Abstract
Spot welding is used in the automotive and aircraft industries, where high-speed, repetitive welding is needed to join thin sections of metal. Epoxy adhesives are applied as sealers to the metal seams. Pulmonary function abnormalities and airway irritation have been reported in spot welders, but no animal toxicology studies exist. Therefore, the goal of this study was to investigate vascular, immune and lung toxicity measures after exposure to these metal fumes in an animal model. Male Sprague-Dawley rats were exposed by inhalation to 25 mg/m3 to either mild-steel spot welding aerosols with sparking (high metal, HM) or without sparking (low metal, LM) for 4 h/d for 3, 8 and 13 d. Shams were exposed to filtered air. Bronchoalveolar lavage (BAL), lung gene expression and ex vivo BAL cell challenge were performed to assess lung toxicity. Lung resistance (RL) was evaluated before and after challenge with inhaled methacholine (MCh). Functional assessment of the vascular endothelium in isolated rat tail arteries and leukocyte differentiation in the spleen and lymph nodes via flow cytometry was also done. Immediately after exposure, baseline RL was significantly elevated in the LM spot welding aerosols, but returned to control level by 24 h postexposure. Airway reactivity to MCh was unaffected. Lung inflammation and cytotoxicity were mild and transient. Lung epithelial permeability was significantly increased after 3 and 8 d, but not after 13 d of exposure to the HM aerosol. HM aerosols also caused vascular endothelial dysfunction and increased CD4+, CD8+ and B cells in the spleen. Only LM aerosols caused increased IL-6 and MCP-1 levels compared with sham after ex vivo LPS stimulation in BAL macrophages. Acute inhalation of mild-steel spot welding fumes at occupationally relevant concentrations may act as an irritant as evidenced by the increased RL and result in endothelial dysfunction, but otherwise had minor effects on the lung.
Keywords: Cardiovascular, inhalation studies, particulate matter, volatile organic compounds, welding
Introduction
Resistance spot welding is a process that joins contacting surfaces of thin sheets of metal by the heat obtained from resistance to electric current (Stout, 1987). Metal work pieces are held together under pressure exerted by two copper alloy electrodes that concentrate a large current into a small spot, generating temperatures high enough to melt the metal and form a weld. Spot welding is most commonly used in the automotive, aircraft and appliance manufacturing industries. During the process, epoxy adhesives are often applied as sealers between the sheets of metal to be welded. Thus, there is an occupational hazard risk for exposure to complex aerosols composed of metal oxide particulates, volatile organic compounds (VOCs) and other gas vapors.
A Health Hazard Evaluation performed by National Institute for Occupational Safety and Health (NIOSH) investigators at an automotive assembly plant found evidence of respiratory illness in body shop workers (Kanwal & Boylstein, 2006). Some chemicals associated with adhesives used in the body shop were detected in the air of the plant. Several of the substances (e.g. methyl methacrylate, acetic acid, phthalic anhydride, formaldehyde and styrene) are classified asthma-gens, potentially causing respiratory irritation, asthma, bronchitis and sinusitis. A cross-sectional study conducted in 2000–2001 in an automotive assembly plant found welders had increased rates of allergy symptoms and cough compared with assembly workers (Hammond et al., 2005). In a cohort of automotive manufacturing workers, Luo et al. (2006) observed evidence of restrictive and obstructive lung abnormalities among spot welders. In addition, a dose–response relationship for airway irritation symptoms (e.g. cough, phlegm production and chronic bronchitis) was seen in spot welders but not unexposed control subjects. In agreement, a survey of workers in an Iranian automotive assembly factory indicated decreased pulmonary function and increased respiratory symptoms (e.g. increased sputum and dyspnea) among spot welders at exposures within American Conference of Governmental Industrial Hygienists limit values (Loukzadeh et al., 2009). Unfortunately, these studies failed to identify which byproduct(s) of the spot welding process was responsible for the respiratory effects observed.
We examined the effects of mild-steel resistance spot welding fumes on lung injury, inflammation and function as well as the effects on the vascular, immune and central nervous systems using the newly developed automated, computer-controlled spot welding aerosol generator and inhalation exposure system (Afshari et al., 2014). The neurotoxicity effects are described in a companion report (Sriram et al., 2014).
Methods
Animals
Male Sprague-Dawley [Hla:(SD) CVF] rats from Hilltop Lab Animals (Scottdale, PA), weighing 200–250 g and free of viral pathogens, parasites, mycoplasmas, Helicobacter and CAR bacillus, were used for all exposures. The rats were acclimated for one week after arrival and were housed in ventilated polycarbonate cages on Diamond Dry cellulose chips and hardwood Sani-chips as bedding, and provided HEPA-filtered air, Harlan 2918 irradiated Teklad Global 18% rodent chow (Harlan Laboratories, Inc., Madison, WI), and tap water ad libitum. The animal facilities are specific pathogen-free, environmentally controlled and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal procedures used during the study were done under a protocol approved by the National Institute for Occupational Safety and Health (NIOSH) Animal Care and Use Committee.
Mild-steel resistance spot welding animal exposure
Animals were exposed by whole-body inhalation using the resistance spot welder and inhalation exposure system described in the submitted companion paper (Afshari et al., 2014). In brief, two rolls that contained strips of low alloy, carbon steel sheet metal were directed by a set of rollers to copper (class I)-tipped electrodes of the welder and spot welded at a determined distance of 20 mm between each spot weld by an automated, computer-controlled resistance spot welding gun [Small new modified “C” style Trans-gun 136 kva-AC (Milco Manufacturing Company, Warren, MI)]. For most of experiments, the spot welder was set at 7.5–10 kA with a welding time of 140 ms, a post-weld holding time of 50 ms and a clamping force of 2.6 KN (600 lbs). The time between welds and amount of dilution air entering the exposure chamber was gradually increased or decreased automatically through software to maintain a constant exposure chamber aerosol concentration. In all cases, an adhesive [Tekoral 2300 (Henkel Surface Technology, Madison Heights, MI)] was injected between the two strips of metal before spot welding.
