γ-Terpinene synthase from T. vulgaris was characterized. The crystal structure was elucidated to almost 1.5 Å resolution.
Keywords: γ-terpinene synthase, thyme, Thymus vulgaris, terpenoid biosynthesis, cyclase, crystal structure, essential oils, geranyl diphosphate, gene expression
Abstract
The biosynthesis of γ-terpinene, a precursor of the phenolic isomers thymol and carvacrol found in the essential oil from Thymus sp., is attributed to the activitiy of γ-terpinene synthase (TPS). Purified γ-terpinene synthase from T. vulgaris (TvTPS), the Thymus species that is the most widely spread and of the greatest economical importance, is able to catalyze the enzymatic conversion of geranyl diphosphate (GPP) to γ-terpinene. The crystal structure of recombinantly expressed and purified TvTPS is reported at 1.65 Å resolution, confirming the dimeric structure of the enzyme. The putative active site of TvTPS is deduced from its pronounced structural similarity to enzymes from other species of the Lamiaceae family involved in terpenoid biosynthesis: to (+)-bornyl diphosphate synthase and 1,8-cineole synthase from Salvia sp. and to (4S)-limonene synthase from Mentha spicata.
1. Introduction
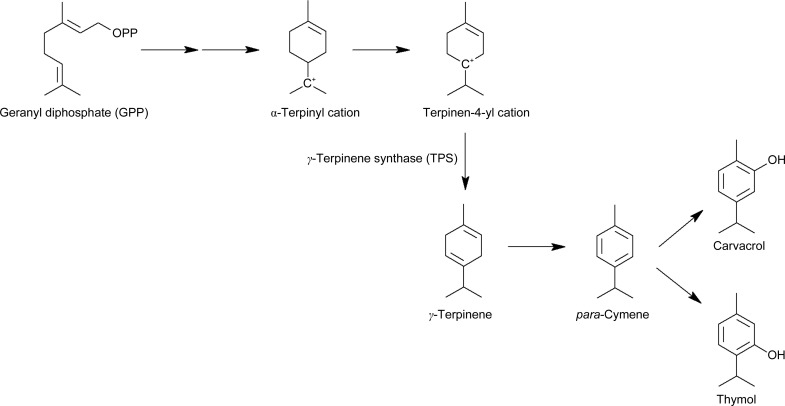
The genus Thymus (belonging to the Lamiaceae family) comprises ∼215 species spread over Mediterranean regions, the northern part of Africa and southern Greenland (Morales, 2002 ▸). A characteristic feature of these plants is the abundance of essential oil localized in the glandular trichomes, special hairs on the surfaces of the leaves. The species T. vulgaris (common thyme) represents the most widely spread and economically interesting species. Several metabolites found in the essential oil extracted from T. vulgaris have become commercially important as natural pesticides, food additives or pharmaceuticals, for example for local, respiratory and systematic infections, as well as stimulating agents with beneficial effects on digestion, circulation and also mental balance (Duke, 1991 ▸; Sefidkon et al., 2005 ▸). The biosynthesis of the components thymol (2-isopropyl-5-methylphenol) and the isomeric carvacrol (5-isopropyl-2-methylphenol), both of which are monoterpene phenols abundantly found in such oil extracts, is so far only poorly understood. Presumably, the precursor geranyl diphosphate (GPP; Poulose & Croteau, 1978 ▸) is initially converted to γ-terpinene (4-methyl-1-isopropyl-1,4-cyclohexadiene) in a series of steps catalyzed by the enzyme γ-terpinene synthase (TPS) involving cyclization and the formation of carbocation intermediates. γ-Terpinene is then converted to para-cymene, which is subsequently hydroxylated to thymol and carvacrol (Fig. 1 ▸; Haudenschild et al., 2000 ▸; Crocoll et al., 2010 ▸; Weitzel & Simonsen, 2015 ▸).
Figure 1.
Proposed reaction scheme of γ-terpinene synthesis, as catalyzed by TvTPS1, providing a precursor for thymol and carvacrol biosynthesis (based on Lima et al., 2013 ▸).
Most aspects of the detailed mechanism of enzymatic γ-terpinene synthesis as well as substrate specificity are still to be elucidated. To date, TPSs have been described as multifunctional enzymes, with single amino-acid substitutions being sufficient to result in an altered metabolic profile (Köllner et al., 2004 ▸), and a number of TPSs and other terpene-modifying enzymes have been described (Keszei et al., 2008 ▸). Recently, TPS genes have been identified and characterized from several plant species from the Lamiaceae family (summarized in Lima et al., 2013 ▸): TPS from Lavandula (Muñoz-Bertomeu et al., 2008 ▸; Lane et al., 2010 ▸), Mentha (Turner & Croteau, 2004 ▸), Origanum (Crocoll et al., 2010 ▸; Lukas et al., 2010 ▸), Perilla (Ito & Honda, 2007 ▸), Coriandrum (Galata et al., 2014 ▸) and Salvia (Kampranis et al., 2007 ▸; Schmiderer et al., 2010 ▸). Within the genus Thymus, only TPS from T. caespititius (including several isoforms) has been described previously (Lima et al., 2010 ▸, 2011 ▸, 2013 ▸; Mendes et al., 2014 ▸). Here, for the first time, the cloning, recombinant production, purification and biochemical characterization as well as the crystal structure elucidation of γ-terpinene synthase from T. vulgaris (TvTPS) is reported.
