Abstract
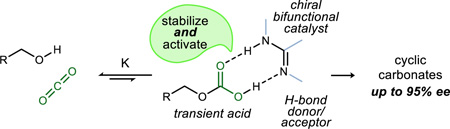
Carbon dioxide exhibits many of the qualities of an ideal reagent – it is nontoxic, plentiful, and inexpensive. Unlike other gaseous reagents, however, it has found limited use in enantioselective synthesis. Moreover, unprecedented is a tool that merges one of the simplest biological approaches to catalysis – Brønsted acid/base activation – with this abundant reagent. We describe a metal-free small molecule catalyst that achieves the three component reaction between a homoallylic alcohol, carbon dioxide, and an electrophilic source of iodine. Cyclic carbonates are formed enantioselectively.
Graphical Abstract
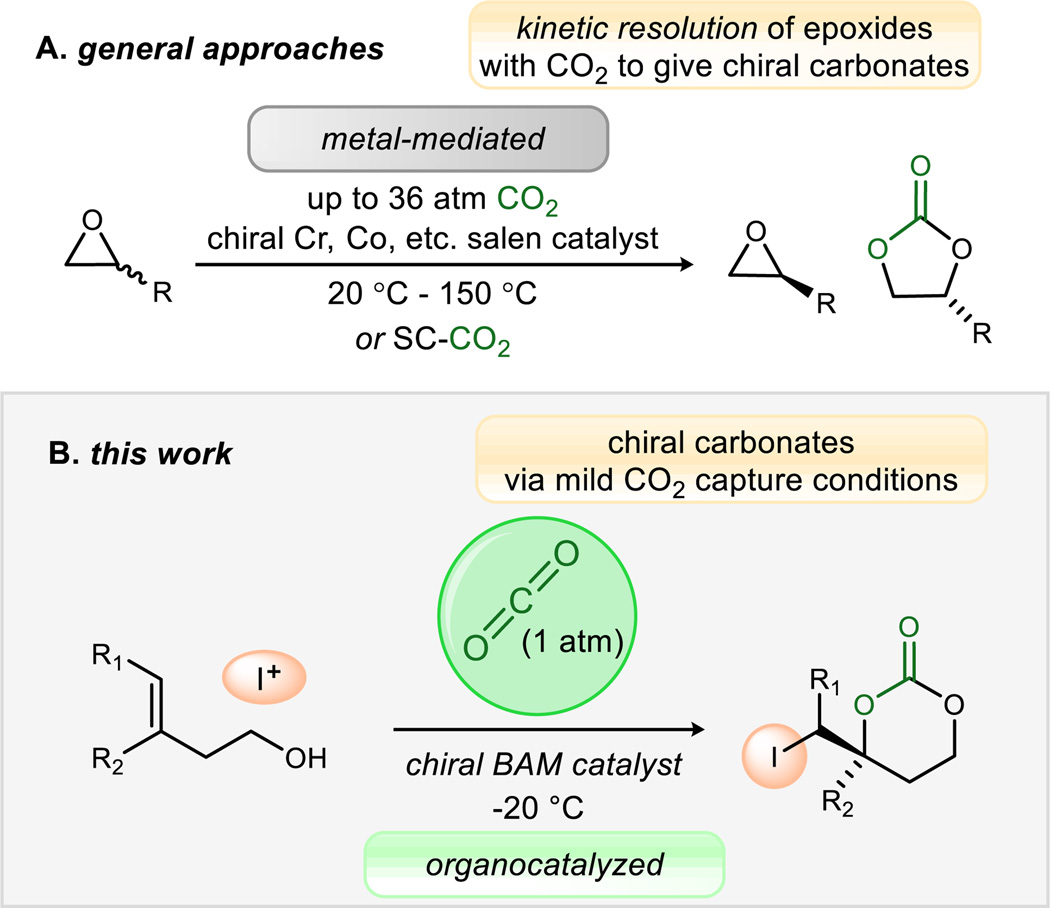
The current global economic and environmental landscape has accelerated research into carbon dioxide (CO2) capture and storage (CCS) technology across a broad range of chemical disciplines. The most notable advancements have been made in the areas of materials chemistry,1 carbon storage engineering, and alkylamine–based “scrubbing” systems.2 The threat of car-bon dioxide accumulation as a greenhouse gas has motivated these sequestration strategies, but this gaseous reagent holds immense potential value as an abundant and nontoxic C1–building block3 for carbon-carbon bond formation and carbon-heteroatom functionalization reactions in chemical synthesis.4,5 Unfortunately, the underlying features that contribute to car-bon dioxide’s low general toxicity and ease of handling render it relatively inert as a chemical reactant.6 This is punctuated by the contrasting abundance of enantioselective chemical reactions using hydrogen (H2),7 oxygen (O2),8 and even carbon monoxide (CO).9 Chemical technologies that preferentially form one handedness (enantiomer) of a chiral product have direct application to drug development and new materials. Despite the virtues of high temperature and/or pressure to address poor reactivity, transformations employing CO2 as a reagent10 are typically limited to either Lewis basic substrates with sufficient nucleophilicity to react with the poorly electrophilic CO2,3 or metal-based reagents to increase the rate of CO2 incorporation, often through a metal carboxylate intermediate (Figure 1A).11,12 We sought a reagent, ideally a catalyst, that could both over-come these barriers to reactivity and/or unfavorable equilibria while simultaneously controlling stereoselection – in essence, a catalyst that could stabilize a substrate-CO2 adduct, but still activate this adduct toward subsequent carbon-oxygen bond formation. Unprecedented is the use of a metal-free catalyst to stabilize the adduct of a weak nucleophile with carbon dioxide, such as a carbonic acid-base complex, while effectively guiding it toward enantioselective carbon-oxygen bond formation. Metal-based systems include CO2 insertion into activated epoxides generating almost exclusively 5-membered cyclic carbonates, including enantioselective kinetic resolutions (Figure 1A).13 Additionally, Yamada has reported a silver(I)-based alcohol desymmetrization using carbon dioxide at high pressure to pre-pare five-membered cyclic carbonates.14