Aerosols generated during welding were transported via tubing from the spot welding fume chamber to an animal exposure chamber. The temperature and relative humidity [Vaisala Temperature-Humidity Probe, model# HMP233 (Woburn, MA)] inside the exposure chamber were measured and continuously recorded. Animals were exposed to one of the two types of spot welding aerosols. Low metal (LM) aerosols were generated when the weld current was set to 7.5–9.0 kA. This lower current setting still produced quality welds, but without the generation of sparking or the expulsion of molten metal. Fewer particles were generated per weld when operating in this mode. The average time between weld was ~5 s to achieve the target exposure concentration of 25 mg/m3. High metal (HM) aerosols were generated when sparking occurred at an average current of 10.5 kA and the average time between each spot weld in this mode was 20 s. Animals were exposed to either LM or HM spot welding aerosols at a target concentration of 25 mg/m3 for 4 h/d for 3, 8 and 13 d. These concentrations represent a dose between those of our previous metal inert gas (MIG) welding fume inhalation exposures, which were 15 and 40 mg/m3 (Antonini et al., 2007, 2009). Table 1 provides actual aerosol exposure concentrations during animal exposures. Shams were exposed to filtered air.
Table 1.
Actual spot welding aerosol exposure concentrations.
| Exposure condition | Fume concentration (mg/m3)a |
|---|---|
| Low metal | 3 d: 19.7 ± 0.9 |
| 8 d: 27.7 ± 6.8 | |
| 13 d: 26.9 ± 4.7 | |
| High metal | 3 d: 32.0 ± 5.0 |
| 8 d: 23.9 ± 2.1 | |
| 13 d: 21.8 ± 2.2 |
Mean fume concentration in exposure chamber ± standard error; measurements were made in duplicate every 30 min during the daily 4 h exposure. Target fume concentration was 25 mg/m3.
To maintain spot welding fume concentrations in the animal exposure chamber, fume was collected through an aerosol delivery line above the welding system at a flow rate of 25 L/min by maintaining a slight negative pressure within the exposure chamber as described in Afshari et al. (2014). The aerosol delivery line mated with dilution air and was allowed to mix before entering the exposure chamber. Dilution airflow rate was adjusted by a 0–20 L/min mass flow controller. The exposure system control software would automatically make adjustments to the dilution air and the time between welds to provide a desired mass concentration (25 mg/m3) in the exposure chamber. The mass concentration in the chamber was monitored in real time by a real time aerosol monitor (DataRAM, MIE, Inc. DR-2000, Bedford, MA). Shams were housed in an air-tight animal chamber that was located in close proximity to the spot welding exposure chamber and received conditioned, filtered air. The control chamber had the same dimensions and was made from identical materials as the spot welding fume animal exposure chamber. The supplied air originated from a water-seal compressor, in-house air-line and was conditioned through a dryer, charcoal filter and HEPA filter.
Mild-steel resistance spot welding aerosol characterization
The physical and chemical characteristics of the generated spot welding aerosols are described in greater detail in Afshari et al. (2014). A brief description of the aerosol characterization results for the LM and HM spot welding aerosols follows and is reported in Table 2.
Table 2.
Resistance spot welding fume characteristics.
| Low metala | High metala | |
|---|---|---|
| Particle morphologyb: | ||
| Micron-sized mode | Spherical particles | Spherical particles |
| Submicron-sized mode | Chain-like aggregates | Chain-like aggregates |
| Nano-sized/ultrafine mode | Chain-like aggregates | Chain-like aggregates |
| Particle size, MMADc: | ||
| Micron-sized mode | 1.66 μm (67% of particle mass) | 3.04 μm (61% of particle mass) |
| Submicron-sized mode | 0.30 μm (33% of particle mass) | 0.25 μm (36% of particle mass) |
| Nano-sized/ultrafine mode | 0.01–0.05 μm (~1% of particle mass) | 0.01–0.1 μm (~3% of particle mass) |
| Particle diameter | 0.18–1.8 μm (less particles) | 0.18–1.8 μm (more particles) |
| Elements (Wt%)d: | ||
| Fe | 99.0% | 99.2% |
| Mn | 0.534% | 0.539% |
| Cu | 0.431% | 0.280% |
| VOCse, Major peaks | Benzene | Benzene |
| Toluene | Toluene | |
| Isopropanol | Isopropanol | |
| 2-butoxyethanol | 2-butoxyethanol | |
| VOCs, Minor peaks | Butanol | Butanol |
| Ethylene glycol | Ethylene glycol |
Resistance spot welding parameters were to set to generate aerosols that contained either low (no sparking) or high (sparking) metal particulates.
Particle morphology was determined from SEM photomicrographs of aerosols and examined under a Hitachi S4800 field emission scanning electron microscope and a Bruker X-ray system.
Particle size distribution was determined in the exposure chamber by using Micro-Orifice Uniform Deposit Impactor (MOUDI) and Nano-MOUDI. Three modes of particle sizes were measured. The mass median aerodynamic diameter (MMAD) was determined for each particle size mode.
The particle samples were digested and the metals analyzed by inductively coupled plasma-atomic emission spectroscopy.
During spot welding, gas samples were collected in thermal desorption tubes for qualitative analysis of volatile organic compounds (VOCs) by thermal desorption gas chromatography/mass spectrometry.
To assess particle morphology, fume was collected during spot welding onto 47-mm Nuclepore polycarbonate filters (Whatman, Clinton, PA), mounted onto aluminum stubs with silver paste and viewed using a Hitachi S4800 field emission scanning electron microscope (FESEM) (Hitachi High Technologies America, Inc., Schaumburg, IL) and X-ray system (Bruker, Madison, WI). Independent of spot welding parameter settings, two distinct particle morphologies for each type of spot welding aerosol generated were observed: a reddish-brown metal particle that predominated in the smaller (nano-mode) particle size fractions and a darker, more black-colored particle that was observed in the larger (micron/submicron modes) particle size fractions. Substantially more particles deposited on each of the filters for the different size fractions when spot welder parameters were set to generate a significant amount of sparking and high amounts of metal (HM) as compared with spot welding at parameters that generated little to no sparking (LM).