2. Materials and methods
2.1. Plant material and RNA extraction
T. vulgaris plants were obtained from OBI GmbH & Co. Deutschland KG. RNA isolation was performed from fresh young leaves only. 0.1 g of plant tissue was ground to a fine powder in liquid nitrogen using a mortar and pestle. Total RNA extraction was carried out with an innuPREP RNA Mini Kit (Analytik Jena AG, Jena, Germany). Messenger RNA was isolated using a cDNA Synthesis Kit H Plus (PEQLAB Biotech GmbH, Erlangen, Germany).
2.2. Cloning, expression and purification
Polymerase-chain-reaction amplifications were performed with Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, England) as described previously (Rudolph et al., 2014 ▸). A peqSTAR 96 Universal Gradient thermocycler (PEQLAB Biotech GmbH, Erlangen, Germany) was used for amplification according to the supplier’s recommendation.
The nucleotide sequence of TvTPS cDNA was determined by DNA sequencing from both the 5′ and the 3′ ends of the cDNA inserted into the pCR 2.1-TOPO cloning vector using M13 forward and M13 reverse primers, respectively. The sequences of the two TvTPS isoforms found (TvTPS1 and TvTPS2) were submitted to GenBank (accession Nos. ALB78115.1 and JQ957864.1 for TvTPS1 and TvTPS2, respectively). Expression-vector constructs were created using the vectors pENTR-D-TOPO and Champion pET300/NT-DEST (Invitrogen, Karlsruhe, Germany), providing a hexahistidine tag (His6) at the N-terminus of TvTPS (Table 1 ▸). To generate the single-amino-acid exchange variants of TvTPS1 (D356G and T565A), site-directed mutagenesis was carried out as described by Bauer et al. (2012 ▸) and Rudolph et al. (2014 ▸).
Table 1. Macromolecule-production information for TvTPS1.
| Source organism | T. vulgaris |
| DNA source | cDNA derived from T. vulgaris mRNA |
| Forward primer† | ATGGCTACCCTTAGCATGCAAGTGTCC |
| Reverse primer† | CACATATGGCTCGAAAATAAGGCCTCCC |
| Forward primer‡ | CACCATGGCTACCCTTAGCATGCAAGTGTCC |
| Reverse primer‡ | CTACACATATGGCTCGAAAATAAGGCCTCCC |
| Cloning vector | pCR 2.1-TOPO |
| Expression vector | pENTR-D-TOPO, Champion pET300/NT-DEST |
| Expression host | E. coli BL21 CodonPlus |
| Complete amino-acid sequence of the construct produced | MHHHHHHITSLYKKAGSMRRSGNYQAPVWNNDFIQSFSTDKYKDEKFLKKKEELIAQVKVLLNTKMEAVKQLELIEDLRNLGLTYYFEDEFKKILTSIYNEHKGFKNEQVGDLYFTSLAFRLLRLHGFDVSEDVFNFFKNEDGSDFKASLGENTKDVLELYEASFLIRVGEVTLEQARVFSTKILEKKVEEGIKDEKLLAWIQHSLALPLHWRIQRLEARWFLDAYKARKDMNPIIYELGKIDFHIIQETQLQEVQEVSQWWTNTNLAEKLPFVRDRIVECYFWALGLFEPHEYGYQRKMAAIIITFVTIIDDVYDVYDTLDELQLFTDAIRKWDVESISTLPYYMQVCYLAVFTYASELAYDILKDQGFNSISYLQRSWLSLVEGFFQEAKWYYAGYTPTLAEYLENAKVSISSPTIISQVYFTLPNSTERTVVENVFGYHNILYLSGMILRLADDLGTTQFELKRGDVQKAIQCYMNDNNATEEEGTEHVKYLLREAWQEMNSAMADPDCPLSEDLVFAAANLGRTSQFIYLDGDGHGVQHSEIHNQMGGLIFEPYV |
Used in TvTPS cDNA cloning.
Used in cloning into expression vector.
Bacterial expression cultures were grown in 1 l LB medium pH 7.5 at 37°C (in a shake flask) to an OD600 of 0.5–0.7. 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) was then added to induce the expression of TvTPS. Cells were subsequently cultivated at 20°C for up to 18 h for optimal expression of recombinant TvTPS (Lima et al., 2013 ▸) and were then harvested by centrifugation for 20 min at 4000g and 4°C. The pellet was frozen and kept at −20°C. After thawing on ice, the cells were resuspended in 10 ml lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole pH 8.0) containing 1 mg ml−1 lysozyme. Cell disruption was performed by ultrasonication (7 × 10 s) at maximum power (Bandelin Sonopuls UW 2070/MS73, Bandelin Electronic, Berlin, Germany). The debris was removed by centrifugation at 4°C for 30 min at 13 000g. His6-tagged TvTPS was purified from the supernatant using an ÄKTApurifier system and a HisTrap HP (1 ml) column (GE Healthcare, Uppsala, Sweden) according to the manufacturer’s manual (QIAexpressionist). After purification, the protein was concentrated to a volume of 200 µl using Amicon Ultra centrifugal filter units (Merck Millipore, Darmstadt, Germany) and loaded for size-exclusion chromatography onto a Superdex 75 10/300 gel-filtration column (GE Healthcare) equilibrated with a buffer consisting of 20 mM Tris–HCl pH 7.8, 1 mM MgCl2, 4 mM DTT. The eluted protein fractions were pooled and the protein was concentrated to 10 mg ml−1 according to quantification using the method of Bradford (1976 ▸).