Figure 1.
Enantioselective methods using CO2 as a reagent, contrasting state-of-the-art metal-mediated reactions with this report of Brønsted acid/base catalysis.
We posited that a properly balanced Brønsted acid-Brønsted base bifunctional catalyst might lower the barrier to CO2 incorporation and/or assist in the stabilization of the resulting adduct15 as a prelude to its use as an oxygen nucleophile in a subsequent enantioselective carbon-oxygen bond-forming step.16 If this could be achieved using a metal-free catalyst – an organocatalyst – the virtues of minimalism (symmetrical catalyst, low temperature, atmospheric pressure, near-neutral pH) would apply, suggesting broad impact. In this report, we validate this design by the development of a carboxylation/alkene function-alization reaction of homoallylic alcohols to produce chiral cyclic carbonates.
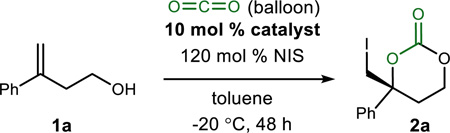
Homoallylic alcohol 1a became the basis for developing a tandem alcohol carboxylation–alkene iodocarbonation reaction due to the lower steric demand of a 1,1-disubstituted alkene. The standard reaction to which others are compared involved chilling (−20 °C) a toluene solution of homoallylic alcohol (1a, 0.4 M) prior to addition of N-iodosuccinimide (Table 1, entry 1) and carbon dioxide (balloon). These catalyst-free conditions returned starting material following a 48 h reaction period. Compared to an otherwise identical reaction, addition of a strong base (sodium hydride, Table 1, entry 2) delivered the desired cyclic carbonate, but in only 16% yield.17 Substitution of a more polar solvent (e.g. THF) for toluene increased the yield marginally (Table 1, entry 3). Based on precedence for Brønsted basic amines to react directly with carbon dioxide, or CO2/H2O combined, several amine bases were examined (Table 1, entries 4–5), as well as hydrogen bond-donor/acceptor amines (e.g. TBD, Table 1, entry 6) in an attempt to accelerate the desired reaction.18, 19 These extensive attempts generally provided three outcomes: 1) return of unreacted homoallylic alcohol, 2) formation of apparent iodoetherification products, or simply 3) low yields (<15%) of the desired carbonate. Similarly, good hydrogen bond-donors, such as TFA or thiourea 320 (Table 1, entries 7–8) failed to deliver any significant amount of carbonate.
Table 1.
