Abstract
Purpose: Toll-like receptors (TLR) are well known components of the innate immune system. Among them, TLR4 is related to the inflammatory processes involved in atherosclerotic plaque formation. Our purpose was to compare the monocytic expression of TLR4 following implantation of drug-eluting (DES) and bare stents (BMS).
Methods: In this study, patients with chronic stable angina undergoing elective percutaneous coronary intervention (PCI) in ShahidMadani Heart Hospital, Tabriz, Iran were included. Ninety-five patients receiving DES and 95 patients receiving BMS were selected between 2012 and 2014.Everolimus eluting stents were implanted for DES group. Both groups received similar medications and procedure. Blood samples were taken before PCI, 2 hours and 4 hours after termination of PCI. Expression of TLR4 on monocytes was measured using flowcytometry. Patients were matched for age, sex and coronary artery disease risk factors, but not for TLR4 expression rate before PCI.
Results: A significant difference was seen between DES and BMS in TLR4 expression before (21.3±2.8% vs. 15.5±2.7%; P< 0.05) and four hours after PCI (30.1 ± 3.3% vs 39.2 ± 3.2%, P< 0.05). Due to the unmatched rate of TLR4+ expression before PCI, we measured the percentage of increase in TLR4 expression between groups. DES compared to BMS significantlycaused less increase in the TLR4 expression (50.23%±10.03% vs. 446.35%±70.58%, p<0.001).
Conclusion: Our findings suggest thateverolimuseluted from the stents can decrease PCI induced increase in the TLR4 expression on the surface of monocytes.
Keywords: Inflammation, Percutaneous coronary intervention, Stent, TLR4
Introduction
Atherosclerosis is a chronic and generalized disease of vessels.1,2 Smoking, hypertension, diabetes, and hypercholesterolemia are well-defined risk factors for Atherosclerosis.3 In line with other important components of atherosclerosis, mononcytes and T cells have distinguished role in initiation and progression of atherogenesis.4 Macrophages can secrete cytokines, hydrolytic enzymes, metalloproteinases, and growth regulating molecules. It is noted that the interaction between macrophages and T helper cells can lead to a chronic inflammatory response.1,4-7
Toll-like receptor4 (TLR4) is a pattern recognition molecule, which consistent with its role in pathogen recognition, is expressed by cells involved in the first line of host defense, including neutrophils, macrophages, and dendritic cells. TLR4 sensitization on the surface of monocytes usually results in production of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α.8-10 It is well established that TLR4 can be activated by a number of endogenous ligands that are formed in pathological conditions.11 It is also found that TLR4 is expressed in endothelial cells of human atherosclerotic lesions. It is suggested that TLR4 is involved in plaque formation and deterioration of the coronary arteries.12,13 It has been shown that patients with acute coronary syndromes (ACS) had an augmented expression of TLR4 on the surface of CD14+ monocytes compared to patients with stable angina.14,15
Reperfusion therapies like coronary artery bypass graft surgery (CABG) or percutaneous coronary interventions (PCI) are used to treat patients with chronic stable angina who are still symptomatic although on routine medical therapy or have dangerous anatomical lesions.15,16 PCI contribution to local or general inflammatory responses is a subject of debate. Our previous works showed that implantation of stents could up-regulate the monocytic expression of TLR4.17
Currently most of PCI procedures are performed using DES. However, Bare Metallic Stents (BMS) are still in use. In general, anti-proliferative drugs are incorporated into DES to inhibit local cell proliferation and blood vessel occlusion. This study was designed to compare effects of DES and BMS on monocytic expression of TLR4 in patients who underwent PCI. Everolimus-eluting stents are among the most popular stents used in PCI. Everolimus is an analogue of sirolimus and a macrolide antibiotic with immunosuppressive and anti-proliferative activities. Everolimus inhibits cell proliferation and suppresses in-stent neointimal growth and significantly improve neointimal healing and reduce of neointimal proliferation.18,19 We focused on understanding whether in this setting everolimuseluted from the stents is able to reduce monocytic expression of TLR4.
Materials and Methods
Study patients
Between 2012 and 2014, patients with chronic stable angina undergoing elective percutaneous coronary intervention (PCI) in ShahidMadani Heart Hospital, Tabriz, Iran were included. Study was designed to include 95 patients who received DES and 95 patients who received BMS for chronic stable angina. Patient selection was done randomly. The exclusion criteria were previous myocardial infarction within 3 months, autoimmune diseases, complete chronic occlusion, advanced hepatic or renal disease, malignant neoplastic disease, receiving hydrocortisone during PCI, valvular heart disease, heart failure, unstable angina, receiving anti-inflammatory and immunosuppressive drugs and using antibiotics. The study was approved by ethical board of Tabriz University of Medical Science and informed consent letter was obtained from all participants.