SDS–PAGE (Fig. 2 ▸) was carried out using a Bio-Rad Mini-PROTEAN 3 slab-gel apparatus (Bio-Rad, Richmond, California, USA) and the Laemmli system (Laemmli, 1970 ▸) to assess protein purity. Molecular-weight markers (SeeBlue Plus2 pre-stained standard; Invitrogen, Karlsruhe, Germany) ranging in size from 14 to 191 kDa were used as molecular-weight standards. 10% Bis-Tris gels were run with MOPS buffer (50 mM MOPS, 50 mM Tris, 1 mM EDTA, 0.1% SDS) at 130 V for 20 min followed by 180 V for 45 min. The gels were washed three times with water (10 min each) and visualized after sensitive Coomassie staining overnight (Kang et al., 2002 ▸). Western blot analysis was performed using antibodies against the His6-tag (Anti-His Antibody Selector Kit, a mixture of RGS-His, tetra-His and penta-His antibodies; Qiagen GmbH, Hilden, Germany). Native protein PAGE was performed as described by Herl et al. (2009 ▸).
Figure 2.
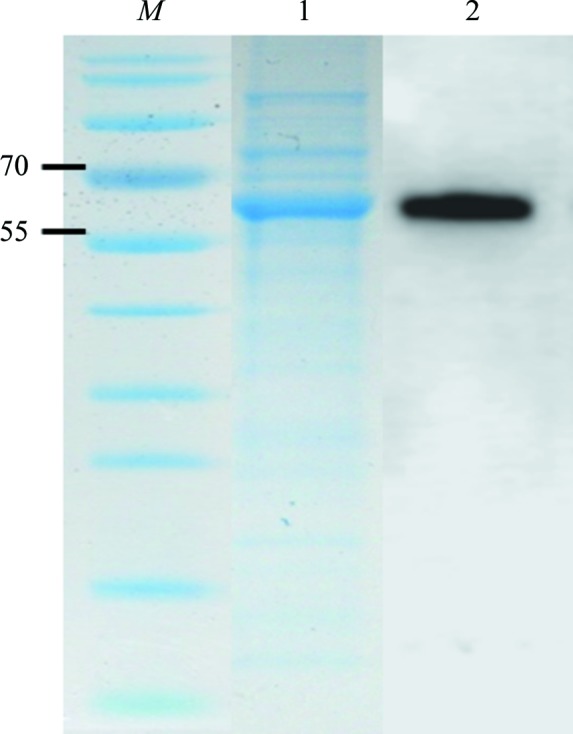
Overexpression of TvTPS1 analyzed by SDS–PAGE and Western blotting. Lane M, molecular-weight marker proteins (labelled in kDa); lane 1, partially purified fraction of TvTPS1; lane 2, Western blot analysis using an anti-His6-tag antibody
2.3. Activity assay
The enzyme activity of the TvTPS variants was assayed in a 1.5 ml vial containing 1.56 µg purified enzyme and filled to 500 µl with assay buffer (10 mM Tris–HCl pH 7.0, 10 mM MgCl2, 10% glycerol, 1 mM DTT). The reaction was initiated by adding GPP (Sigma, Taufkirchen, Germany) to a final concentration of 60 µM and was incubated at 25°C for 30 min. Terpene products were collected in 100 µl n-pentane. The upper layer was analyzed by GC-MS (Shimadzu GmbH, Duisburg, Germany). The area under the curve was integrated with the GCMSsolution software (v.2.70; Shimadzu) using thymol as an internal standard. To confirm the product identity, a sample reference of pure γ-terpinene was used (Fluka AG, St. Gallen, Switzerland).
2.4. Crystallization
Crystals of purified TvTPS1 were obtained using commercially available crystallization screens from Hampton Research (Crystal Screen, Index, PEGRx and PEG/Ion). The screens were set up in 96-well plates (sitting-drop method, 70 µl reservoir, drop volumes 0.4–0.6 µl, protein:reservoir ratios of 2:1, 1:1 and 1:2, protein concentration 10 mg ml−1) using a crystallization robot (Phoenix, Art Robbins) at a temperature of 19°C. Crystals appeared within 3–12 d in many different conditions in various shapes and sizes. The crystallization conditions of the crystal used for data collection and structure elucidation are given in Table 2 ▸.