Development of an Enantioselective CO2-Capture Reaction using a Homoallylic Alcohol
 | |||||
|---|---|---|---|---|---|
| entry | ligand (base) | acida | notesb | yieldc | ee (%)d |
| 1 | none | – | – | 0% | – |
| 2 | none | – | NaHe | 16% | – |
| 3 | none | – | NaH (THF)e | 25% | – |
| 4 | DMAP (5)f | – | – | trace | – |
| 5 | DBU (6)f | – | – | trace | – |
| 6 | TBD (4)f | – | – | 11% | – |
| 7 | TFAf | – | – | 6% | – |
| 8 | 3f | – | – | 4% | – |
| 9 | PBAM (7) | – | – | 18% | 39 |
| 10 | StilbPBAM (8) | – | – | 33% | 36 |
| 11 | 8 | – | MS | 35% | 60 |
| 12 | 8 | HOTf | MS | 62% | 74 |
| 13 | 8 | H2NTf | MS | 52% | 62 |
| 14 | 8 | F6C3(SO2)2NH | MS | 70% | 86 |
| 15 | 8g | HNTf2 | MS, 0.4 M | 95% | 91 |
| 16 | 8 | HNTf2 | MS, 0.2 M | 51% | 89 |
| 17 | 8 | HNTf2 | MS, 0.1 M | 10% | 79 |
| 18 | 5f | – | – | trace | – |
| 19 | 4f | – | MS | 30% | – |
| 20 | 6f | – | MS | 13% | – |
| 21 | 6f | ½ HNTf2 | MS | 4% | – |
Catalyst prepared as the 1:1 acid salt, except entry 21.
MS denotes molecular sieves 4A, employed at a concentration of 1 g/mmol relative to the alcohol.
Isolated yield.
Enantiomeric excess (ee) determined by HPLC using a chiral stationary phase. Reactions are 0.4 M toluene unless otherwise noted.
Reaction temp = 0 °C. 3-Methyl-3-buten-1-ol was converted in 60% yield under identical conditions.
20 mol % catalyst employed.
Our ultimate goal was to explore the ability of a Brønsted ac-id/base combination to promote the reaction. Use of pyrroli-dine-substituted bis(amidine) 7 (‘PBAM’) resulted in an 18% yield of 2a, however the carbonate was formed in a promising 39% ee (Table 1, entry 9) at −20 °C. The analogous catalyst incorporating trans-stilbene diamine (‘StilbPBAM’ (8)) instead of trans-cyclohexane diamine provided the product in 33% yield and similar ee (36% ee, Table 1, entry 10). It was noted in these early experiments that the addition of molecular sieves (MS 4A) resulted in more consistent reactions as judged by conversion and/or yield (Table 1, entry 11). In reactions with-out sieves, formation of a precipitate appeared to correlate with lower yields, and varying enantioselectivity (particularly with free base (7 or 8; vide infra). Exploration of strong Brønsted acid additives (1:1 ligand:acid) led to moderate differences in enantioselection (Table 1, entries 12–15), with catalyst complex 8·HNTf2 providing product with 91% ee (Table 1, entry 15). Some sensitivity of both yield and selectivity to concentration was noted,21 with lower concentrations leading to depressed yield and selectivity (Table 1, entries 16–17). We reinvestigated several achiral amine bases under these optimized conditions, with only marginal improvement in yield (Table 1, entries 18–21). Attempts were also made to simulate the Brønsted acid/base character of catalyst 8·HNTf2 using monobasic amines in combination with varying amounts of Brønsted acid (e.g. Table 1, entry 21), none resulting in significant improvement of yield. Collectively, these results suggest an underlying order to the hydrogen bonding network in the key selectivity-determining step, if not unique reactivity associated with the proper positioning of a Brønsted acid and base in the same molecule, in this carbon dioxide–fixating reaction.
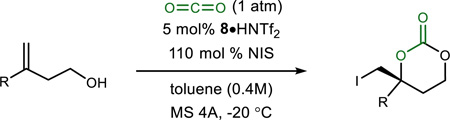
Application of conditions optimized for homoallylic alcohol 1a to a range of similar substrates is outlined in Table 2. α-Substituted styrene derivatives were scrutinized using the mild conditions developed (2a–m, Table 2). Nominal substitution of the aromatic ring led to equally positive outcomes, with 2a–2c formed in 91–95% ee, and high chemical yield (82–96% yield) (Table 2, entries 1, 3–5). Substitution near the alkene was not tolerated, as no substrate conversion was observed to produce 2d or 2e (Table 2, entries 6–7). Increasing the reaction temperature to 0 °C led to complex mixtures suggestive of competing intermolecular iodoetherification. However, a β-naphthyl-substituted alkene led to good enantioselection and yield (2f, 90% ee, 88% yield) (Table 2, entry 8). Anisole derivatives (meta-and para-substituted) provided generally good enantioselectivity (80–90% ee) and higher chemical yield (97%, 2h), but lower yield for 2g (Table 2, entries 9–10). Halogen-substituted arenes led to a range of results, mostly related to reactivity; while selectivity remained high, some reached only partial conversion de-spite extended reaction times. Halogen substitution meta and para to the alkene provided consistently good enantioselection (87–90% ee) (Table 2, entries 11–14). Reactivity varied greatly among 2i–2l, however, and suggested that the alkene nucleophilicity might be a key determinant of reactivity. Carbonate 2m was produced in nearly quantitative yield and 91% ee (Table 2, entry 15).