The size distribution of the spot welding aerosols inside the exposure chamber was determined by using a Micro-Orifice Uniform Deposit Impactor (MOUDI, MSP Model 110, MSP Corporation, Shoreview, MN) and a Nano-MOUDI (MSP Model 115). Three modes of particle sizes were measured when spot welding parameters were to set to generate aerosols that contained either high (HM) or low (LM) levels of metal particles. The mass median aerodynamic diameter (MMAD) was determined for each particle size mode. For the LM group, the MMAD was 1.66 μm for the micron-sized mode and accounted for 67% of the particle mass, 0.30 μm for the submicron-sized mode and accounted for 33% of the particle mass and ~0.01–0.05 μm for the nano-sized mode and accounted for <1% of the particle mass. For the HM group, the MMAD was 3.04 μm for the micron-sized mode and accounted for 61% of the particle mass, 0.25 μm for the submicron-sized mode and accounted for 36% of the particle mass and ~0.01–0.1 μm for the nano-sized mode and accounted for <3% of the particle mass.
To determine elemental composition of the LM and HM samples, aerosols were collected in the breathing zone of the animals inside the exposure chamber onto 5 μm polyvinyl chloride membrane filters. The particle samples were digested and the metals analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) by Bureau Veritas North America, Inc. (Novi, MI), according to NIOSH method 7303 (NIOSH, 1994). The generated particles were composed of 99% of iron, regardless of the spot welding parameter settings, with <1% of the composition being manganese and copper.
During the spot welding process, gas samples were collected on thermal desorption tubes in the animal exposure chamber for qualitative identification of VOCs by thermal desorption gas chromatography/mass spectrometry (TD-GC–MS). Independent of spot welding parameter settings, most major peaks present were siloxanes and silicon-containing compounds, benzene, toluene, isopropanol and 2-butoxyethanol.
Respiratory mechanics and reactivity to methacholine after 8 d of exposure
At 0 and 24 h after 8 d (4 h/d) of spot welding aerosol exposure, random sets of rats were anesthetized (80 mg/kg ketamine, 10 mg/kg xylazine; ip), instrumented and placed in plethysmographs for measurement of lung resistance (RL) and dynamic compliance (Cdyn) (Buxco Electronics, Inc., Wilmington, NC). Rats were ventilated: maximum mouth pressure, 40 cm H2O; maximum tidal volume, 3 ml; respiratory rate, 90 bpm. After recording baseline values of RL and Cdyn delivery of saline vehicle as a control, a range of increasing methacholine (MCh) aerosol concentrations (0.10–30 mg/ml) were delivered at 5 min intervals to generate dose–response curves. Maximum RL and minimum Cdyn values were logged at 5 s intervals.
Partial lung bronchoalveolar lavage after 3, 8 and 13 d of exposure
At 24 h after 3, 8 and 13 d (4 h/d) of spot welding aerosol exposure, separate sets of rats were deeply anesthetized with an overdose of Sleepaway (26% sodium pentobarbital, 7.8% isopropyl alcohol and 20.7% propylene glycol; ip) (Fort Dodge Animal Health, Fort Dodge, IA). Blood samples were collected via the vena cava, then the abdominal aorta was cut to exsanguinate the rat. The left bronchus was clamped and bronchoalveolar lavage (BAL) was done on the right lung lobes with Ca2+ and Mg2+-free phosphate buffered saline (PBS). The right lung was lavaged with 4 ml of PBS, kept separate on ice, followed by four washes (5 ml/wash). Both lavage fractions were then centrifuged (500g, 10 min, 4 °C) and the acellular supernatant of the first lavage used for evaluation of lung toxicity (below). Finally, the cell pellets of the first and subsequent washes were combined then suspended in 1 ml PBS for cell counts and differentials.
Assessment of lung toxicity
Harvested BAL cells were counted using a Coulter Multisizer II and AccuComp software (Beckman Coulter Inc., Hialeah, FL) and a hemocytometer. Cytospin slides for BAL cell differentials were prepared as described previously (Zeidler-Erdely et al., 2011). A minimum of 300 cells were identified and counted under light microscopy.
Alterations in alveolar–capillary barrier permeability and cytotoxicity in the lung were assessed by measuring albumin levels and lactate dehydrogenase (LDH) activity, respectively, in the acellular BAL fluid. Measurements were performed with a COBAS c111 analyzer (Roche Diagnostic Systems, Indianapolis, IN) as previously described (Zeidler-Erdely et al., 2011).
Serum samples after 8 d of exposure and BAL samples after 8 and 13 d of exposure to both aerosol types were analyzed by Myriad RBM (Austin, TX) on the 58-biomarker Multi-Analyte Profile (MAP) for rodents or RodentMAP v3.0 (Myriad Rules Based Medicine, Austin, TX).
Relative lung mRNA expression after 8 d of exposure
Lung RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer's directions. Evaluation of gene expression was determined by custom designed TaqMan® array (Erdely et al., 2009) using the ABI 7900 (Applied Biosystems, Grand Island, NY). Samples were run according to the Applied Biosystems TaqMan® array user bulletin. For our samples, a total of 1 μg of total RNA converted to cDNA in a final volume of 200 μl (96 a format) was used. The reference gene contained within the array used for normalization was hypoxanthine-guanine phosphoribosyltransferase (HPRT). Relative mRNA expression was calculated using the comparative threshold method (2 –DDCt) with sham animals serving as the reference group.
Ex vivo stimulation of harvested BAL cells after 13 d of exposure
Harvested BAL cells were counted via a hemocytometer, plated at 5 × 105 cells/well onto 24 well plates and allowed to attach for 2 h at 37 °C in 1 ml Eagle's modified essential medium (Biowhittaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum, 1 mM glutamine, 100 U/ml penicillin/streptomycin and 10 mM HEPES at pH 7.2. Cells were then washed three times with 37 °C PBS to remove any non-adherent cells which resulted in a pure culture of lung macrophages. Lipopolysaccharide (LPS; 1 μg/ml) was then added to the cells in fresh media. Supernatants were harvested at 18 h for cytokine analysis. Cytokine protein concentrations were determined with enzyme-linked immunosorbent assay (ELISA) kits that were specifically used to identify rat interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α) according to the manufacturer's instructions (Invitrogen Corporation, Camarillo, CA). Absorbance was read at 450 nm with a Spectramax Plus 384 spectrophotometer using Softmax Pro 5.4.1 software (Molecular Devices Corp., Sunnyvale, CA).
