Abstract
Purpose: Umbilical cord blood (UCB) is an alternative source of hematopoietic stem cell (HSC) transplantation for the treatment of patients with leukemia if matched donor is not available. CD34+ is a pan marker for human hematopoietic stem cells, including umbilical cord blood stem cell. In comparison to other sources, cord blood CD34+ cells proliferate more rapidly and produce large number of progeny cells. For ex vivo expansion of Umbilical Cord Blood- HSCs/HPCs, different combinations of cytokines have been used in many laboratories. IL2rg cytokines, including IL2, IL7 and IL15, are key cytokines in the regulation of differentiation, proliferation and survival of immune cells. IL2 is important cytokine for T cell survival and proliferation, IL7 involve in B cell development and IL15 is a key cytokine for NK cell development. In this study we evaluated the generation of T cells derived from CD34+ and CD34- cord blood mononuclear cells by using combination of cytokines including IL2, IL7 and IL15.
Methods: Cultured cord blood mononuclear cells were evaluated at distinct time points during 21 days by using flow cytometry.
Results: Present study showed that differentiation of T cells derived from CD34+ cord blood mononuclear cells increased by using IL2 and IL7 at different time points. In the other hand IL15 did not show any significant role in generation of T cells from CD34+ cord blood mononuclear cells.
Conclusion: Taken together, our data illustrated that either IL2 or IL7 versus other cytokine combinations, generate more T cell from cord blood CD34 cells, probably this cytokines can be the best condition for ex vivo expansion of UCB HSCs.
Keywords: Umbilical cord blood, Hematopoietic cells, CD34+ cells, T cells, Immune cells, Cytokine
Introduction
Hematopoietic stem cell (HSC) have been used in clinical transplantation for curative treatment of malignant and non-malignant diseases.1,2 Umbilical cord blood (UCB) is an alternative source of HSCs transplantation in treatment of leukemia. For a long time CD34 positive has been accepted as a pan marker of HSC in bone marrow and UCB. However, CD34+ fraction is a heterogeneous population of immature stem and progenitor cells. In comparison to bone marrow cells, CD34+ UCB cells proliferate more rapidly and produce large numbers of progeny cells.2-5 Developing T cells from a common lymphoid progenitor cells pass through a series of distinct phases that are marked by changes in the expression of the T-cell receptor and cell-surface proteins such as CD3 complex during functional maturation.6
Previous studies showed that the IL2rg cytokines(common cytokine receptor γ chain) are essential for the function of several cytokines, including IL-2, IL-7, IL-15 and some others.7 IL-2 plays an essential role in regulating the size of the peripheral T cell pool8 and is reported to influence NK cell differentiation and function.9 IL-7 is the major IL2rg cytokine which involved in B and T cell development.10-12 It has been shown that IL-7 knockout mice have a 10-fold reduction in B cells.12,13 However it is unclear the role of these cytokines on T cell development from cord blood cells.
In adults, setting of CB transplantation influences apoptosis in T cells following stem cell transplantation more than adult peripheral blood T cells and these cells are also more susceptible to apoptosis after being allo-priming in vitro.14
Cord blood is one of stem cell sources for transplantation in leukemia patients. However the number of getting cell per each baby delivery is limited. In order to get more effective CB transplantation, many laboratories do ex vivo expansion of HSCs/HPCs. Therefore, they apply some extrinsic regulators such as stem cell factor (SCF) which improve homing and proliferation of UCB cells in preclinical models15,16 and Fetal liver tyrosine kinase-3-ligand (Flt3) to enhance short-term expansion, proliferation and differentiation of HSCs.17 As it is known, IL-7 has a mutual role in B cell development as well as in induced NK cell differentiation.18,19 IL-15 is also a crucial cytokine for NK cell differentiation.19-21 Furthermore, IL2 which is a T cell growth factor mediates in activated B cell proliferation and NK cells differentiation.22-24
Therefore, it is important to understand the effect of these cytokines on the T cell expansion in cord blood context, since T cell is important player in immunity. In this study, we evaluated the potential of CD34+ cord blood cells differentiation to T cells. We also established the best cytokine condition for development of T cells derived from cord blood mononuclear cells.
