism of thrombin-induced vasodilation in human coronary arterioles. Am J Physiol Heart Circ Physiol. 2003;284(4):H1080–1086. doi: 10.1152/ajpheart.00465.2002. [DOI] [PubMed] [Google Scholar]
- 14.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol. 2012;302(3):H634–642. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317(5843):1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 16.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135(5):1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78(3):415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 19.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8(5-6):691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 20.Chadha PS, Haddock RE, Howitt L, Morris MJ, Murphy TV, Grayson TH, Sandow SL. Obesity up-regulates intermediate conductance calcium-activated potassium channels and myoendothelial gap junctions to maintain endothelial vasodilator function. J Pharmacol Exp Ther. 2010;335(2):284–293. doi: 10.1124/jpet.110.167593. [DOI] [PubMed] [Google Scholar]
- 21.Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, Grayson TH, Murphy TV, Sandow SL. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K+ channel and nitric oxide. J Pharmacol Exp Ther. 2011;336(3):701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- 22.Chan KY, Edvinsson L, Eftekhari S, Kimblad PO, Kane SA, Lynch J, Hargreaves RJ, de Vries R, Garrelds IM, van den Bogaerdt AJ, Danser AH, Maassenvandenbrink A. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther. 2010;334(3):746–752. doi: 10.1124/jpet.110.165993. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100(3):1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaytor AT, Bakker LM, Edwards DH, Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol. 2005;144(1):108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry. 2011;50(18):3840–3848. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251(4 Pt 2):H779–788. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- 27.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531(Pt 2):359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csanyi G, Pagano PJ. Strategies Aimed at Nox4 Oxidase Inhibition Employing Peptides from Nox4 B-Loop and C-Terminus and p22 (phox) N-Terminus: An Elusive Target. Int J Hypertens. 2013;2013:842827. doi: 10.1155/2013/842827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459(6):897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 30.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291(2):H517–531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 31.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74(2):226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- 32.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102(10):1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013;20(3):239–247. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dworakowski R, Alom-Ruiz SP, Shah AM. NADPH oxidase-derived reactive oxygen species in the regulation of endothelial phenotype. Pharmacol Rep. 2008;60(1):21–28. [PubMed] [Google Scholar]
- 35.Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J Cardiovasc Pharmacol. 2011;57(2):148–153. doi: 10.1097/FJC.0b013e3181f580d9. [DOI] [PubMed] [Google Scholar]
- 36.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 37.Eckman DM, Hopkins N, McBride C, Keef KD. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: role of epoxyeicosatrienoic acid. Br J Pharmacol. 1998;124(1):181–189. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards DH, Li Y, Ellinsworth DC, Griffith TM. The effect of inorganic arsenic on endothelium-dependent relaxation: role of NADPH oxidase and hydrogen peroxide. Toxicology. 2013;306:50–58. doi: 10.1016/j.tox.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008;28(10):1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- 40.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396(6708):269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 41.Edwards G, Feletou M, Gardener MJ, Glen CD, Richards GR, Vanhoutte PM, Weston AH. Further investigations into the endothelium-dependent hyperpolarizing effects of bradykinin and substance P in porcine coronary artery. Br J Pharmacol. 2001;133(7):1145–1153. doi: 10.1038/sj.bjp.0704157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellinsworth DC. Arsenic, reactive oxygen, and endothelial dysfunction. J Pharmacol Exp Ther. 2015;353(3):458–464. doi: 10.1124/jpet.115.223289. [DOI] [PubMed] [Google Scholar]
- 43.Ellinsworth DC, Earley S, Murphy TV, Sandow SL. Endothelial control of vasodilation: integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids. Pflugers Arch. 2014;466(3):389–405. doi: 10.1007/s00424-013-1303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. Interactions between thromboxane A(2), thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res. 2014;102(1):9–16. doi: 10.1093/cvr/cvu015. [DOI] [PubMed] [Google Scholar]