Table 2. Crystallization.
| Method | Sitting drop |
| Plate type | 96-well |
| Temperature (K) | 292 |
| Protein concentration (mg ml−1) | 10 |
| Buffer composition of protein solution | 20 mM Tris–HCl pH 7.8, 1 mM MgCl2, 4 mM DTT |
| Composition of reservoir solution | 0.2 M ammonium tartrate dibasic pH 7.0, 20%(w/v) polyethylene glycol 3350 |
| Volume and ratio of drop | 0.4–0.6 µl, 1:1 and 1:2 |
| Volume of reservoir (µl) | 70 |
2.5. Data collection and processing
Crystals of TvTPS1 were analyzed for X-ray diffraction using an in-house copper rotating-anode generator (Rigaku MicroMax-007 HF) equipped with an image-plate detector (Rayonix MAR345). The best diffracting crystals of dimensions of up to 200 µm (Fig. 3 ▸) could be harvested from condition No. 30 of the PEG/Ion 2 screen (Table 2 ▸). As they were not single crystals but were composed of several layers of crystals, an acupuncture needle was used to isolate pieces without visible defects. After cryoprotection for 10 min in a mixture consisting of 80%(v/v) reservoir and 20%(v/v) ethylene glycol, the crystals were flash-cooled in liquid nitrogen. An initial data set (1.9 Å resolution) was collected in-house and processed with the XDS package (Kabsch, 2010 ▸). This data set was complemented by a higher resolution data set (1.65 Å) acquired on beamline P13 at the PETRA III synchrotron source (MX1, EMBL, Hamburg, Germany) using a Pilatus 6M-F pixel detector (Dectris). Data processing was carried out using XDS. The final data set (1.65 Å resolution) was obtained by merging the in-house and synchrotron data sets with XSCALE (Kabsch, 2010 ▸) as the merged data set provided higher completeness and redundancy compared with the synchrotron data set alone. Data-collection parameters and processing statistics for the three data sets are given in Table 3 ▸.
Figure 3.

Triclinic crystals of recombinant TvTPS1 used for structure elucidation.
Table 3. Data-collection and processing statistics.
Values in parentheses are for the outer shell.
| Data set | In-house | Synchrotron | Merged |
|---|---|---|---|
| Diffraction source | Rigaku MicroMax-007 HF | P13, PETRA III | — |
| Wavelength (Å) | 1.5418 | 0.97731 | — |
| Temperature (K) | 100 | 100 | — |
| Detector | Rayonix MAR345 | Dectris Pilatus 6M-F | — |
| Crystal-to-detector distance (mm) | 130 | 293 | — |
| Rotation range per image (°) | 1 | 0.2 | — |
| Total rotation range (°) | 180 | 360 | — |
| Exposure time per image (s) | 900 | 0.1 | — |
| Space group | P1 | ||
| a, b, c (Å) | 50.11, 79.29, 93.10 | 50.20, 79.61, 93.32 | |
| α, β, γ (°) | 96.09, 103.23, 105.46 | 94.79, 103.31, 105.56 | |
| Mosaicity (°) | 0.1 | ||
| Resolution range (Å) | 20.6–1.90 (2.02–1.90) | 46.7–1.65 (1.75–1.65) | 46.7–1.65 (1.75–1.65) |
| Total No. of reflections | 194409 (30017) | 555250 (88301) | 745728 (88882) |
| No. of unique reflections | 96757 (14985) | 146156 (23393) | 154186 (23289) |
| Completeness (%) | 92.6 (88.7) | 90.1 (89.0) | 95.7 (89.2) |
| Multiplicity | 2.0 (2.0) | 3.8 (3.8) | 4.8 (3.8) |
| 〈I/σ(I)〉 | 10.35 (1.49) | 11.74 (1.72) | 9.19 (1.96) |
| R meas | 0.094 (0.819) | 0.070 (0.779) | 0.115 (0.757) |
| CC1/2 | 99.7 (63.6) | 99.8 (74.1) | 99.7 (76.2) |
| Overall B factor from Wilson plot (Å2) | 23.0 | 22.4 | 22.9 |
2.6. Structure solution and refinement
The crystal structure of TvTPS1 was solved by molecular replacement using Phaser (McCoy et al., 2007 ▸) and the crystal structure of (4S)-limonene synthase from Mentha spicata (Hyatt et al., 2007 ▸; PDB entry 2ong; 47% sequence identity to TvTPS1). The asymmetric unit contained two molecules of TvTPS1. The phased structure was further completed by automated model building using phenix.autobuild from the PHENIX suite (Adams et al., 2010 ▸). The model was finished by manual building with Coot (Emsley et al., 2010 ▸) and refined using phenix.refine (PHENIX suite), employing TLS refinement but with no use of NCS restraints. Structure validation was performed with MolProbity (Chen et al., 2010 ▸); refinement statistics are shown in Table 4 ▸. Preparation of molecular representations and structural superpositions as well as the calculation of r.m.s.d. values were performed using PyMOL (v.1.7.4; Schrödinger). The atomic coordinates and structure factors have been deposited in the Protein Data Bank as entry 5c05.
Table 4. Structure-solution and refinement statistics.