Materials and Methods
Cell isolation
Cord blood samples collected from full-term normal deliveries, were diluted 2:1 with phosphate-buffered saline (PBS) (SIGM). Subsequently, mononuclear cells were isolated by centrifugation on Ficoll-paque (GE healthcare, 1.078 g/ml) at 850 gm for 25 minutes. The mononuclear cells were collected, washed twice and resuspended in RPMI1640 (Gibco) supplemented with 10% FBS (Gibco) either for culture or for freezing.
Cell culture and culture condition
The 105 cord blood mononuclear cells were seeded in 96-well plates in 250 µL of RPMI1640 (Gibco) containing 20% fetal bovine serum (FBS; Gibco), 1% penicillin/streptomycin (Gibco), supplemented with cytokines with final concentrations: SCF (40ng/ml), Flt3 ligand (FL, 40 ng/mL), interleukin-7 (IL-7, 40 ng/mL), IL-15 (40 ng/mL), and IL-2 (40 ng/mL) (all cytokines purchased from PeproTech). Cells were cultured at 37°C for 21 days, and half of the culture medium was replaced weekly. At indicated days (day 7, 14 and 21), cells were harvested, staind by antibody and analyzed by FACS for T (CD3) and CD34 positive cells.
Monoclonal antibodies and flow cytometry
Monoclonal antibodies (conjugated with different fluorochromes) used to stain cell-surface antigens were: CD34 (581; Abcam) and CD3 (UCHT1; R&D).
We evaluated the cultured cells by flow cytometeric analysis every week. Propidium iodide (1.0 mg/mL; Invitrogen) were used to exclude dead cells from the analysis. Cells were analyzed by BD caliber (BD ebioscience), between10000 to 30000 events were collected and analyses were performed using flowing software (Perttu Terho, version: 2.5.1.).
Statistical analysis
All results are expressed as mean (SD). The statistical significances between groups were determined using the Student t test and one-way ANOVA. P < 0.05 was considered to be statistically significant. The analysis performed by GraphPad Prism software (version: 5.04).
Experimental Ethical matters have been approved by Ethical committee of Tabriz University of medical Sciences.
Results
Role of cytokines in generation of T cells from cord blood CD34+/- cells