- 45.Eryanni-Levin S, Khatib S, Levy-Rosenzvig R, Tamir S, Szuchman-Sapir A. 5,6-delta-DHTL, a stable metabolite of arachidonic acid, is a potential substrate for paraoxonase 1. Biochim Biophys Acta. 2015;1851(9):1118–1122. doi: 10.1016/j.bbalip.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Fawcett DW. The fine structure of capillaries, arterioles and small arteries. In: Reynolds SRM, Zweifach BW, editors. The Microcirculation. University of Illinois press; 1959. pp. 1–27. [Google Scholar]
- 47.Feher A, Rutkai I, Beleznai T, Ungvari Z, Csiszar A, Edes I, Bagi Z. Caveolin-1 limits the contribution of BK(Ca) channel to EDHF-mediated arteriolar dilation: implications in diet-induced obesity. Cardiovasc Res. 2010;87(4):732–739. doi: 10.1093/cvr/cvq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5'-6'- epoxyeicosatrienoic acid. J Gen Physiol. 2008;131(5):i2. doi: 10.1085/JGP1315OIA2. [DOI] [PubMed] [Google Scholar]
- 49.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88(1):44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 50.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(12):2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 51.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459(6):923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 52.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115(5):525–532. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukao M, Mason HS, Kenyon JL, Horowitz B, Keef KD. Regulation of BK(Ca) channels expressed in human embryonic kidney 293 cells by epoxyeicosatrienoic acid. Mol Pharmacol. 2001;59(1):16–23. doi: 10.1124/mol.59.1.16. [DOI] [PubMed] [Google Scholar]
- 54.Fulton D, Quilley J. Evidence against anandamide as the hyperpolarizing factor mediating the nitric oxide-independent coronary vasodilator effect of bradykinin in the rat. J Pharmacol Exp Ther. 1998;286(3):1146–1151. [PubMed] [Google Scholar]
- 55.Gamliel-Lazarovich A, Abassi Z, Khatib S, Tavori H, Vaya J, Aviram M, Keidar S. Paraoxonase1 deficiency in mice is associated with hypotension and increased levels of 5,6-epoxyeicosatrienoic acid. Atherosclerosis. 2012;222(1):92–98. doi: 10.1016/j.atherosclerosis.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 56.Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc Res. 2009;84(2):218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge ZD, Zhang XH, Fung PC, He GW. Endothelium-dependent hyperpolarization and relaxation resistance to N(G)-nitro-L-arginine and indomethacin in coronary circulation. Cardiovasc Res. 2000;46(3):547–556. doi: 10.1016/s0008-6363(00)00040-7. [DOI] [PubMed] [Google Scholar]
- 58.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res. 1998;35(4):274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- 59.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141(6):881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci U S A. 2002;99(9):6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatoum OA, Binion DG, Miura H, Telford G, Otterson MF, Gutterman DD. Role of hydrogen peroxide in ACh-induced dilation of human submucosal intestinal microvessels. Am J Physiol Heart Circ Physiol. 2005;288(1):H48–54. doi: 10.1152/ajpheart.00663.2004. [DOI] [PubMed] [Google Scholar]
- 62.Hatton CJ, Peers C. Effects of cytochrome P-450 inhibitors on ionic currents in isolated rat type I carotid body cells. Am J Physiol. 1996;271(1 Pt 1):C85–92. doi: 10.1152/ajpcell.1996.271.1.C85. [DOI] [PubMed] [Google Scholar]
- 63.Heberlein KR, Han J, Straub AC, Best AK, Kaun C, Wojta J, Isakson BE. A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction. Arterioscler Thromb Vasc Biol. 2012;32(5):1271–1279. doi: 10.1161/ATVBAHA.112.246371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heberlein KR, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, Meher A, Leitinger N, Isakson BE. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res. 2010;106(6):1092–1102. doi: 10.1161/CIRCRESAHA.109.215723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation. 2009;16(4):307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heusch G. Control of coronary vasomotor tone in ischaemic myocardium by local metabolism and neurohumoral mechanisms. Eur Heart J 12 Suppl F. 1991:99–106. doi: 10.1093/eurheartj/12.suppl_f.99. [DOI] [PubMed] [Google Scholar]
- 67.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96(3):376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol. 2010;299(5):H1439–1450. doi: 10.1152/ajpheart.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, Welsh DG, Plane F. Endothelial feedback and the myoendothelial projection. Microcirculation. 2012;19(5):416–422. doi: 10.1111/j.1549-8719.2012.00187.x. [DOI] [PubMed] [Google Scholar]