Flow cytometry analysis after 13 d of exposure
Single-cell suspensions of lung-associated lymph nodes (LALN) were prepared by disruption of tissue between the frosted ends of microscopic slides and spleens were prepared by processing tissue through 70 μm nylon mesh screens in PBS. Cells were counted on a Coulter Counter Z1 following lysis of red blood cells with Zap-o-globin (Beckman Coulter Inc., Hialeah, FL). About 106 cells were resuspended in PBS containing 1% bovine serum albumin and 0.1% sodium azide (FACS Buffer). To prevent nonspecific binding to Fc receptors, the cells were incubated with anti-rat CD32 (clone D34-485, BD Biosciences, San Jose, CA) in FACS buffer for 10 min on ice. Cells were then centrifuged and resuspended in FACS buffer containing fluorescently conjugated monocolonal antibodies (BD Biosciences) including: anti-rat CD45RAPE, clone OX-33; CD3-FITC, clone G4.18; CD4-APC, clone OX-35 and CD8-PerCP, clone OX-8. The cells were protected from light and incubated on ice for 20 min. The cells were then washed and resuspended in BD Cytofix buffer (BD Biosciences) and incubated for 20 min on ice. Cells were then washed and resuspended in 0.3 ml of FACS buffer and analyzed on a LSR II flow cytometer (BD Biosciences). Cells were discriminated by forward and side light scatter and doublets excluded by side scatter area versus side scatter height. Lymphocyte populations were identified by their phenotype as follows: B cells (CD45A+ CD3negative), CD4 T cells (CD3+ CD4+) and CD8 T cells (CD3+ CD8+).
Endothelium function assessment after 3 d of exposure
Rat tail arteries were harvested 24 h after 3 d of LM or HM aerosol exposure and isolated in DMEM containing glucose. Endothelium-dependent vasodilation was assessed using Living Systems Instrumentation (St. Albans, VT). Arteries were pressurized to 60 mmHg for 1 h then constricted to 50% of initial diameter with phenylephrine. Following constriction, arteries were exposed to increasing concentrations of acetylcholine (ACh) and percent relaxation was calculated as [(DSS – DCon)/(DI – DCon)] × 100. DSS is the steady-state diameter during the experimental period taken as the average diameter of the final 2 min of the 5 min period. DI is the initial vessel diameter measured after the 1 h equilibration and DCon is the steady-state diameter measured over a 5 min period once 50% constriction had been obtained.
Statistics
The analysis of measures of percent relaxation was generated using SAS/STAT software, Version 9.1 (SAS Institute, Cary, NC). All other data were analyzed using a Student's t-test with significance set at p<0.05.
Results
Effects on respiratory mechanics and reactivity to MCh after 8 d of exposure to mild-steel spot welding aerosols
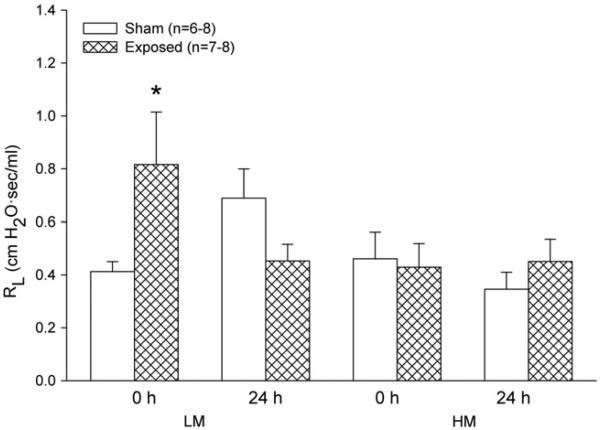
Immediately after the 8 d spot welding aerosol exposure, baseline RL was increased significantly in the group exposed to the LM, but not the HM, aerosols (Figure 1). At 24 h, however, there was no difference from sham in baseline RL with either group. No changes in Cdyn were found (data not shown). Airway reactivity to increasing concentrations of MCh was not affected at any time point after either aerosol exposure (data not shown).
Figure 1.
Effect of LM and HM resistance spot welding aerosols on baseline lung resistance (RL) in rats immediately (0 h) or 24 h after exposure to 25 mg/m3 for 4 h/d for 8 d. Values are mean ± standard error (n = 6–8 per group). Significance from sham 0 h at *p<0.05.
Lung injury and inflammation assessment of mild-steel spot welding aerosols
Numerous macrophages recovered by BAL from both the HM and LM groups at 24 h after 3 and 13 d of exposure were observed to contain phagocytized spot welding particles (Figure 2). As expected, significantly more metal particles were present in the macrophages recovered from the HM group compared with the LM group. No neutrophils were observed among the recovered BAL cells after exposure to either the HM or LM aerosols, indicating a lack of an overt inflammatory response. BAL total cell counts were unaffected and recovered cells consisted of >99% macrophages at 24 h after exposure to 3, 8 or 13 d of LM or HM resistance spot welding aerosols, which confirmed the lack of inflammatory cells observed in the cytospins (Figure 3, panel A). In addition, 3, 8 or 13 d of exposure to LM or HM aerosols had no effect on LDH activity (Figure 3, panel B). Alveolar epithelial barrier damage, measured as albumin, was significantly increased after 3 and 8 d of exposure to the HM aerosols only (Figure 3, panel C).
Figure 2.
Representative images of cytospin-stained lung BAL cells recovered at 24 h after 3 and 13 d of exposure to 25 mg/m3 for 4 h/d to LM (panels A and C) and HM (panels B and D) spot welding aerosols.
Figure 3.
Effect of LM and HM resistance spot welding aerosols on total cell counts (panel A), LDH (panel B) and albumin (panel C) measured in the BAL at 24 h after 3, 8 and 13 d of exposure to 25 mg/m3 for 4 h/d. Data are presented as percent of sham and values are mean ± standard error (n = 4–8 per group). Significance at *p<0.05.