Table 2.
Initial Scope of an Enantioselective CO2-Capture Reaction using a Homoallylic Alcohola
 | |||||
|---|---|---|---|---|---|
| entry | R | product | time (h) | ee (%) | yield (%) |
| 1 | C6H5 | 2a | 48 | 91 | 95 |
| 2b | C6H5 | 2a | 48 | 89 | 79 |
| 3 | pMeC6H4 | 2b | 48 | 91 | 96 |
| 4 | pMeC6H4 | 2b | 72d | 95 | 82 |
| 5 | mMeC6H4 | 2c | 48 | 93 | 96 |
| 6 | oMeC6H4 | 2d | >96 | - | - |
| 7 | 1Np | 2e | >96 | - | - |
| 8 | 2Np | 2f | 48 | 90 | 88 |
| 9 | pMeOC6H4 | 2g | 48 | 80 | 26e |
| 10 | mMeOC6H4 | 2h | 48 | 90 | 97 |
| 11 | pBrC6H4 | 2i | 48 | 90 | 65 |
| 12c | mClC6H4 | 2j | 5 d | 87 | 44 |
| 13c | mFC6H4 | 2k | 96 | 89 | 40 |
| 14 | pFC6H4 | 2l | 48 | 90 | 54 |
| 15 | p((CH3)3C)C6H4 | 2m | 48 | 91 | 99 |
| 16 | PhCH2CH2 | 2n | 72 | 67 | 71 |
| 17 | Me | 2o | 48 | 68 | 72 |
| 18c | Cy | 2p | 48 | 74 | 76 |
Enantiomeric excess (ee) determined by HPLC using a chiral stationary phase. Reactions are 0.4 M in toluene. Isolated yields are listed. See SI for complete experimental details. Absolute configuration for 2a assigned using X-ray analysis, remaining examples assigned by analogy.
6.8 mmol (1.0 g) substrate was employed under the optimized conditions (1 atm CO2) utilizing 3.0 mol % catalyst for 48 h.
10 mol % catalyst loading.
Reaction temperature was −50 °C.
It was noted that purified 2g was prone to decomposition.
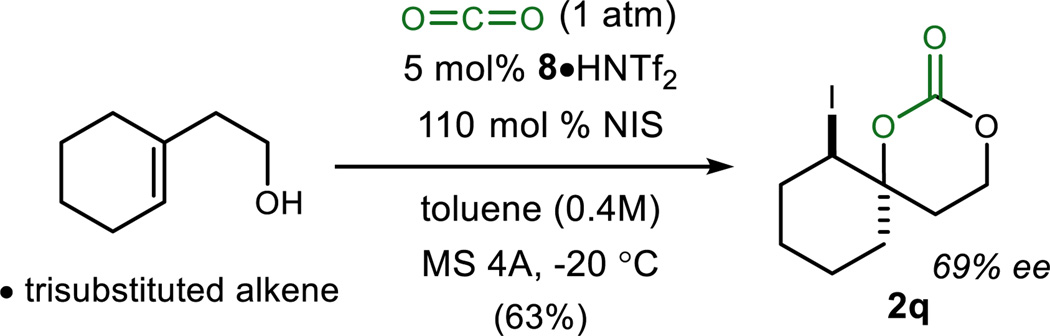
Alkenes bearing aliphatic substituents are often regarded as challenging substrates in stereoselective difunctionalization reactions.22 3-Alkyl butenols were prepared and converted to carbonates 2n–2p with promising levels of enantioselectivity (up to 74% ee) (Table 2, entries 16–18). Iodocarbonate 2o, derived from the sterically–unencumbered 3-methyl-but-3-ene-1-ol, a widely available isoprenyl feedstock, formed in a moderate 68% ee. Although not a focus of these investigations, allylic alcohols reacted sluggishly but exhibited good yield and lower enantioselection. In an effort to probe the adaptation of this method to larger amounts, a gram-scale experiment using 3 mol % catalyst led to the carbonate in 89% ee and 79% yield (Table 2, entry 2). Finally, spirocyclic carbonate 2q was prepared from the corresponding trisubstituted alkene in moderate yield (63%) and encouraging enantioselection (69% ee) (Scheme 1).
Scheme 1.
Iodocarbonation of a Trisubstituted Alkene.