Several cytokines are known to up regulate and control the generation of T cells. For example IL2 and IL7 are T cell growth factors involved in proliferation and survival of T cell.22-24
We cultured 1x 105 cord blood mononuclear cells for 21 days in presence of different combination of SCF, FL, IL2, IL7, and IL15. Harvested cells evaluated by FACS at distinct time points gating on lymphoid mononuclear cells. We gated CD3+ cells on CD34+ and CD34- fractions separately to evaluate the percentage of CD3+ T cells derived from cultured mononuclear cells (Figure 1). As shown in (Figure 1), CD34+ fraction significantly produce CD3+ cells about 60 t0 80 percent more than CD34- fraction.
Figure 1.
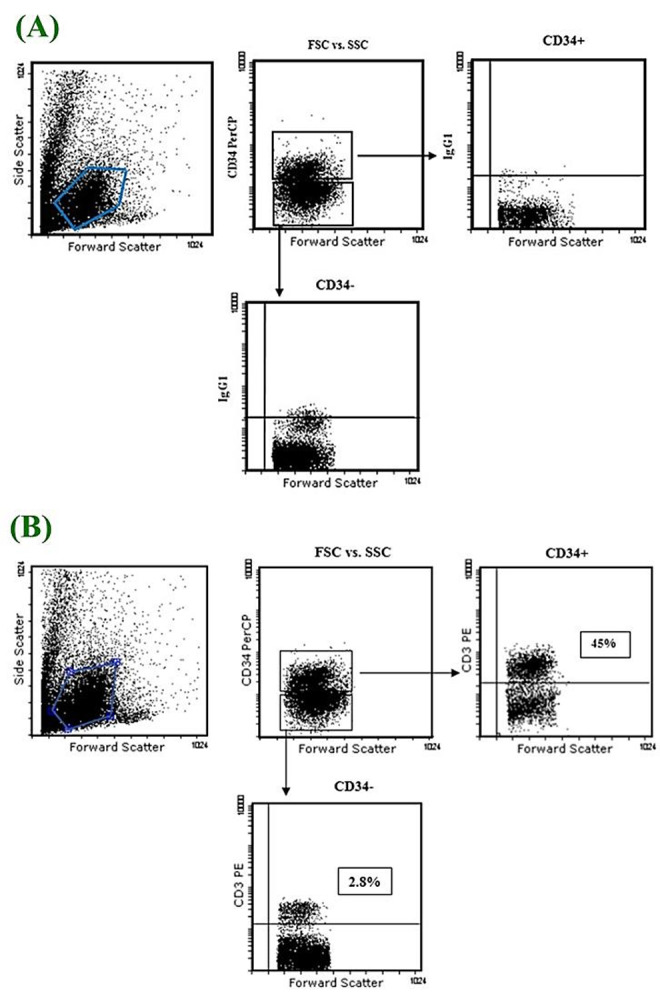
Representative FACS profile of 105 cultured cord blood mononuclear cells. A. Control. B. Sample
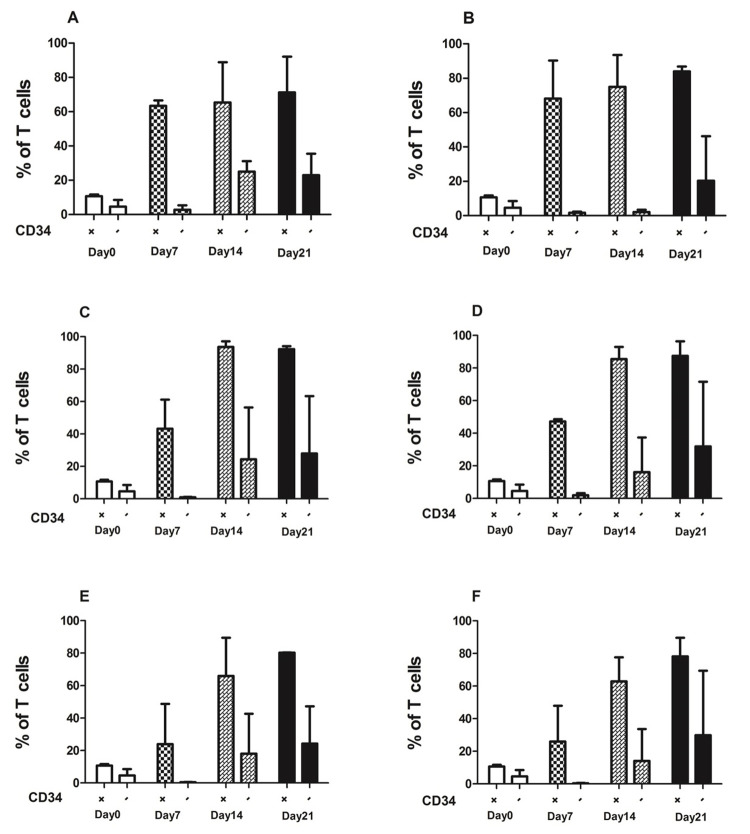
In presence of the combination of all cytokines, the percentage of T cells increased from day 7 to day 21 significantly from 10% to around 93% (Figure 2). IL-2 in compare to IL7 and IL15, produced higher CD34+T cells (92.4%) (Figure 2C). However with addition of IL7 and IL15 the percentages of CD34+T cells were 87.4% and 80.3% respectively (Figure 2D, E). Moreover in combination of all cytokines the percentage of T cells was (78.2%) (Figure 2F). CD3+CD34- cells increased in presence of different combination of mentioned cytokines from day 7 to day 21 as like as CD3+CD34+ cells. There is significant difference between expansion of CD3+CD34+ and CD3+CD34- in different time points (Figure 2A, B, C, D, and F).
Figure 2.
Percentage of CD3+CD34+ and CD3+CD34- cells derived from cord blood mononuclear cells.
Flow cytometry and mean (SD) were used to evaluate the expression of CD3+ cells in different time point in presence of different combination of cytokines: no cytokines (A), SCF+FL (B), SCF+FL+IL2 (C), SCF+FL+IL7 (D) SCF+FL+IL15 (E), and SCF+FL+IL2+IL7+IL15 (F). The percentage of CD3+CD34+ and CD3+CD34- Cells increased after 21 days of culture in the presence of cytokines.