- 70.Kerr PM, Wei R, Tam R, Sandow SL, Murphy TV, Ondrusova K, Lunn SE, Tran CH, Welsh DG, Plane F. Activation of endothelial IK channels underlies NO-dependent myoendothelial feedback. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.09.001. (In Press). DOI: doi: 10.1016/j.vph.2015.09.00110.1016/j.vph.2015.09.001, 10.1016/j.vph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Kokusho Y, Komaru T, Takeda S, Takahashi K, Koshida R, Shirato K, Shimokawa H. Hydrogen peroxide derived from beating heart mediates coronary microvascular dilation during tachycardia. Arterioscler Thromb Vasc Biol. 2007;27(5):1057–1063. doi: 10.1161/ATVBAHA.0000261570.85983.4f. [DOI] [PubMed] [Google Scholar]
- 72.Koller A, Bagi Z. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 2004;287(6):H2461–2467. doi: 10.1152/ajpheart.00295.2004. [DOI] [PubMed] [Google Scholar]
- 73.Kristek F, Gerova M. Myoendothelial relations in the conduit coronary artery of the dog and rabbit. J Vasc Res. 1992;29(1):29–32. doi: 10.1159/000158928. [DOI] [PubMed] [Google Scholar]
- 74.Lacza Z, Puskar M, Kis B, Perciaccante JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol. 2002;283(1):H406–411. doi: 10.1152/ajpheart.00007.2002. [DOI] [PubMed] [Google Scholar]
- 75.Lang NN, Luksha L, Newby DE, Kublickiene K. Connexin 43 mediates endothelium-derived hyperpolarizing factor-induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. Am J Physiol Heart Circ Physiol. 2007;292(2):H1026–1032. doi: 10.1152/ajpheart.00797.2006. [DOI] [PubMed] [Google Scholar]
- 76.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr., Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol. 2009;29(5):739–745. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102(1):59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290(2):H491–499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105(28):9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80(6):877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 81.Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85(4):349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108(5):566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol. 2009;104(3):211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput. 2008;46(5):469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93(6):573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 86.Lock JT, Sinkins WG, Schilling WP. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J Physiol. 2012;590(Pt 15):3431–3447. doi: 10.1113/jphysiol.2012.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80(3):445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 88.Lu T, He T, Katusic ZS, Lee HC. Molecular mechanisms mediating inhibition of human large conductance Ca2+-activated K+ channels by high glucose. Circ Res. 2006;99(6):607–616. doi: 10.1161/01.RES.0000243147.41792.93. [DOI] [PubMed] [Google Scholar]
- 89.Ma Y, Zhang P, Li J, Lu J, Ge J, Zhao Z, Ma X, Wan S, Yao X, Shen B. Epoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteries. Biochim Biophys Acta. 2015;1852(3):552–559. doi: 10.1016/j.bbadis.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol. 2008;295(1):C221–230. doi: 10.1152/ajpcell.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcus ML. Autoregulation in the coronary circulation. In: Brehm JJ, Schwartz M, editors. The coronary circulation in health and disease. McGraw-Hill Book Co.; New York: 1983. pp. 93–112. [Google Scholar]
- 92.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106(12):1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller FJ, Jr., Dellsperger KC, Gutterman DD. Pharmacologic activation of the human coronary microcirculation in vitro: endothelium-dependent dilation and differential responses to acetylcholine. Cardiovasc Res. 1998;38(3):744–750. doi: 10.1016/s0008-6363(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 94.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92(2):e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 95.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation. 1999;99(24):3132–3138. doi: 10.1161/01.cir.99.24.3132. [DOI] [PubMed] [Google Scholar]
- 96.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr., Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation. 2001;103(15):1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 97.Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 2011;120(4):131–141. doi: 10.1042/CS20100384. [DOI] [PubMed] [Google Scholar]
- 98.Moore DH, Ruska H. The fine structure of capillaries and small arteries. J Biophys Biochem Cytol. 1957;3(3):457–462. doi: 10.1083/jcb.3.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakashima Y, Toki Y, Fukami Y, Hibino M, Okumura K, Ito T. Role of K+ channels in EDHF-dependent relaxation induced by acetylcholine in canine coronary artery. Heart Vessels. 1997;12(6):287–293. doi: 10.1007/BF02766805. [DOI] [PubMed] [Google Scholar]
- 100.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286(2):C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 101.Nishikawa Y, Stepp DW, Chilian WM. In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol. 1999;277(3 Pt 2):H1252–1259. doi: 10.1152/ajpheart.1999.277.3.H1252. [DOI] [PubMed] [Google Scholar]