Values in parentheses are for the outer shell.
| Resolution range (Å) | 29.84–1.65 (1.69–1.65) |
| Completeness (%) | 95.6 (90.0) |
| No. of reflections, working set | 152073 (10298) |
| No. of reflections, test set | 1990 (126) |
| Final R cryst | 0.164 (0.2534) |
| Final R free | 0.186 (0.2450) |
| No. of non-H atoms | |
| Protein | 8664 |
| Buffer components | 32 |
| Solvent | 942 |
| Total | 9638 |
| R.m.s. deviations | |
| Bonds (Å) | 0.011 |
| Angles (°) | 1.080 |
| Average B factors (Å2) | |
| Protein | 32.2 |
| Buffer components | 40.3 |
| Ramachandran plot | |
| Most favoured (%) | 98.7 |
| Allowed (%) | 0.9 |
3. Results and discussion
3.1. Cloning and production of TvTPS and analysis of catalytic activity
Two distinct TvTPS nucleotide sequences each of 1788 bp in length, corresponding to 596 amino acids (542 amino acids in the recombinant forms), were cloned from cDNA generated from T. vulgaris leaf-extract mRNA and sequenced. Interestingly, the translated proteins (presumably isoforms, termed TvTPS1 and TvTPS2) differed at only two amino-acid positions, resulting in Asp356/Thr565 for TvTPS1 and Gly356/Ala565 for TvTPS2. The nucleotide sequences were submitted to GenBank with accession IDs ALB78115.1 for TvTPS1 and JQ957864.1 for TvTPS2. Recombinant His6-tagged TvTPS1 and TvTPS2 as well as the single-amino-acid exchange variants TvTPS1-D356G and TvTPS1-T565A were overexpressed as soluble proteins in Escherichia coli, followed by purification by Ni2+ metal-chelate affinity chromatography (IMAC) and size-exclusion chromatography. The purity and identity of His6-tagged TvTPS was assessed by SDS–PAGE and Western blotting (Fig. 2 ▸). The activity of the purified TvTPSs was analysed by GC-MS and showed enzymatic conversion of the substrate GPP to γ-terpinene by TvTPS1 (Fig. 4 ▸). The identity of the product was confirmed by a GC-MS reference analysis of pure γ-terpinene. Surprisingly, TvTPS2 showed no conversion of the substrate GPP (data not shown). As TvTPS1 and TvTPS2 differ at only two positions (residues 356 and 565), the single-amino-acid exchange variants TvTPS1-D356G and TvTPS1-T565A were generated and purified to assess the individual influence of the amino-acid exchanges on TPS activity.
Figure 4.
GC-MS analysis of the TPS activity in (a) single ion-monitoring mode and (b) total ion-monitoring mode. Thymol was used as an internal standard (Rf = 14.674); γ-terpinene (Rf = 9.568) was identified as the product of GPP conversion catalyzed by TvTPS1.
While mutation of TvTPS1 at position 565 (TvTPS1-T565A) resulted in a decrease in activity to 18% of that of the wild-type TvTPS1, the enzymatic activity of TvTPS1-D356A was completely abolished (data not shown).
3.2. Crystal structure of TvTPS1
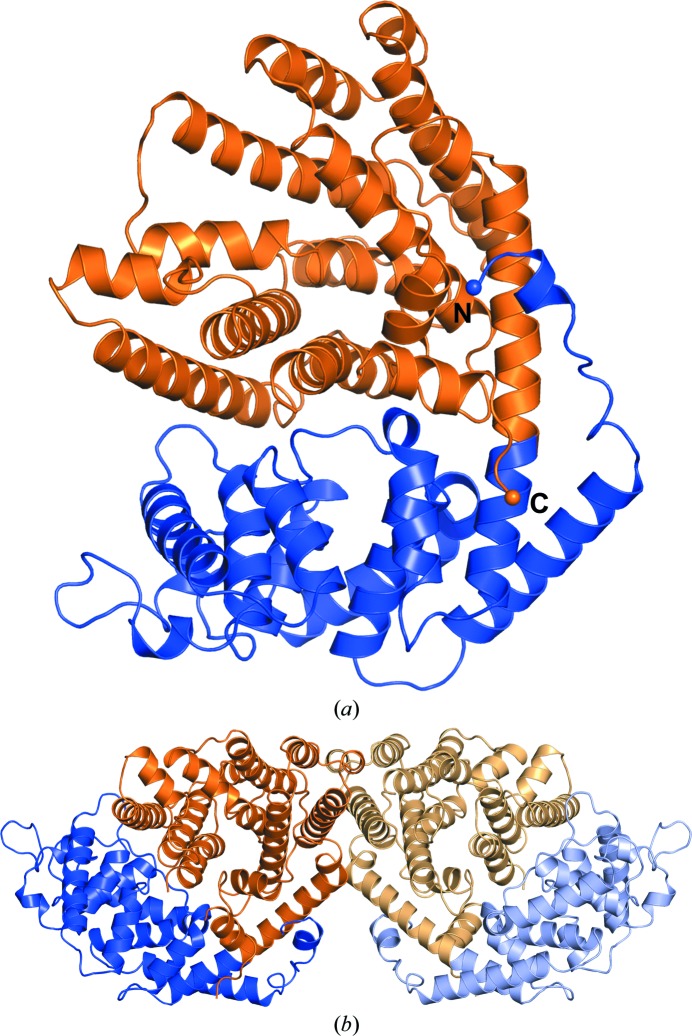
The structure of TvTPS1 determined from a triclinic crystal (Fig. 3 ▸) revealed two molecules (monomers A and B) in the asymmetric unit (Fig. 5 ▸). For both monomers the refined model starts at residue Val65 and includes residues up to the C-terminal residue Val596, with the exception of two unresolved regions spanning Gln499–Val507 and Gly573–Ser581 in monomer A and Gly573–Ser581 in monomer B owing to poor electron density. The structures of monomers A and B are highly similar (apart from residues Gln499–Val507, which are only present in monomer B) and superimpose with an r.m.s.d. of 0.344 Å (based on structural alignment of 513 Cα atoms). Both monomers share a common molecular interface, burying a total surface area of 2229 Å2 as calculated with the PISA software (Krissinel & Henrick, 2007 ▸). This suggests a TvTPS1 dimer as the biologically relevant oligomeric state, confirming previous indications of a dimeric state from native PAGE analysis (data not shown). Presumably, the visibility of residues Gln499–Val507 in the structural model of monomer B can be explained by additional crystal contacts formed by these residues to a symmetry-related molecule, which was not observed for monomer A.