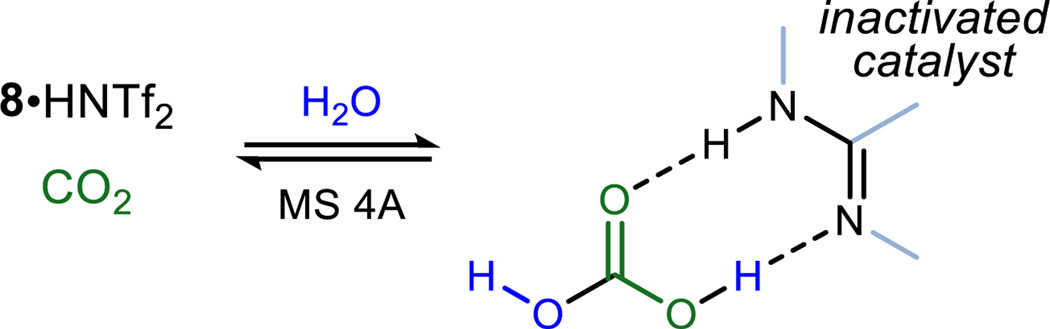
Several experimental observations are worth mention in ad-dition to the trends summarized above. Foremost among these, use of a carbon dioxide balloon attached to a degassed reaction established that the steady-state carbon dioxide concentration is significant in chilled toluene and could be reached within 40 minutes – far shorter than the time to complete conversion to carbonate (monitored by in situ IR). The rate of CO2 absorption was affected insignificantly by most every factor examined, including temperature, the presence of MS 4A, and stirring rate. The correlation between high chemical yield and molecular sieves can be explained by the formation of a complex between the catalyst, adventitious water, and carbon dioxide, which we hypothesize to be the carbonic acid salt (Figure 2)23. This complex precipitates from the reaction mixture when using 8·HNTf2, but its formation appears reversible, reverting to active catalyst when a dessicant (MS 4A) and dry gas (argon) are added. Although a non-covalent complex is hypothesized in our work, some nucleophilic amines can form a covalent adduct with CO2.24 When not in competition with water, the alcohol substrate can entrain carbon dioxide, forming an intermediate and transient alkyl carbonic acid salt with the bifunctional catalyst. This intermediate, in reaction with NIS, forms a complex which then collapses to the cyclic carbonate either stepwise or directly.25
Figure 2.
Catalyst Inactivation Pathway and its Recovery Using MS 4A
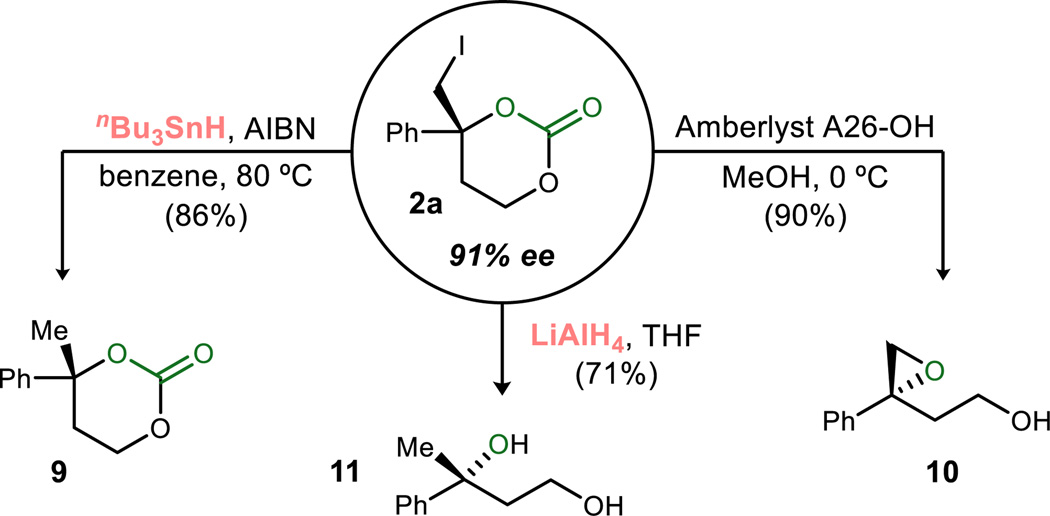
While cyclic carbonates are valuable in their own right,26 and they are prepared here using carbon dioxide as a phosgene surrogate, Scheme 2 details several notable transformations. First, reduction by stannane provided carbonate 9 in 86% yield when applied to iodocarbonate 2a. Straightforward carbonate hydrolysis with a basic resin in methanol led to the versatile epoxide 10. This is particularly significant from the viewpoint that carbon dioxide is effectively used as an equivalent to epoxidation, which normally requires an electrophilic source of oxygen (e.g. a peracid or dioxirane). Full reduction employing a stronger reducing agent (LiAlH4) leads to tertiary alcohol 11 in 71% yield. This carbon dioxide fixation method therefore offers a simple two step equivalent to metal-free oxidations of homoallylic alcohols,27 for which carbon dioxide is converted to either dialkyl carbonate or methanol.