Discussion
Stem cell transplantation is a standard treatment for hematological disorders. Umbilical cord blood is an alternative source of hematopoietic stem cell and it is currently used for transplantation.25 However, the limiting number of cells in cord blood is a challenging factor in transplantation especially in adult recipients.26 In this study, we evaluated the potential of UCB CD34+ cells to differentiate to T cells. We cultured mononuclear cord blood cells in presence of IL2, IL7 and IL15. We compared percentage of T cells derived from CD34+ and CD34- at different time points.
Previous studies showed relationship between IL2 and IL7 with T cell differentiation,22-24 It has been reported that knockout in IL7 gene reduces mature T cell dramatically and also cause decline in the size of thymus.27,28 IL2 deficiency cause reduction in the T cell progenitors and block the T cell maturation.29 It is very important to understand whether T cell derived cord blood cells are affected by IL2, IL7 and IL15. We tested T cell differentiation from cord blood mononuclear cells in presence of IL2 and IL7. We found that IL-2 in comparison to IL7 has more influence on T cell differentiation from CD34+ cord blood cells. We obtained same result in agreement with previous studies which have displayed that the percentage of T cells increased in presence of IL7 (Marcel R. M. and colleagues in 2001).30 However IL15 did not increase the T cell differentiation. This finding is in agreement with other studies which have shown that IL15 is a key cytokines for NK cell development. Therefore our data illustrated that this cytokines can be used in ex vivo expansion of UCB HSCs.
These findings are worth for clinicians, who are working on cord blood transplantation. It is important to understand, whether they should use cytokines for the expansion of cord blood cells before transplantation or in vivo injection during transplantation. As shown IL2 and IL7 alone increase the T cell differentiation from cord blood cells more than other combinations. Although Donor T cell from bone marrow transplantation attack and kill the recipient cells and cause the relapse because of the graft versus host disease, however in cord blood transplantation these negative effect is not severe and clinicians can prevent the minor negative effect of derived T cell in cord blood transplantation by injection of T cell preventive drugs. These findings could be helpful to improve the cord blood transplantation for leukemia patients and probably it should be replaced with other types of transplantation. However, still the types of T cell derived from cord blood CD34 positive cells remain unknown.
Conclusion
In summary, we illustrated that either IL2 or IL7 versus other cytokine combinations, generate more T cell from cord blood CD34 cells, probably this cytokines can be useful for ex vivo and in vivo expansion of cord blood stem cell. However it is crucial to understand whether T cell derived cells are cytolytic or other types.
Acknowledgments
The authors thank Nazli Saeedi for helpful flowcytometry and Farinaz Barghi for her editorial help .This work has been approved by School of Advanced Medical Sciences and financially supported by Research Council of Medical Sciences. Hojjatollah Nozad Charoudeh has an Assistant Professor position in Faculty of Medicine. Zeynab Aliyari is spending her master of sciences.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE. et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–75. doi: 10.1056/nejmoa041276. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94(10):5320–5. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Link H, Arseniev L, Bahre O, Kadar JG, Diedrich H, Poliwoda H. Transplantation of allogeneic CD34+ blood cells. Blood. 1996;87(11):4903–9. [PubMed] [Google Scholar]
- 4.Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995;86(10):3745–53. [PubMed] [Google Scholar]
- 5.Delaney C, Ratajczak MZ, Laughlin MJ. Strategies to enhance umbilical cord blood stem cell engraftment in adult patients. Expert Rev Hematol. 2010;3(3):273–83. doi: 10.1586/ehm.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24(1):287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 7.Vosshenrich CA, Di Santo JP. Interleukin signaling. Curr Biol. 2002;12(22):R760–3. doi: 10.1016/s0960-9822(02)01286-1. [DOI] [PubMed] [Google Scholar]