Cardiovascular risk factors, medications, sex, age and previous medical history were obtained by questionnaire. In addition to home medications, all of the patients were treated with standard PCI medications including heparin, aspirin and clopidogrel.
Blood collection and processing
Blood sampling was performed in a time dependent manner: time of admission (0 h), 2 hour and 4 hour (2h, 4h) after PCI procedure. Two ml blood was taken from patients and kept in EDTA anticoagulant tubes for flowcytometry.
Stenting procedure
Patients received intravenous heparin (10,000 U) prior the stenting procedure. Patients took aspirin (80 mg per day) and clopidogrel (75 mg per day) after implantation of stents. All PCI procedures were performed according to the routine protocols of ShahidMadani Heart Hospital, Tabriz, Iran.
Measurement of TLR4 expression on the surface of monocytes
Briefly, cells were stained for 40 minutes with monoclonal antibodies for human CD14 (BD, USA) conjugated with fluorescein isothiocyanate (FITC), and hTLR4 (BD, USA) conjugated with phycoerythrin (PE) at room temperature in the dark. FITC and PE-conjugated non-specific mouse IgG2a antibodies were used for isotype controls (Dako, Denmark). Cell- associated fluorescence was measured using FACSCalibur flow cytometer (BD, USA). Data were analyzed using CellQuest software (BD, USA).
Statistical analysis
Data are presented as mean ± SD. Unpaired t-test and chi-square were used to compare differences between two groups. Statistical significance was considered as P<0.05. Analyses were performed using SPSS software version 16.
Results
Characteristics of patients
Clinical and angiographic data of 190 patients are presented in Table 1. No significant difference was seen in the use of medications, white blood cell count or the risk factors.
Table 1. Baseline and angiographic characteristics of patients .
| - | DES (n=95) | BMS (n = 95) | P value |
| Age (years) | 58.8 ± 6.6 | 59.5 ± 8.3 | 0.7 |
| Male | 68 | 72 | 0.3 |
| Hypertension | 46 | 54 | 0.8 |
| Hyperlipidemia | 28 | 25 | 0.7 |
| Smoking | 29 | 28 | 0.9 |
| Diabetes | 44 | 37 | 0.6 |
| Aspirin | 83 | 88 | 0.3 |
| Beta-blockers | 58 | 61 | 0.6 |
| Calcium Channel Blocker | 20 | 16 | 0.7 |
| Nitroglycerin | 50 | 54 | 0.6 |
| Statins | 80 | 84 | 0.5 |
| Clopidogrel | 63 | 60 | 0.3 |
| ACE inhibitor | 45 | 49 | 0.6 |
| WBC | 7.1±0.4 | 7.2±0.5 | 0.4 |
| HDL | 41±8 | 44±3 | 0.3 |
| LDL | 140±31 | 134±29 | 0.7 |
| Glucose | 191±40 | 109±18 | 0.6 |
| Cholesterol | 107±21 | 189±34 | 0.07 |
| One-vessel | 60 | 54 | 0.5 |
| Two-vessel | 33 | 39 | 0.8 |
| Three-vessel | 2 | 2 | 0.0 |
Data are shown in mean ± SD and number
ACE: angiotensin converting enzyme.
TLR4 monocyte expression
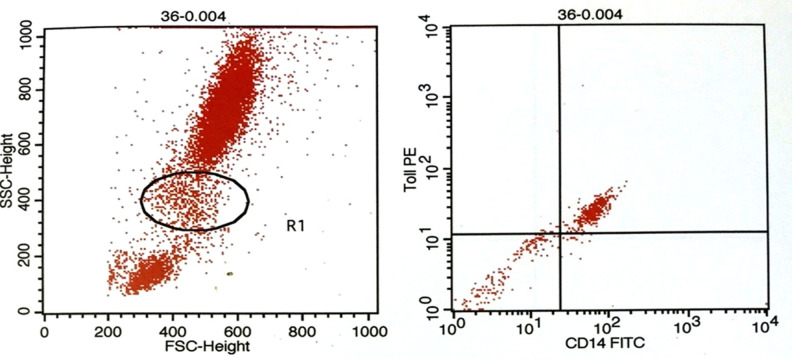
Flowcytometry was used to quantify TLR4 expression on CD14+ monocytes (Figure 1). As shown in Table 2, before PCI, TLR4+/CD14+ monocytes were 15.5±2.7% in patients implanted with BMS. Two hours and 4 hours after PCI it was 33.90±3.6.% and 39.5±3.2%, respectively. In DES group, TLR4+/CD14+ monocytic expressionat different time pointswere 21.3±2.8%, 33.1±3.2% and 30.1±3.3%, respectively. Difference between two groups was significant before and 4 hours after PCI (P< 0.05). Due to the differences between TLR4+/CD14+ monocytic expression before PCI in DES and BMS, we measured the percent of increase in TLR4 expression. Findings showed that DES compared to BMS had significantly less increase in percent of TLR4 expression (50.23%±10.03% vs. 446.35%±70.58%, p<0.001).