- 102.Paolocci N, Pagliaro P, Isoda T, Saavedra FW, Kass DA. Role of calcium-sensitive K(+) channels and nitric oxide in in vivo coronary vasodilation from enhanced perfusion pulsatility. Circulation. 2001;103(1):119–124. doi: 10.1161/01.cir.103.1.119. [DOI] [PubMed] [Google Scholar]
- 103.Park Y, Capobianco S, Gao X, Falck JR, Dellsperger KC, Zhang C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;295(5):H1982–1988. doi: 10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Popp R, Brandes RP, Ott G, Busse R, Fleming I. Dynamic modulation of interendothelial gap junctional communication by 11,12-epoxyeicosatrienoic acid. Circ Res. 2002;90(7):800–806. doi: 10.1161/01.res.0000015328.20581.d6. [DOI] [PubMed] [Google Scholar]
- 105.Popp R, Fleming I, Busse R. Pulsatile stretch in coronary arteries elicits release of endothelium-derived hyperpolarizing factor: a modulator of arterial compliance. Circ Res. 1998;82(6):696–703. doi: 10.1161/01.res.82.6.696. [DOI] [PubMed] [Google Scholar]
- 106.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012;18(2):286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of Dynamic Endothelial Ca(2+) Signals by the TRPA1 Channel Activator AITC in Rat Cerebral Arteries. Microcirculation. 2013;20(2):138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229(1):114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- 109.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31(6):1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 110.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18(1):181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 111.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr., Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291(5):H2473–2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 112.Saetrum Opgaard O, Edvinsson L. Mechanical properties and effects of sympathetic co-transmitters on human coronary arteries and veins. Basic Res Cardiol. 1997;92(3):168–180. doi: 10.1007/BF00788634. [DOI] [PubMed] [Google Scholar]
- 113.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol. 2009;297(1):H1–7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- 114.Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function? J Anat. 2009;214(2):258–266. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol. 2009;36(1):67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 116.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86(3):341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 117.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60(3):643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 118.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209(5):689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19(5):403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 120.Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28(2):61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 121.Santiago E, Contreras C, Garcia-Sacristan A, Sanchez A, Rivera L, Climent B, Prieto D. Signaling pathways involved in the H2O2-induced vasoconstriction of rat coronary arteries. Free Radic Biol Med. 2013;60:136–146. doi: 10.1016/j.freeradbiomed.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 122.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol. 2008;8(2):153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 123.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res. 2012;110(9):1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 124.Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, Murphy TV, Tare M, Birnbaumer L, Marrelli SP, Sandow SL. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res. 2012;95(4):439–447. doi: 10.1093/cvr/cvs208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sherf L, Ben-Shaul Y, Lieberman Y, Neufeld HN. The human coronary microcirculation: an electron microscopic study. Am J Cardiol. 1977;39(4):599–607. doi: 10.1016/s0002-9149(77)80172-0. [DOI] [PubMed] [Google Scholar]
- 126.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch. 2010;459(6):915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 127.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336(6081):597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sosa-Melgarejo JA, Berry CL. Myoendothelial contacts in arteriolosclerosis. J Pathol. 1992;167(2):235–239. doi: 10.1002/path.1711670213. [DOI] [PubMed] [Google Scholar]
- 129.Spagnoli LG, Villaschi S, Neri L, Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982;38(1):124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- 130.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun L, Yau HY, Lau OC, Huang Y, Yao X. Effect of hydrogen peroxide and superoxide anions on cytosolic Ca2+: comparison of endothelial cells from large-sized and small-sized arteries. PLoS One. 2011;6(9):e25432. doi: 10.1371/journal.pone.0025432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Svendsen E, Austarheim AM, Haugen B, Dalen H, Dregelid E. Myoendothelial junctions in human saphenous veins. Acta Anat (Basel) 1990;138(2):150–153. doi: 10.1159/000146932. [DOI] [PubMed] [Google Scholar]