After 8 d of exposure, both the LM and HM aerosol exposures significantly increased vascular endothelial growth factor A (VEGF-A) in the BAL at 24 h postexposure. LM and HM VEGF-A levels were increased 1.81 ± 0.19-fold (mean ± standard error) and 1.79 ± 0.27-fold compared with sham, respectively. Serum eotaxin levels were also significantly increased after exposure to the HM and LM spot welding aerosols. Levels were increased 1.32 ± 0.09 and 1.30 ± 0.10-fold compared with sham for the LM and HM groups, respectively. At 24 h after 13 d of exposure, no changes were found in the BAL fluid for the 58 biomarkers measured on the RodentMAP panel (data not shown).
Alterations in relative lung mRNA expression
Exposure to spot welding aerosols for 8 d resulted in few changes in lung inflammatory gene expression. In addition, no genes were altered greater than 2-fold, which confirmed an absence of an overt inflammatory response in the BAL. Tissue inhibitor of metalloproteinase 2 (Timp2) was significantly increased from 1.00 ± 0.08 to 1.40 ± 0.08 after LM aerosol exposure. HM aerosol exposure significantly increased relative mRNA expression of arginase 1 (Arg1), arginase 2 (Arg2) and chemokine (C-X-C motif) ligand 6 (Cxcl6) from 1.00 ± 0.01 to 1.77 ± 0.20, 1.00 ± 0.08 to 1.35 ± 0.08 and 1.00 ± 0.16 to 1.47 ± 0.10, respectively. No other genes in the LDA panel were significantly altered (data not shown).
Ex vivo LPS stimulation of lung macrophages after 13 d of exposure to mild-steel spot welding aerosols
Lung macrophages from LM spot welding aerosol exposure produced significantly greater levels of IL-6 and MCP-1 compared with sham in response to LPS stimulation ex vivo (Figure 4, panels A and B). In contrast, HM aerosol-exposed cells produced similar levels to sham of IL-6 and MCP-1 after secondary stimulation (Figure 4, panels C and D). TNF-α levels were not increased after LPS stimulation in either the LM or HM aerosol-exposed cells (data not shown).
Figure 4.
Effect of ex vivo stimulation by LPS on IL-6 and MCP-1 protein levels from lung macrophages harvested by BAL 24 h after 13 d of exposure to 25 mg/m3 for 4 h/d to LM (panels A and B) and HM (panels C and D) resistance spot welding aerosols. Values are mean ± standard error (n = 4 per group). Significance at *p<0.05 between LPS-stimulated and non-stimulated (i.e. baseline) groups. Significance at #p<0.05 between LPS-stimulated sham and LM groups.
Effects on splenocytes after 13 d of mild-steel spot welding aerosol exposure
No changes in lung-associated lymph node cell populations or total cell numbers were found after either exposure (data not shown). Only HM aerosol exposure increased the total numbers of splenocytes compared with sham (Figure 5, panel A). LM aerosol exposure increased the number of CD4+ T cells, while HM exposure increased B cells in the spleen (Figure 5, panels B and C).
Figure 5.
Effect of LM and HM resistance spot welding aerosols on total splenocyte number (panel A) and populations of CD4+, CD8+ and B cells (panels B and C) harvested from spleens 24 h after 13 d of exposure to 25 mg/m3 for 4 h/d. Values are mean ± standard error (n = 4 or 8 per group). Significant difference from sham at *p<0.05.
Effects on endothelium-dependent vasodilation
Pre-constricted rat tail arteries harvested from the HM group 24 h after a 3 d spot welding aerosol exposure showed reduced responsiveness to increasing concentrations of ACh compared with sham (Figure 6, panel A). No change in reactivity to ACh was observed in arteries recovered from the LM group at 24 h after spot welding aerosol exposure (Figure 6, panel B).
Figure 6.
Pre-constricted rat tail arteries after exposure to 25 mg/m3 for 4 h/d of HM (panel A) and LM (panel B) resistance spot welding aerosols to increasing concentrations of ACh. Values are mean ± standard error (n = 4 per group). Significance from sham at *p<0.05.
Discussion
Several studies have indicated that automotive assembly workers exposed to aerosols generated during resistance spot welding using adhesives develop respiratory abnormalities, such as airway irritation, reduced lung function and bronchitis (Hammond et al., 2005; Loukzadeh et al., 2009; Luo et al., 2006). A goal of this study was to determine the contribution of each component (metal fume versus VOCs) of generated spot welding aerosol in relation to a potential toxicological response in an animal model. By controlling various combinations of parameters (current and the time between spot welds) with the previously developed spot welding aerosol generator, it was possible to produce aerosols with different characteristics (Afshari et al., 2014). When parameters were selected for a significant amount of sparking, an aerosol with a substantially greater amount of metal fume was generated (HM sample) when compared with spot welding conditions with little to no sparking (LM sample). For all exposures, the application of adhesive to the metal strips liberated VOCs into the inhalation exposure system.
Similarities and differences were observed after examining the aerosol characteristics of the LM and HM samples (Afshari et al., 2014). Particle size distribution and morphology, elemental composition, and the VOCs profile were similar when comparing the LM and HM aerosols. Besides, the presence of more large spherical particles after the generation of the HM aerosol due to spatter caused by sparking, particles from both aerosols appeared as chain-like agglomerates formed by numerous ultrafine primary particles with a tri-modal size distribution. Regardless of the welding parameter settings, the metal composition of the LM and HM samples was 99% iron and siloxanes, silicon-containing compounds, benzene, toluene, isopropanol and 2-butoxyethanol were the primary VOCs present. However, the HM aerosols, generated with significant sparking, contained nearly four times more metal than the LM aerosols. Thus, the formed HM aerosols were composed of a significantly higher metal content compared with the LM aerosol that contained less metal particles but had a greater volatile component. As expected, significantly more metal particles were observed in the lung macrophages recovered by BAL from the HM group compared with the LM group after each of the exposure regimens (Figure 2).