Figure 5.
Overall structure of TvTPS1, a two-domain protein. (a) Overall structure of a TvTPS1 monomer (chain B) with the N-terminal domain coloured blue and the C-terminal domain coloured orange. The N- and C-termini are depicted as spheres and labelled. (b) Dimer of TvTPS1 as observed in the asymmetric unit of the triclinic crystal with molecule A shown in lighter colours and molecule B in darker colours using the same domain-colouring scheme as in (a).
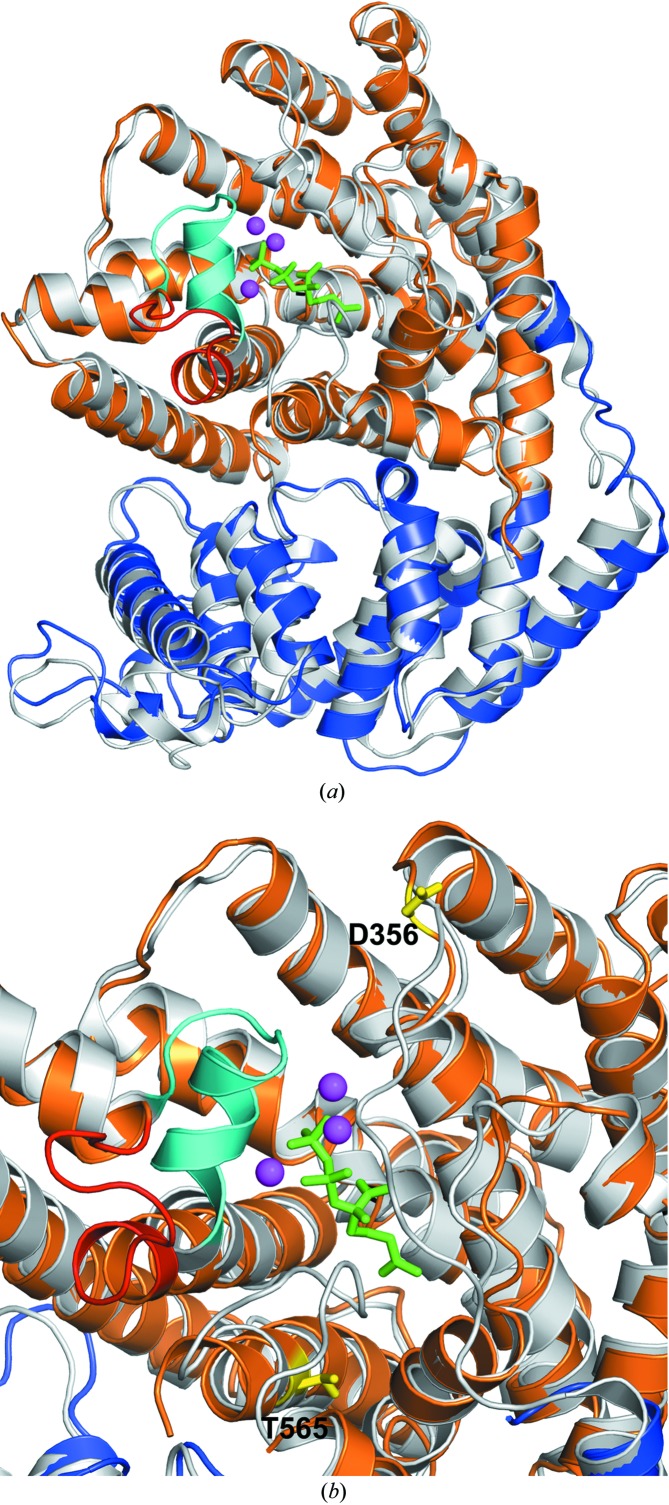
The overall structure of each TvTPS1 monomer comprises two α-helical domains: an N-terminal domain comprising an ‘α/α barrel’ and a C-terminal ‘orthogonal bundle’ domain. This architecture is shared by several other enzymes involved in plant terpenoid (or isoprenoid) synthesis, as identified by a search for structurally related proteins using the DALI server (Holm & Rosenström, 2010 ▸) with the structure of TvTPS1 chain B as a search template. The four closest structural homologues (ranked by the DALI Z-score) were (+)-bornyl diphosphate synthase from Salvia officinalis (Whittington et al., 2002 ▸; r.m.s.d. 1.5 Å), 1,8-cineole synthase from S. fruticosa (Kampranis et al., 2007 ▸; r.m.s.d. 1.5 Å), (4S)-limonene synthase from M. spicata (Hyatt et al., 2007 ▸; r.m.s.d. 1.5 Å) and isoprene synthase from Populus canescens (Köksal et al., 2010 ▸; r.m.s.d. 2.1 Å). Based on these related structures, of which some were elucidated in complex with substrates (or substrate analogues), the location of the putative active site of TvTPS1 could be assigned (Fig. 6 ▸ a). Interestingly, the disordered regions in the structure of TvTPS (residues Gly573–Ser581 and Gln499–Val507, resolved only in monomer B) are located in close proximity to the putative active site of TvTPS1. Remarkably, the loop region Gln499–Val507, which is visible in monomer B, shows a significantly different conformation to the homologous region in (4S)-limonene synthase, with the latter being elucidated in complex with the substrate analogue 2-fluorogeranyl disphosphate (Fig. 6 ▸ b). While the conformation of this loop observed in TvTPS (in the absence of any substrate) could represent an ‘open conformation’ in which the active site is accessible to the substrate, it cannot be excluded at this point that it is merely a consequence of the crystal-packing interactions observed in this region fixing an otherwise flexible loop region. In fact, the latter assumption is supported by the crystal structures of ligand-free (+)-bornyl diphosphate synthase and 1,8-cineole synthase, in which the corresponding residues were not resolved in the electron density, presumably owing to conformational flexibility of this loop in the ligand-free state. When a ligand is bound, the loop adopts a well ordered ‘closed conformation’, for example as seen in the substrate analogue-bound crystal structure of (4S)-limonene synthase and that of (+)-bornyl diphosphate synthase from S. officinalis bound to (+)-bornyl diphosphate. Formation of this structure upon substrate binding is also observed for the second unresolved loop region near the active site (corresponding to TvTPS residues Gly573–Ser581), for example as seen in the substrate analogue-bound structure of (4S)-limonene synthase (Hyatt et al., 2007 ▸). In this study, pronounced effects of amino-acid exchanges on TPS activity were observed. As for the enzymatically inactive TvTPS2 (differing from TvTPS1 only at positions 356 and 565) a lack of and a significantly lower TPS activity was observed for the derived single-amino-acid exchange variants TvTPS1-D356G and TvTPS1-T565A, respectively. Even with the structure of TvTPS1 to hand, it remains very difficult to explain the observed loss of activity, as the respective residues are not in very close proximity to the putative active site (Fig. 6 ▸). Nevertheless, mutations at these positions could influence enzymatic activity, substrate binding or even the substrate specificity of the enzyme by indirect effects, possibly by altering interactions with neighbouring residues, which in turn form part of the substrate-binding site and catalytic centre (Kampranis et al., 2007 ▸). To date, the question is still open as to how this class of enzymes (TPSs) accomplishes the formation of a simple and/or complex pattern of terpenoid metabolites with different functions. To understand the differences in the biosynthesis catalysed by TPSs from different plants, site-directed mutagenesis of the enzymes will enable us to modulate or direct pathways for an optimized metabolite composition.
Figure 6.
Structural superposition of TvTPS (monomer B; N-terminal domain coloured blue, C-terminal domain coloured orange) with the crystal structure of ligand-bound (4S)-limonene synthase (coloured light grey; PDB entry 2ong, monomer A). (a) The structural alignment reveals the putative active centre of TvTPS, which is located near the binding site of the substrate analogue 2-fluorogeranyl diphosphate (shown in green sticks), and three coordinated Mn2+ ions (magenta spheres) bound to (4S)-limonene synthase. In proximity, a loop (coloured in cyan) covers the active site of ligand-bound (4S)-limonene synthase. In ligand-free TvTPS1 this loop shows a different conformation (coloured red). (b) Close-up view of the active-site regions of TvTPS1 and (4S)-limonene synthase. In addition, the side chains of residues Asp356 and Thr565, which were mutated in this study, are shown as yellow sticks and labelled.
Supplementary Material
PDB reference: TvTPS1, 5c05
Acknowledgments
We are grateful to Bionorica SE (Neumarkt, Germany) for financial support to KR. We thank Guillaume Pompidor from the EMBL Outstation Hamburg for help with data collection on beamline P13 of the PETRA III synchrotron ring at DESY. The European Community’s Seventh Framework Programme (FP7/2007–2013) under BioStruct-X (grant agreement No. 283570) supported synchrotron data collection. No conflicts of interest are declared. This article is dedicated to Professor Dr Benno Parthier.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Bauer, P., Rudolph, K., Müller-Uri, F. & Kreis, W. (2012). Phytochemistry, 77, 53–59. [DOI] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Crocoll, C., Asbach, J., Novak, J., Gershenzon, J. & Degenhardt, J. (2010). Plant Mol. Biol. 73, 587–603. [DOI] [PubMed]
- Duke, S. O. (1991). Handbook of Natural Toxins, Vol. 6, edited by R. F. Keeler & A. T. Tu, pp. 269–296. New York: Marcel Dekker.