- 8.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74(6):961–5. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 9.Schimpl A, Hunig TM, Elbe A, Berberich I, Kramer S, Merz H. Development and Function of the Immune System in Mice with Targeted Disruption of the Interleukin 2 Gene. San Diego: Academic Press; 1994. [Google Scholar]
- 10.Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo IL-7 deprivation on T cell maturation. J Exp Med. 1995;181(4):1399–409. doi: 10.1084/jem.181.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Freeden-Jeffry U, Vieira P, Lucian LA, Mcneil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194(8):1141–50. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SJ, Cheng PJ, Hsiao SS, Lin HH, Hung PF, Kuo ML. Differential effect of IL-15 and IL-2 on survival of phytohemagglutinin-activated umbilical cord blood T cells. Am J Hematol. 2005;80(2):106–12. doi: 10.1002/ajh.20431. [DOI] [PubMed] [Google Scholar]
- 15.Ueda T, Tsuji K, Yoshino H, Ebihara Y, Yagasaki H, Hisakawa H. et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105(7):1013–21. doi: 10.1172/jci8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Z, Jiang Y. Umbilical Cord Blood Hematopoietic Stem Cell Expansion Ex Vivo. J Blood Disord Transfus. 2013;S3:004. doi: 10.4172/2155-9864.s3-004. [DOI] [Google Scholar]
- 17.Hofmeister CC, Zhang J, Knight KL, Le P, Stiff PJ. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39(1):11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]
- 18.Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW. et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182(7):4255–66. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavazzana-Calvo M, Hacein-Bey S, De Saint Basile G, De Coene C, Selz F, Le Deist F. et al. Role of interleukin-2 (IL-2), IL-7, and IL-15 in natural killer cell differentiation from cord blood hematopoietic progenitor cells and from gamma c transduced severe combined immunodeficiency X1 bone marrow cells. Blood. 1996;88(10):3901–9. [PubMed] [Google Scholar]
- 20.Vitale M, Sivori S, Pende D, Augugliaro R, Di Donato C, Amoroso A. et al. Physical and functional independency of p70 and p58 natural killer (NK) cell receptors for HLA class I: their role in the definition of different groups of alloreactive NK cell clones. Proc Natl Acad Sci U S A. 1996;93(4):1453–7. doi: 10.1073/pnas.93.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng M, Charoudeh HN, Brodin P, Tang Y, Lakshmikanth T, Hoglund P. et al. Distinct and overlapping patterns of cytokine regulation of thymic and bone marrow-derived NK cell development. J Immunol. 2009;182(3):1460–8. doi: 10.4049/jimmunol.182.3.1460. [DOI] [PubMed] [Google Scholar]
- 22.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 23.Meazza R, Azzarone B, Orengo AM, Ferrini S. Role of common-gamma chain cytokines in NK cell development and function: perspectives for immunotherapy. J Biomed Biotechnol. 2011;2011:861920. doi: 10.1155/2011/861920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carayol G, Robin C, Bourhis JH, Bennaceur-Griscelli A, Chouaib S, Coulombel L. et al. NK cells differentiated from bone marrow, cord blood and peripheral blood stem cells exhibit similar phenotype and functions. Eur J Immunol. 1998;28(6):1991–2002. doi: 10.1002/(SICI)1521-4141(199806)28:06<1991::AID-IMMU1991>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Kawase T, Morishima Y, Matsuo K, Kashiwase K, Inoko H, Saji H. et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. 2007;110(7):2235–41. doi: 10.1182/blood-2007-02-072405. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009;94(4):451–4. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S, Hollander GA. et al. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta+ intraepithelial lymphocytes. J Immunol. 2013;190(12):6173–9. doi: 10.4049/jimmunol.1202573. [DOI] [PubMed] [Google Scholar]
- 28.Munitic I, Williams JA, Yang Y, Dong B, Lucas PJ, El Kassar N. et al. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood. 2004;104(13):4165–72. doi: 10.1182/blood-2004-06-2484. [DOI] [PubMed] [Google Scholar]
- 29.Popmihajlov Z, Xu D, Morgan H, Milligan Z, Smith KA. Conditional IL-2 Gene Deletion: Consequences for T Cell Proliferation. Front Immunol. 2012;3:102. doi: 10.3389/fimmu.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alpdogan O, Schmaltz C, Muriglan SJ, Kappel BJ, Perales MA, Rotolo JA. et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98(7):2256–65. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]