Scheme 2.
Conversions of Carbonate Products (Conservation of Enantiomeric Excess (ee) Observed in All Cases)
In summary, a mild and operationally straightforward carbon dioxide fixation reaction has been developed using a dual Brønsted acid/base catalyst that presents hydrogen bond-donor and acceptor functionality to activate and orient substrates in an enantioselective reaction. This metal-free method employs relatively weak nucleophiles (homoallylic alcohols) in the CO2 fixation step, generating transient acids that add to an alkene in combination with N-iodosuccinimide. The catalysts deployed here use the virtues of Brønsted acid/base activation alone to achieve highly enantioselective carbonate synthesis.28 From a different viewpoint, this carbon dioxide fixation method circumvents approaches dependent on phosgene29 as a source of carbonate protecting group for 1,3-diol prepared through stereoselective synthesis. Numerous enantioenriched small molecules might be prepared using carbon dioxide as a source for carbon-oxygen bond formation.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIH GM 084333). WWW was supported by an HHMI Fellowship (Kalamazoo College). We are grateful to Dr. Yasunori Toda and Dr. Roozbeh Yousefi for the preparation of several alkene substrates and insightful conversations, and to Dr. Maren Pink (Indiana University Molecular Structure Center) for X-ray analysis.
Footnotes
Experimental procedures and spectroscopic data for all new compounds, and X-ray data (cif) for 2a. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Li J-R, Kuppler RJ, Zhou H-C. Chem. Soc. Rev. 2009;38:1477. doi: 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]; Ramdin M, de Loos TW, Vlugt TJH. Ind. Eng. Chem. Res. 2012;51:8149. [Google Scholar]; Gassensmith JJ, Furukawa H, Smaldone RA, Forgan RS, Botros YY, Yaghi OM, Stoddart JF. J. Am. Chem. Soc. 2011;133:15312. doi: 10.1021/ja206525x. [DOI] [PubMed] [Google Scholar]
- 2.Rochelle GT. Science. 2009;325:1652. doi: 10.1126/science.1176731. [DOI] [PubMed] [Google Scholar]
- 3. Sakakura T, Choi J-C, Yasuda H. Chem. Rev. 2007;107:2365. doi: 10.1021/cr068357u. Additional uses of CO2 as a feedstock: Lindsey AS, Jeskey H. Chem. Rev. 1957;57:583. Greenhalgh MD, Thomas SP. J. Am. Chem. Soc. 2012;134:11900. doi: 10.1021/ja3045053. Luo J, Preciado S, Larrosa I. J. Am. Chem. Soc. 2014;136:4109. doi: 10.1021/ja500457s. Liu Q, Wu L, Jackstell R, Beller M. Nat. Commun. 2015;6 doi: 10.1038/ncomms6933. Reduction of CO2 for commodity-based chemical production: Studt F, Sharafutdinov I, Abild-Pedersen F, Elkjær CF, Hummelshøj JS, Dahl S, Chorkendorff I, Nørskov JK. Nature Chem. 2014;6:320. doi: 10.1038/nchem.1873. Graciani J, Mudiyanselage K, Xu F, Baber AE, Evans J, Senanayake SD, Stacchiola DJ, Liu P, Hrbek J, Sanz JF, Rodriguez JA. Science. 2014;345:546. doi: 10.1126/science.1253057. For recent advances adapting CO2 in continuous flow synthesis: Kozak JA, Wu J, Su X, Simeon F, Hatton TA, Jamison TF. J. Am. Chem. Soc. 2013;135:18497. doi: 10.1021/ja4079094. Wu J, Kozak JA, Simeon F, Hatton TA, Jamison TF. Chem. Sci. 2014;5:1227.