Multiple parameters of lung function after acute inhalation exposure to different spot welding aerosols were examined in animals in this study. The HM and LM spot welding aerosols had little effect on lung function measurements in the exposed animals. Baseline RL was significantly elevated in the group exposed to the LM immediately after exposure, but not the HM, spot welding aerosols (Figure 1). The presence of a higher volatile component of the LM aerosol likely induced a pulmonary irritant effect during exposure that resolved after the animals were removed from the exposure. Although the studies are few, ambient VOCs have been associated with increased respiratory symptoms in asthmatic children (Delfino et al., 2003a,b). Indeed, the presence of benzene in the LM aerosol may have contributed to the irritant effect as significant associations between ambient benzene and hospital admissions for respiratory disease (Hagen et al., 2000), asthma exacerbations (Thompson et al., 2001) and childhood wheezing (Buchdahl et al., 2000) have been reported.
Due to the minimal effect of the spot welding aerosols on lung function, it was not surprising that both LM and HM spot welding exposures did not result in lung inflammation and/or cytotoxicity (Figure 3). Despite the relatively high aerosol concentration used in the study, no increase in lung inflammatory cell influx or death was evident in the BAL after either spot welding exposure. Changes in lung permeability did occur at 24 h post-exposure to 3 and 8 d of HM aerosol exposure. However, this effect was not evident at 24 h post-exposure to 13 d of HM exposure. This suggests an acute lung response that was not maintained with longer exposure periods. These results were not unexpected as mild-steel, MIG welding aerosols composed of >80% iron also have shown minimal changes in lung injury, inflammation and BAL levels of IL-6, MCP-1 and TNF-α in rodent inhalation studies at exposure levels of 40 mg/m3 (Antonini et al., 2009, 2011). In agreement with previous studies, the production of IL-6, MCP-1 and TNF-α after ex vivo LPS stimulation of BAL cells recovered from the animals exposed to the HM fume was not significantly different from sham. Interestingly, the ex vivo production of both IL-6 and MCP-1 were increased after stimulation of BAL cells harvested from the animals exposed to the LM aerosol, indicating that the VOC component of the aerosol may alter lung immune cell function and cytokine production (Figure 4). Airway inflammation and an increase in the production of pro-inflammatory cytokines have been shown after inhalation exposure to VOC mixtures in animal models (Bonisch et al., 2012; Wang et al., 2012).
Elevations in specific cytokines involved in systemic inflammation were observed in the animals exposed to the spot welding fumes. Serum eotaxin and BAL VEGF-A levels were increased significantly after exposure to the HM and LM spot welding aerosols. Increased eotaxin has been observed following gas metal arc-stainless steel and manual metal arc-stainless steel welding fume exposure (our unpublished observations). In addition, serum eotaxin increased with exposure to other insoluble particulates such as multi-walled carbon nanotubes (Erdely et al., 2009), indicating a mechanism secondary to the ongoing pulmonary response. The exact role of increased serum eotaxin following a pulmonary exposure is unknown, but elevated serum eotaxin levels have been associated with cardiomyopathy (Vistnes et al., 2010). Also, CCR3-dependent chemokines regulate migration of bone-marrow derived progenitor cells to ischemic myocardium (Bonaros et al., 2008), indicating a potential link to cardiovascular function. Indeed, an increased risk of adverse cardiovascular effects has been observed in populations of welders (Hilt et al., 1999; Ibfelt et al., 2010; Sjogren et al., 2002). VEGF-A promotes angiogenesis and is involved in new vessel formation, endothelial cell migration and activation, stem cell recruitment and tissue regeneration (Taimeh et al., 2013). VEGF has been observed to be a key mediator in airway remodeling and chronic inflammation in asthma patients (Meyer & Akdis, 2013). Stark et al. (2009) observed alterations in VEGF expression in welders with normal pulmonary function after short-term exposure to aluminum/iron welding fumes. Therefore, the mechanisms of action for increased serum eotaxin and VEGF-A following a pulmonary particulate exposure warrant further investigation.
It is well established that pulmonary particulate exposures are associated with an increased risk of cardiovascular dysfunction (Brook et al., 2010). Both animal and human studies have shown adverse cardiovascular effects following welding fume exposure (du Plessis et al., 2010; Erdely et al. 2011a,b; Fang et al., 2010; Kim et al., 2005). In this study, as expected, the HM exposure resulted in significant endothelial-dependent dysfunction (Figure 6). The dysfunction was present despite very limited indications of pulmonary inflammation, an occurrence that has been previously noted (Nurkiewicz et al., 2008). Interestingly, the LM exposure did not result in any adverse effects. Other than a vapor consisting of VOCs, the LM group had very little particulate exposure after 3 d of inhalation. Although the studies are few, VOCs from air pollution have been linked to cardiovascular dysfunction and mortality (Ma et al., 2010; Seilkop et al., 2012; Tsai et al., 2010; Vedal et al., 2013; Weichenthal et al., 2012). More specifically, Campen et al. (2005) showed that naïve vessels exposed directly to the VOC component of diesel exhaust displayed endothelial dysfunction. The present model examined vascular effects from excised conduit vessels following whole-body inhalation potentially accounting for the differing results. In addition, the VOCs identified in this exposure were directly related to the adhesive utilized and were not as diverse as compared with studies related to air pollution. Although the LM exposure did not result in measurable endothelial dysfunction in this study, the role of VOCs as a mixture including metal-rich particulates (e.g. HM exposure) enhancing cardiovascular dysfunction should not be excluded. Recent detailed studies evaluating the components of air pollution have concluded that a gas–PM interaction likely accounts for maximal adverse cardiovascular outcomes (Seilkop et al., 2012; Vedal et al., 2013).
Epidemiology and animal studies have indicated that inhalation of specific welding fumes can cause both local and systemic alterations in immune cell response (Zeidler-Erdely et al., 2012). Anderson et al. (2007) demonstrated that pharyngeal aspiration of a soluble stainless steel welding fume in a mouse immunotoxicity model reduced systemic and pulmonary humoral immune responses despite causing significant elevations in absolute lymph node cell numbers for B- and T-cells, including, CD4+ and CD8+ subsets. Conversely, repeated pulmonary treatment of rats with a welding fume that contained elevated levels of manganese caused a reduction in circulating total lymphocytes, primarily CD4+ and CD8+ T lymphocytes (Antonini et al., 2012). Inhalation of stainless steel and mild-steel welding fumes has been shown to suppress lung immune responses and reduce the pulmonary clearance of a bacterial pathogen in an animal infectivity model (Antonini et al., 2007, 2009). Exposure to the spot welding aerosols in this study also induced alterations in the immune cell response. Inhalation of the HM spot welding fume increased total splenocytes as well as B cells, whereas the LM aerosol exposure increased the number of CD4+ T cells in the spleen (Figure 5).