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Galata, M., Sarker, L. S. & Mahmoud, S. S. (2014). Phytochemistry, 102, 64–73. [DOI] [PubMed]
- Haudenschild, C. D., Schalk, M., Karp, F. & Croteau, R. (2000). Arch. Biochem. Biophys. 379, 127–136. [DOI] [PubMed]
- Herl, V., Fischer, G., Reva, V. A., Stiebritz, M., Muller, Y. A., Müller-Uri, F. & Kreis, W. (2009). Biochimie, 91, 517–525. [DOI] [PubMed]
- Holm, L. & Rosenström, P. (2010). Nucleic Acids Res. 38, W545–W549. [DOI] [PMC free article] [PubMed]
- Hyatt, D. C., Youn, B., Zhao, Y., Santhamma, B., Coates, R. M., Croteau, R. B. & Kang, C. (2007). Proc. Natl Acad. Sci. USA, 104, 5360–5365. [DOI] [PMC free article] [PubMed]
- Ito, M. & Honda, G. (2007). Phytochemistry, 68, 446–453. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kampranis, S. C., Ioannidis, D., Purvis, A., Mahrez, W., Ninga, E., Katerelos, N. A., Anssour, S., Dunwell, J. M., Degenhardt, J., Makris, A. M., Goodenough, P. W. & Johnson, C. B. (2007). Plant Cell, 19, 1994–2005. [DOI] [PMC free article] [PubMed]
- Kang, D., Gho, Y. S., Suh, M. & Kand, C. (2002). Bull. Korean Chem. Soc. 23, 1511–1512.
- Keszei, A., Brubaker, C. L. & Foley, W. J. (2008). Aust. J. Bot. 56, 197–213.
- Köksal, M., Zimmer, I., Schnitzler, J. P. & Christianson, D. W. (2010). J. Mol. Biol. 402, 363–373. [DOI] [PMC free article] [PubMed]
- Köllner, T. G., Schnee, C., Gershenzon, J. & Degenhardt, J. (2004). Plant Cell, 16, 1115–1131. [DOI] [PMC free article] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed]
- Lane, A., Boecklemann, A., Woronuk, G. N., Sarker, L. & Mahmoud, S. S. (2010). Planta, 231, 835–845. [DOI] [PubMed]
- Lima, A. S., Schimmel, J., Lukas, B., Novak, J., Barroso, J. B., Figueiredo, A. C., Pedro, L. G., Degenhardt, J. & Trindade, H. (2013). Planta, 238, 191–204. [DOI] [PubMed]
- Lima, A. S., Lukas, B., Novak, J., Figueiredo, A. C., Pedro, L. G., Barroso, J. G. & Trindade, H. (2011). Planta Medica, 77, PI9.
- Lima, A. S., Trindade, H., Figueiredo, A. C., Barroso, J. B. & Pedro, L. G. (2010). Acta Hortic. 860, 81–85.
- Lukas, B., Samuel, R. & Novak, J. (2010). Isr. J. Plant Sci. 58, 211–220.
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Mendes, M. D., Barroso, J. G., Oliveira, M. M. & Trindade, H. (2014). J. Plant Physiol. 171, 1017–1027. [DOI] [PubMed]
- Morales, R. (2002). Thyme: The Genus Thymus, edited by E. Stahl-Biskup & F. Sáez, pp. 1–43. London: Taylor & Francis.
- Muñoz-Bertomeu, J., Ros, R., Arrillaga, I. & Segura, J. (2008). Metab. Eng. 10, 166–177. [DOI] [PubMed]
- Poulose, A. J. & Croteau, R. (1978). Arch. Biochem. Biophys. 191, 400–411. [DOI] [PubMed]
- Rudolph, K., Bauer, P., Schmid, B., Mueller-Uri, F. & Kreis, W. (2014). Biochimie, 101, 31–38. [DOI] [PubMed]
- Schmiderer, C., Grausgruber-Gröger, S., Grassi, P., Steinborn, R. & Novak, J. (2010). J. Plant Physiol. 167, 779–786. [DOI] [PubMed]
- Sefidkon, F., Kalvandi, R., Atri, M. & Barazandeh, M. M. (2005). Flavour Fragr. J. 20, 521–524.
- Turner, G. W. & Croteau, R. (2004). Plant Physiol. 136, 4215–4227. [DOI] [PMC free article] [PubMed]
- Weitzel, C. & Simonsen, H. T. (2015). Phytochem. Rev. 14, 7–24.
- Whittington, D. A., Wise, M. L., Urbansky, M., Coates, R. M., Croteau, R. B. & Christianson, D. W. (2002). Proc. Natl Acad. Sci. USA, 99, 15375–15380. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: TvTPS1, 5c05