- 4.Tsuji Y, Fujihara T. Chem. Commun. 2012;48:9956. doi: 10.1039/c2cc33848c. [DOI] [PubMed] [Google Scholar]
- 5.Cokoja M, Bruckmeier C, Rieger B, Herrmann WA, Kühn FE. Angew. Chem. Int. Ed. 2011;50:8510. doi: 10.1002/anie.201102010. [DOI] [PubMed] [Google Scholar]
- 6.Omae I. Coord. Chem. Rev. 2012;256:1384. [Google Scholar]
- 7.Cui X, Burgess K. Chem. Rev. 2005;105:3272. doi: 10.1021/cr0500131. [DOI] [PubMed] [Google Scholar]
- 8. Punniyamurthy T, Velusamy S, Iqbal J. Chem. Rev. 2005;105:2329. doi: 10.1021/cr050523v. For a metal-free example, see: Yang Y, Moinodeen F, Chin W, Ma T, Jiang Z, Tan C-H. Org. Lett. 2012;14:4762. doi: 10.1021/ol302030v.
- 9. Nozaki K, Sato N, Takaya H. J. Am. Chem. Soc. 1995;117:9911. Brookhart M, Wagner MI, Balavoine GGA, Haddou HA. J. Am. Chem. Soc. 1994;116:3641. Reviews: Tietze LF, Ila H, Bell HP. Chem. Rev. 2004;104:3453. doi: 10.1021/cr030700x. Bianchini C, Meli A. Coord. Chem. Rev. 2002;225:35.
- 10.Kielland N, Whiteoak CJ, Kleij AW. Adv. Synth. Catal. 2013;355:2115. [Google Scholar]
- 11.Decortes A, Castilla AM, Kleij AW. Angew. Chem. Int. Ed. 2010;49:9822. doi: 10.1002/anie.201002087. [DOI] [PubMed] [Google Scholar]
- 12.Maeda C, Miyazaki Y, Ema T. Catal. Sci. Technol. 2014;4:1482. [Google Scholar]
- 13.Paddock RL, Nguyen ST. J. Am. Chem. Soc. 2001;123:11498. doi: 10.1021/ja0164677. [DOI] [PubMed] [Google Scholar]; Lu X-B, Liang B, Zhang Y-J, Tian Y-Z, Wang Y-M, Bai C-X, Wang H, Zhang R. J. Am. Chem. Soc. 2004;126:3732. doi: 10.1021/ja049734s. [DOI] [PubMed] [Google Scholar]; Berkessel A, Brandenburg M. Org. Lett. 2006;8:4401. doi: 10.1021/ol061501d. [DOI] [PubMed] [Google Scholar]; Luinstra GA, Haas GR, Molnar F, Bernhart V, Eberhardt R, Rieger B. Chem. Eur. J. 2005;11:6298. doi: 10.1002/chem.200500356. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Fukui K, Kikuchi S, Yamada T. J. Am. Chem. Soc. 2010;132:4072. doi: 10.1021/ja1007118. [DOI] [PubMed] [Google Scholar]
- 15.Direct spectroscopic identification of an alkyl carbonic acid is limited by an equilibrium that generally favors free CO2 and the corresponding organic alcohol: Gassensmith JJ, Furukawa H, Smaldone RA, Forgan RS, Botros YY, Yaghi OM, Stoddart JF. J. Am. Chem. Soc. 2011;133:15312. doi: 10.1021/ja206525x. Formation of alkyl organic carbonates is possible under supercritical CO2 conditions: West KN, Wheeler C, McCarney JP, Griffith KN, Bush D, Liotta CL, Eckert CA. J. Phys. Chem. A. 2001;105:3947.
- 16.Selected references: Dobish MC, Johnston JN. J. Am. Chem. Soc. 2012;134:6068. doi: 10.1021/ja301858r. Davis TA, Wilt JC, Johnston JN. J. Am. Chem. Soc. 2010;132:2880. doi: 10.1021/ja908814h. Toda Y, Pink M, Johnston JN. J. Am. Chem. Soc. 2014;136:14734. doi: 10.1021/ja5088584. Denmark SE, Kuester WE, Burk MT. Angew. Chem. Int. Ed. 2012;51:10938. doi: 10.1002/anie.201204347. Whitehead DC, Yousefi R, Jaganathan A, Borhan B. J. Am. Chem. Soc. 2010;132:3298. doi: 10.1021/ja100502f. Tan CK, Zhou L, Yeung Y-Y. Synlett. 2011;2011:1335. Paull DH, Fang C, Donald JR, Pansick AD, Martin SF. J. Am. Chem. Soc. 2012;134:11128. doi: 10.1021/ja305117m. Cheng YA, Yu WZ, Yeung Y-Y. Org. Biomol. Chem. 2014;12:2333. doi: 10.1039/c3ob42335b.
- 17.This particular substrate (3-phenyl-3-butenol) has not been reported with NaH in THF ,but the analogous 3-methyl-3-butenol was used under similar conditions: Bongini A, Cardillo G, Orena M, Porzi G, Sandri S. J. Org. Chem. 1982;47:4626.