- 133.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11(2):171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- 134.Tare M, Coleman HA, Parkington HC. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am J Physiol Heart Circ Physiol. 2002;282(1):H335–341. doi: 10.1152/ajpheart.2002.282.1.H335. [DOI] [PubMed] [Google Scholar]
- 135.Tiefenbacher CP, Friedrich S, Bleeke T, Vahl C, Chen X, Niroomand F. ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. Am J Physiol Heart Circ Physiol. 2004;286(4):H1425–1432. doi: 10.1152/ajpheart.00783.2003. [DOI] [PubMed] [Google Scholar]
- 136.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 137.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30(11):860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 138.Ueda A, Ohyanagi M, Koida S, Iwasaki T. Enhanced release of endothelium-derived hyperpolarizing factor in small coronary arteries from rats with congestive heart failure. Clin Exp Pharmacol Physiol. 2005;32(8):615–621. doi: 10.1111/j.0305-1870.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- 139.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 140.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101(1):396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 142.Weaver H, Shukla N, Ellinsworth D, Jeremy JY. Oxidative stress and vein graft failure: a focus on NADH oxidase, nitric oxide and eicosanoids. Curr Opin Pharmacol. 2012;12(2):160–165. doi: 10.1016/j.coph.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 143.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005;145(6):775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie W, Parker JL, Heaps CL. Effect of exercise training on nitric oxide and superoxide/H(2)O(2) signaling pathways in collateral-dependent porcine coronary arterioles. J Appl Physiol (1985) 2012;112(9):1546–1555. doi: 10.1152/japplphysiol.01248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie W, Parker JL, Heaps CL. Exercise training-enhanced, endothelium-dependent dilation mediated by altered regulation of BK(Ca) channels in collateral-dependent porcine coronary arterioles. Microcirculation. 2013;20(2):170–182. doi: 10.1111/micc.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yada T, Shimokawa H, Hiramatsu O, Haruna Y, Morita Y, Kashihara N, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Cardioprotective role of endogenous hydrogen peroxide during ischemia-reperfusion injury in canine coronary microcirculation in vivo. Am J Physiol Heart Circ Physiol. 2006;291(3):H1138–1146. doi: 10.1152/ajpheart.00187.2006. [DOI] [PubMed] [Google Scholar]
- 147.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, Ogasawara Y, Kajiya F. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation. 2003;107(7):1040–1045. doi: 10.1161/01.cir.0000050145.25589.65. [DOI] [PubMed] [Google Scholar]
- 148.Yada T, Shimokawa H, Hiramatsu O, Shinozaki Y, Mori H, Goto M, Ogasawara Y, Kajiya F. Important role of endogenous hydrogen peroxide in pacing-induced metabolic coronary vasodilation in dogs in vivo. J Am Coll Cardiol. 2007;50(13):1272–1278. doi: 10.1016/j.jacc.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 149.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci. 2011;124(Pt 20):3477–3483. doi: 10.1242/jcs.087221. [DOI] [PubMed] [Google Scholar]
- 150.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 151.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285(2):H793–803. doi: 10.1152/ajpheart.01155.2002. [DOI] [PubMed] [Google Scholar]
- 152.Yamanaka A, Ishikawa T, Goto K. Characterization of endothelium-dependent relaxation independent of NO and prostaglandins in guinea pig coronary artery. J Pharmacol Exp Ther. 1998;285(2):480–489. [PubMed] [Google Scholar]
- 153.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res. 2011;110(3):471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng X, Zinkevich NS, Gebremedhin D, Gauthier KM, Nishijima Y, Fang J, Wilcox DA, Campbell WB, Gutterman DD, Zhang DX. Arachidonic acid-induced dilation in human coronary arterioles: convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J Am Heart Assoc. 2013;2(3):e000080. doi: 10.1161/JAHA.113.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng Y, Shen X. H2O2 directly activates inositol 1,4,5-trisphosphate receptors in endothelial cells. Redox Rep. 2005;10(1):29–36. doi: 10.1179/135100005X21660. [DOI] [PubMed] [Google Scholar]
- 156.Zhou Z, Merkus D, Cheng C, Duckers HJ, Jan Danser AH, Duncker DJ. Uridine adenosine tetraphosphate is a novel vasodilator in the coronary microcirculation which acts through purinergic P1 but not P2 receptors. Pharmacol Res. 2013;67(1):10–17. doi: 10.1016/j.phrs.2012.09.011. [DOI] [PubMed] [Google Scholar]