Figure 1.
Gating monocyte population (A). Representative dot plot showing TLR4+/CD14+ monocytes (B). Ten thousand cells were analyzed by flowcytometry.
Table 2. Frequency of TLR4+/CD14+ on circulating monocytes at different time points (%).
| Group | 0h | 2h | 4h |
| BMS (n=95) | 15.5 ± 2.7* | 33.9 ± 3.6 | 39.2 ± 3.2** |
| DES (n=95) | 21.3 ± 2.8 | 33.1 ± 3.2 | 30.1 ± 3.3 |
*P< 0.05 vs. DES at 0h; **P< 0.05 vs. DES at 4h.
Discussion
Everolimus is a proliferation signal inhibitor that acts on several cell types including vascular smooth muscle cells. The antiproliferative properties of the drug inhibit in-stent neointimal growth in experimental models and coronary vessels following stent implantation.18,19 Everolimus is highly lipophilic, potent, and rapidly absorbed into tissue making it a desirable drug for intravascular delivery.
Evidence from literature suggests that inflammation has a critical role in re-stenosis following BMS or DES implantation.20,21 In this study, patients with chronic stable angina were included to compare effects of BMS and everolimus eluting stent as the DES on monocyte expression of TLR4 at different time intervals.
We previously reported that PCI as an invasive method can positively regulate monocyte expression of TLR4.17 In contrast, some investigations demonstrated that various inflammatory cells were down-regulated following PCI procedure.16 In contrast, some investigations demonstrated that various inflammatory cells were down-regulated following PCI procedure.The study byTiong and colleagues revealed that neutrophil Mac-1 was deactivated and plasma matrix metalloproteinase-9 (MMP-9) was unchanged in patients that underwent PCI.22
Generally, at the time of stent implantation the overall inflammatory status is not equivalent in all patients. Our findings in link with other studies may partially explain the mechanism of restenosis after PCI in which inflammation plays a pivotal role.
In the present study, we found that both BMSs and DESs could significantly up-regulate CD14+/TLR4+ monocytes after PCI procedure. However, BMS caused a greater up-regulation of CD14+/TLR4+ monocytes. In fact, DES contribution to partial suppression of local inflammation may be related to the relative down regulation of monocytic expression of TLR4 and of courses its related pro-inflammatory cytokines.
Reperfusion following a successful PCI can harm the myocardium through propagation of inflammation. Notably, Yang et al. proved that fibrinolytic therapy in patients with ST elevation MI could significantly up-regulate monocyte expression of TLR4.14
Evidence suggests that inflammatory responses following BMS are greater than DES. It is proposed that vascular injury by BMS can initiate a local inflammation.23 However, recent study showed that DES could cause a late increase in the levels of the pro-inflammatory cytokines compared with BMS.24 Moreover, another study reported that sirolimus- eluting stents and paclitaxel – eluting stents are associated with higher risk of late thrombosis.25 This finding may partially explain the difference between BMS and DES in healing of the injured endothelial cells.26
The results of the present study provide evidence that eluted drugs in stents can partially suppress the early inflammation following PCI. The primary mechanism of action of such drugs is inhibition of T cell proliferation through blockade of the mammalian target of rapamycin (mTOR).27 However, the exact mechanism of action in this setting remains unknown. Further experimental and clinical studies should address concomitant use of medications that target PCI related inflammation. It is also beneficial that larger trials clarify a strong perception about the prognostic value of TLR4 in patients undergoing PCI.It is postulated that systemic use of immunosuppressive drugs can improve clinical outcome of patients following PCI or other craniological procedures that are associated with inflammation.28,29 Unfortunately, systemic use of such drugs have had no considerable effect on clinical status of patients so far. Some limitation of our study should be acknowledged;Due to limited number of patients, our data should be confirmed in larger studies. Obviously our findings are representative for the majority of male patients with IHD in Iran. Finally, the activation mechanism of TLR4 in non-infectious conditions like PCI should be further investigated.
Acknowledgments
This article was written based on a dataset of cardiology residency thesis, registered in Tabriz University of Medical Sciences.This study was supported by a grant from Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical Issues
Permission to conduct the study was obtained from the Ethics Committee at Tabriz University of Medical Sciences.