- 18.Das Neves Gomes C, Jacquet O, Villiers C, Thuéry P, Ephritikhine M, Cantat T. Angew. Chem. Int. Ed. 2012;51:187. doi: 10.1002/anie.201105516. [DOI] [PubMed] [Google Scholar]; Ma J, Zhang X, Zhao N, Al-Arifi ASN, Aouak T, Al-Othman ZA, Xiao F, Wei W, Sun Y. J. Mol. Catal. A. Chem. 2010;315:76. [Google Scholar]
- 19.Hypoiodous acid: Minakata S, Sasaki I, Ide T. Angew. Chem. Int. Ed. 2010;49:1309. doi: 10.1002/anie.200906352.
- 20.Okino T, Hoashi Y, Takemoto Y. J. Am. Chem. Soc. 2003;125:12672. doi: 10.1021/ja036972z. [DOI] [PubMed] [Google Scholar]
- 21.Interestingly, the electrophile NIS is sparingly soluble under the optimized concentration in toluene (0.4 M), suggesting possible phasetransfer-like activation of NIS by the soluble catalyst, as seen by Jacobsen: Brindle CS, Yeung CS, Jacobsen EN. Chem. Sci. 2013;4:2100. doi: 10.1039/C3SC50410G.
- 22.Sakakura A, Ukai A, Ishihara K. Nature. 2007;445:900. doi: 10.1038/nature05553. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh A-AG, Sivaram S. Chem. Rev. 1996;96:951. doi: 10.1021/cr950067i. [DOI] [PubMed] [Google Scholar]
- 24.Villiers C, Dognon J-P, Pollet R, Thuéry P, Ephritikhine M. Angew. Chem. Int. Ed. 2010;49:3465. doi: 10.1002/anie.201001035. [DOI] [PubMed] [Google Scholar]; Heldebrant DJ, Jessop PG, Thomas CA, Eckert CA, Liotta CL. J. Org. Chem. 2005;70:5335. doi: 10.1021/jo0503759. [DOI] [PubMed] [Google Scholar]
- 25. Denmark SE, Burk MT, Hoover AJ. J. Am. Chem. Soc. 2010;132:1232. doi: 10.1021/ja909965h. Müller CH, Rösner C, Hennecke U. Chem. Asian J. 2014;9:2162. doi: 10.1002/asia.201402229. Wu J, Wang YM, Drljevic A, Rauniyar V, Phipps RJ, Toste FD. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13729. doi: 10.1073/pnas.1304346110. Yousefi R, Ashtekar KD, Whitehead DC, Jackson JE, Borhan B. J. Am. Chem. Soc. 2013;135:14524. doi: 10.1021/ja4072145.
- 26.For recent advances in carbonate utilization: Chung G-C, Kim H-J, Jun S-H, Kim M-H. Electrochem. Commun. 1999;1:493. Sanders DP, Fukushima K, Coady DJ, Nelson A, Fujiwara M, Yasumoto M, Hedrick JL. J. Am. Chem. Soc. 2010;132:14724. doi: 10.1021/ja105332k. Edward JA, Kiesewetter MK, Kim H, Flanagan JCA, Hedrick JL, Waymouth RM. Biomacromolecules. 2012;13:2483. doi: 10.1021/bm300718b.
- 27.1,1-Disubstituted olefins have generally been challenging for asymmetric epoxidation: Wang Z-X, Shi Y. J. Org. Chem. 1997;62:8622. doi: 10.1021/jo962392r. Xia QH, Ge HQ, Ye CP, Liu ZM, Su KX. Chem. Rev. 2005;105:1603. doi: 10.1021/cr0406458. Wang B, Wong OA, Zhao M-X, Shi Y. J. Org. Chem. 2008;73:9539. doi: 10.1021/jo801576k.
- 28.It is worth noting the magnesium-dependent enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO). RuBisCO incorporates CO2 through carbon-carbon bond formation into glucose precursors. Berry JA, Lorimer GH, Pierce J, Seemann JR, Meek J, Freas S. Proc. Natl. Acad. Sci. U. S. A. 1987;84:734. doi: 10.1073/pnas.84.3.734. Stec B. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18785. doi: 10.1073/pnas.1210754109.
- 29.McGhee W, Riley D, Christ K, Pan Y, Parnas B. J. Org. Chem. 1995;60:2820. [Google Scholar]; Fukuoka S, Kawamura M, Komiya K, Tojo M, Hachiya H, Hasegawa K, Aminaka M, Okamoto H, Fukawa I, Konno S. Green Chem. 2003;5:497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.