Conclusions
Acute exposure to an iron-rich spot welding aerosol generated in the presence of an adhesive had a transient irritant effect in the lungs, induced a mild local and systemic inflammatory effect as indicated by an increase in pro-inflammatory cytokine production, affected systemic immune cell response by altering the number and profile of specific spleen lymphocytes and caused vascular endothelial dysfunction. The pulmonary irritant effect seemed to be due to the liberation of VOCs from adhesives used during the spot welding process, whereas the effect on vascular endothelial function was due primarily to the metal particle component. A combination of metal particle and VOCs exposure likely contributed to the pro-inflammatory and systemic immune responses observed after inhalation of the different spot welding aerosols. Despite these toxicological responses, most of the observed effects were short term and minimal compared with what has been reported in some automotive assembly workers. The responses in this animal study may not have been as dramatic because of the elemental composition of the strips of metal (primarily iron; studies with galvanized metal are ongoing) that were welded together and the acute nature of the exposure used in this animal inhalation study compared with longer, more sustained exposures (e.g. days versus months/years) in workplaces where spot welding is continually occurring.
Acknowledgements
We thank Amy Cumpston, Jared L. Cumpston and H. Donny Leonard for their expert assistance with the animal inhalation exposures.
Footnotes
Declaration of interest
No competing financial interests are declared. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Afshari A, Zeidler-Erdely PC, McKinney W, et al. Development and characterization of a resistance spot welding aerosol generator and inhalation exposure system. Inhal Toxicol. 2014 doi: 10.3109/08958378.2014.941118. in press. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Meade BJ, Butterworth LF, Munson AE. The humoral immune response of mice exposed to manual metal arc stainless steel-welding fumes. J Immunotoxicol. 2007;4:15–23. doi: 10.1080/15476910601115119. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Stone S, Roberts JR, et al. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol Appl Pharmacol. 2007;223:234–45. doi: 10.1016/j.taap.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, et al. Short-term inhalation exposure to mild steel welding fume had no effect on lung inflammation and injury but did alter defense responses to bacteria in rats. Inhal Toxicol. 2009;21:182–92. doi: 10.1080/08958370802360661. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, et al. Persistence of deposited metals in the lungs after stainless steel and mild steel welding fume inhalation in rats. Arch Toxicol. 2011;85:487–98. doi: 10.1007/s00204-010-0601-1. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Zeidler-Erdely PC, Young SH, Roberts JR, Erdely A. Systemic immune cell response in rats after pulmonary exposure to manganese-containing particles collected from welding aerosols. J Immunotoxicol. 2012;9:184–92. doi: 10.3109/1547691X.2011.650733. [DOI] [PubMed] [Google Scholar]
- Bonaros N, Sondermeijer H, Schuster M, et al. Ccr3- and cxcr4-mediated interactions regulate migration of cd34+ human bone marrow progenitors to ischemic myocardium and subsequent tissue repair. J Thorac Cardiovasc Surg. 2008;136:1044–53. doi: 10.1016/j.jtcvs.2007.12.067. [DOI] [PubMed] [Google Scholar]
- Bonisch U, Bohme A, Kohajda T, et al. Volatile organic compounds enhance allergic airway inflammation in an experimental mouse model. PLoS One. 2012;7:e39817. doi: 10.1371/journal.pone.0039817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the american heart association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Buchdahl R, Willems CD, Vander M, Babiker A. Associations between ambient ozone, hydrocarbons, and childhood wheezy episodes: a prospective observational study in south east London. Occup Environ Med. 2000;57:86–93. doi: 10.1136/oem.57.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, Babu NS, Helms GA, et al. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from apoe−/− mice. Toxicol Sci. 2005;88:95–102. doi: 10.1093/toxsci/kfi283. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Gong H, Jr, Linn WS, Pellizzari ED, et al. Asthma symptoms in hispanic children and daily ambient exposures to toxic and criteria air pollutants. Environ Health Perspect. 2003a;111:647–56. doi: 10.1289/ehp.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Gong H, Linn WS, et al. Respiratory symptoms and peak expiratory flow in children with asthma in relation to volatile organic compounds in exhaled breath and ambient air. J Expo Anal Environ Epidemiol. 2003b;13:348–363. doi: 10.1038/sj.jea.7500287. [DOI] [PubMed] [Google Scholar]
- du Plessis L, Laubscher P, Jooste J, et al. Flow cytometric analysis of the oxidative status in human peripheral blood mono-nuclear cells of workers exposed to welding fumes. J Occup Environ Hyg. 2010;7:367–74. doi: 10.1080/15459621003724108. [DOI] [PubMed] [Google Scholar]
- Erdely A, Hulderman T, Salmen R, et al. Cross-talk between lung and systemic circulation during carbon nanotube respiratory exposure. Potential biomarkers. Nano Lett. 2009;9:36–43. doi: 10.1021/nl801828z. [DOI] [PubMed] [Google Scholar]