Conflict of Interest
Authors declare no conflict of interest in this study.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W Jr, Rosenfeld ME. et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1994;89(5):2462–78. doi: 10.1161/01.Cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW. Established risk factors and coronary artery disease: The framingham study. Am J Hypertens. 1994;7(7 Pt 2):7S–12S. doi: 10.1093/ajh/7.7.7s. [DOI] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104(4):503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 5.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41(4 Suppl S):15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 6.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM. et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92(9):3893–7. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 10.Imler JL, Hoffmann JA. Toll and toll-like proteins: An ancient family of receptors signaling infection. Rev Immunogenet. 2000;2(3):294–304. [PubMed] [Google Scholar]
- 11.Akira S. Mammalian toll-like receptors. Curr Opin Immunol. 2003;15(1):5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 12.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 13.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL. et al. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg. 2004;128(2):170–9. doi: 10.1016/j.jtcvs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Jin LY, Ding JW, Zhou YQ, Yang J, Rui Y. et al. Expression of toll-like receptor 4 on peripheral blood mononuclear cells and its effects on patients with acute myocardial infarction treated with thrombolysis. Arch Med Res. 2010;41(6):423–9. doi: 10.1016/j.arcmed.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111(20):2654–61. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 16.Versteeg D, Hoefer IE, Schoneveld AH, de Kleijn DP, Busser E, Strijder C. et al. Monocyte toll-like receptor 2 and 4 responses and expression following percutaneous coronary intervention: Association with lesion stenosis and fractional flow reserve. Heart. 2008;94(6):770–6. doi: 10.1136/hrt.2007.117259. [DOI] [PubMed] [Google Scholar]
- 17.Bagheri B, Sohrabi B, Movassaghpur A, Mashayekhi S, Garjani A, Shokri M. et al. Monocyte expression of toll-like receptor-4 in patients with stable angina undergoing percutanoeus coronary intervention. Iran J Immunol. 2012;9(3):149–58. [PubMed] [Google Scholar]
- 18.Waksman R, Pakala R, Baffour R, Hellinga D, Seabron R, Kolodgie F. et al. Optimal dosing and duration of oral everolimus to inhibit in-stent neointimal growth in rabbit iliac arteries. Cardiovasc Revasc Med. 2006;7(3):179–84. doi: 10.1016/j.carrev.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Carter AJ, Brodeur A, Collingwood R, Ross S, Gibson L, Wang CA. et al. Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc Interv. 2006;68(1):97–103. doi: 10.1002/ccd.20769. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda D, Shimada K, Tanaka A, Kawarabayashi T, Yoshiyama M, Yoshikawa J. Circulating monocytes and in-stent neointima after coronary stent implantation. J Am Coll Cardiol. 2004;43(1):18–23. doi: 10.1016/j.jacc.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Toutouzas K, Colombo A, Stefanadis C. Inflammation and restenosis after percutaneous coronary interventions. Eur Heart J. 2004;25(19):1679–87. doi: 10.1016/j.ehj.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Tiong AY, Lowe HC, Freedman SB, Brieger DB. Lack of widespread inflammation after contemporary pci. Int J Cardiol. 2010;140(1):82–7. doi: 10.1016/j.ijcard.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC. et al. Drug-eluting stent and coronary thrombosis: Biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–8. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 24.Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK. et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: An autopsy study. Circulation. 2008;118(11):1138–45. doi: 10.1161/CIRCULATIONAHA.107.762047. [DOI] [PubMed] [Google Scholar]
- 25.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C. et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet. 2007;369(9562):667–78. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 26.Laarman GJ, Suttorp MJ, Dirksen MT, van Heerebeek L, Kiemeneij F, Slagboom T. et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355(11):1105–13. doi: 10.1056/NEJMoa062598. [DOI] [PubMed] [Google Scholar]
- 27.Pasceri V, Granatelli C, Pristipino C, Pelliccia F, Speciale G, Pironi B. et al. A randomized trial of a Rapamycin-Eluting stent in acute myocardial infarction: preliminary results. Am J Cardiol. 2003;92(Suppl. 6A):1L. [Google Scholar]
- 28.Bagheri B, Sohrabi B, Movassaghpour AA, Mashayekhi S, Garjani A, Shokri M. et al. Hydrocortisone reduces toll-like receptor 4 expression on peripheral cd14+ monocytes in patients undergoing percutaneous coronary intervention. Iran Biomed J. 2014;18(2):76–81. doi: 10.6091/ibj.12752.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagheri B, Sohrabi B, Movassaghpur A, Mashayekhi S, Garjani A, Shokri M. et al. Association of monoctye expression of Toll-like receptor 4 and its related cytokines with coronary luminal stenosis. Adv Biosci Biotechnol. 2013;4:19–25. doi: 10.4236/abb.2013.47a1004. [DOI] [Google Scholar]