- Erdely A, Hulderman T, Salmen-Muniz R, et al. Inhalation exposure of gas-metal arc stainless steel welding fume increased atherosclerotic lesions in apolipoprotein e knockout mice. Toxicol Lett. 2011a;204:12–16. doi: 10.1016/j.toxlet.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Erdely A, Salmen-Muniz R, Liston A, et al. Relationship between pulmonary and systemic markers of exposure to multiple types of welding particulate matter. Toxicology. 2011b;287:153–9. doi: 10.1016/j.tox.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Fang SC, Eisen EA, Cavallari JM, et al. Circulating adhesion molecules after short-term exposure to particulate matter among welders. Occup Environ Med. 2010;67:11–16. doi: 10.1136/oem.2008.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen JA, Nafstad P, Skrondal A, et al. Associations between outdoor air pollutants and hospitalization for respiratory diseases. Epidemiology. 2000;11:136–40. doi: 10.1097/00001648-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Hammond SK, Gold E, Baker R, et al. Respiratory health effects related to occupational spray painting and welding. J Occup Environ Med. 2005;47:728–39. doi: 10.1097/01.jom.0000165748.31326.e8. [DOI] [PubMed] [Google Scholar]
- Hilt B, Qvenild T, Romyhr O. Morbidity from ischemic heart disease in workers at a stainless steel welding factory. Norsk Epidemiol. 1999;9:21–6. [Google Scholar]
- Ibfelt E, Bonde JP, Hansen J. Exposure to metal welding fume particles and risk for cardiovascular disease in Denmark: a prospective cohort study. Occup Environ Med. 2010;67:772–7. doi: 10.1136/oem.2009.051086. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Boylstein RJ. NIOSH health hazard evaluation report: HETA #2006-0059-3009. Daimler-Chrysler Jefferson North assembly plant; Detroit, MI. Washington, DC: 2006. [18 Jul 2014]. Available from: http://www.cdc.gov/niosh/hhe/reports/pdfs/2006-0059-3009.pdf. [Google Scholar]
- Kim JY, Chen JC, Boyce PD, Christiani DC. Exposure to welding fumes is associated with acute systemic inflammatory responses. Occup Environ Med. 2005;62:157–63. doi: 10.1136/oem.2004.014795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukzadeh Z, Sharifian SA, Aminian O, et al. Pulmonary effects of spot welding in automobile assembly. Occup Med (Lond) 2009;59:267–9. doi: 10.1093/occmed/kqp033. [DOI] [PubMed] [Google Scholar]
- Luo JC, Hsu KH, Shen WS. Pulmonary function abnormalities and airway irritation symptoms of metal fumes exposure on automobile spot welders. Am J Ind Med. 2006;49:407–16. doi: 10.1002/ajim.20320. [DOI] [PubMed] [Google Scholar]
- Ma CM, Lin LY, Chen HW, et al. Volatile organic compounds exposure and cardiovascular effects in hair salons. Occup Med (Lond) 2010;60:624–30. doi: 10.1093/occmed/kqq128. [DOI] [PubMed] [Google Scholar]
- Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep. 2013;13:1–9. doi: 10.1007/s11882-012-0317-9. [DOI] [PubMed] [Google Scholar]
- NIOSH Elements by ICP (hot block/HCL/HNO3 digestion): Method 7303. [18 Jul 2014];NIOSH manual of analytical methods. 1994 Available from: http://www.cdc.gov/niosh/docs/2003-154/.
- Nurkiewicz TR, Porter DW, Hubbs AF, et al. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilkop SK, Campen MJ, Lund AK, et al. Identification of chemical components of combustion emissions that affect pro-atherosclerotic vascular responses in mice. Inhal Toxicol. 2012;24:270–87. doi: 10.3109/08958378.2012.667455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren B, Fossum T, Lindh T, Weiner J. Welding and ischemic heart disease. Int J Occup Environ Health. 2002;8:309–11. doi: 10.1179/107735202800338597. [DOI] [PubMed] [Google Scholar]
- Sriram K, Jefferson AM, Lin GX, et al. Neurotoxicity following acute inhalation of aerosols generated during resistance spot weld-bonding of carbon steel. Inhal Toxicol. 2014 doi: 10.3109/08958378.2014.954654. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M, Zubareb J, Jacovovitz R, et al. Ho-1 and vegf gene expressions are time dependant during exposure to welding fumes. Cytokine. 2009;46:290–5. doi: 10.1016/j.cyto.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Stout RD. Weldability of steels. 4th edn. Welding Research Council; New York: 1987. The welding processes in relation to weldability. pp. 20–2. [Google Scholar]
- Taimeh Z, Loughran J, Birks EJ, Bolli R. Vascular endothelial growth factor in heart failure. Nat Rev Cardiol. 2013;10:519–30. doi: 10.1038/nrcardio.2013.94. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Shields MD, Patterson CC. Acute asthma exacerbations and air pollutants in children living in Belfast, northern Ireland. Arch Environ Health. 2001;56:234–41. doi: 10.1080/00039890109604447. [DOI] [PubMed] [Google Scholar]
- Tsai DH, Wang JL, Chuang KJ, Chan CC. Traffic-related air pollution and cardiovascular mortality in central Taiwan. Sci Total Environ. 2010;408:1818–23. doi: 10.1016/j.scitotenv.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Vedal S, Campen MJ, McDonald JD, et al. Research report number 178. Health Effects Institute; Boston, MA: 2013. National particle component toxicity (npact) initiative report on cardiovascualr effects. [PubMed] [Google Scholar]
- Vistnes M, Waehre A, Nygard S, et al. Circulating cytokine levels in mice with heart failure are etiology dependent. J Appl Physiol. 2010;108:1357–64. doi: 10.1152/japplphysiol.01084.2009. [DOI] [PubMed] [Google Scholar]
- Wang F, Li C, Liu W, Jin Y. Effect of exposure to volatile organic compounds (vocs) on airway inflammatory response in mice. J Toxicol Sci. 2012;37:739–48. doi: 10.2131/jts.37.739. [DOI] [PubMed] [Google Scholar]
- Weichenthal S, Kulka R, Belisle P, et al. Personal exposure to specific volatile organic compounds and acute changes in lung function and heart rate variability among urban cyclists. Environ Res. 2012;118:118–23. doi: 10.1016/j.envres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Battelli LA, Stone S, et al. Short-term inhalation of stainless steel welding fume causes sustained lung toxicity but no tumorigenesis in lung tumor susceptible A/J mice. Inhal Toxicol. 2011;23:112–20. doi: 10.3109/08958378.2010.548838. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Erdely A, Antonini JM. Immunotoxicology of arc welding fume: worker and experimental animal studies. J Immunotoxicol. 2012;9:411–25. doi: 10.3109/1547691X.2011.652783. [DOI] [PMC free article] [PubMed] [Google Scholar]