Abstract
Objective
Patient values and preferences are an important component to decision making when tradeoffs exist that impact quality of life, such as tradeoffs between stroke prevention and hemorrhage in patients with atrial fibrillation (AF) contemplating anticoagulant therapy. Our objective is to describe the development of an Atrial Fibrillation Guideline Support Tool (AFGuST) to assist the process of integrating patients’ preferences into this decision.
Materials and Methods
CHA2DS2VASc and HAS-BLED were used to calculate risks for stroke and hemorrhage. We developed a Markov decision analytic model as a computational “engine” to integrate patient-specific risk for stroke and hemorrhage and individual patient values for relevant outcomes in decisions about anticoagulant therapy.
Results
Individual patient preferences for health-related outcomes may have greater or lesser impact on the choice of optimal antithrombotic therapy, depending upon the balance of patient-specific risks for ischemic stroke and major bleeding. These factors have been incorporated into patient-tailored booklets which, along with an informational video were developed through an iterative process with clinicians and patient focus groups.
Key Limitations
Current risk prediction models for hemorrhage, such as the HAS-BLED, used in the AFGuST, do not incorporate all potentially significant risk factors. Novel oral anticoagulant agents recently approved for use in the United States, Canada, and Europe have not been included in the AFGuST. Rather, warfarin has been used as a conservative proxy for all oral anticoagulant therapy.
Conclusions
We present a proof of concept that a patient-tailored decision-support tool could bridge the gap between guidelines and practice by incorporating individual patient’s stroke and bleeding risks and their values for major bleeding events and stroke to facilitate a shared decision making process. If effective, the AFGuST could be used as an adjunct to published guidelines to enhance patient-centered conversations about the anticoagulation management.
Keywords: Decision Support Tools, Decision Analysis, Shared Decision Making, Atrial Fibrillation
Background and Significance
Atrial fibrillation (AF) is the most common significant cardiac rhythm disorder and is also the most powerful common risk factor for stroke: about 15% of all strokes in the U.S. are attributable to AF. Its frequency increases strikingly with age, reaching a prevalence of 10% in those over age 80.1 With the aging of the U.S. population, the prevalence of AF will increase substantially from over 2.2 million currently to more than 3 million Americans by the year 2020.1 Over the past decade, numerous randomized trials have established that anticoagulation can reduce significantly the stroke risk posed by AF. However, studies in community settings have demonstrated that inappropriate treatment is common and that there is wide variation in adherence to practice guidelines.2 Surveys exploring this gap have identified the pivotal physician-related factor to be an “insufficiently balanced evaluation of the risk versus benefit” of oral anticoagulant therapy.3,4
Patient values and preferences are an important component to decision-making when there are tradeoffs that impact quality of life, such as the tradeoffs between stroke prevention and major hemorrhage in patients with AF contemplating long-term anticoagulant therapy.5 While clinical trial data may report major outcomes, such as deaths, non-fatal strokes, and non-fatal major hemorrhages, techniques are needed to help determine the tradeoffs patients are willing to make between these outcomes. Guidelines such as the 9th edition of the American College of Chest Physician’s (ACCP) Antithrombotic and Thrombolytic Therapy Guidelines sought to incorporate patient values and preferences in a far more explicit manner than in the past.6 However, we still lack convenient tools that can facilitate the incorporation of individual patient’s values and preferences for health outcomes into the decision-making process and discussion. Decision analysis is a technique that can facilitate the formal incorporation of patient values (utilities) into the decision making process. Life spent in less-than-perfect states of health, such as life following a non-fatal stroke, can be valued through multi-attribute metrics, such as quality-adjusted life expectancy, to facilitate explicit tradeoffs between the risks and benefits of therapies.7 Furthermore, patients differ in their underlying risk for ischemic stroke8, and their risk of major bleeding from anticoagulants 9. Thus, the decision to treat AF patients with antithrombotic therapy is ideally suited to a patient-centered decision analytic approach that incorporates both patient-to-patient variability in risk factor profiles and in values and preferences for health outcomes.5
Decision analysis has been suggested as an approach for involving patients in a shared decision-making process.10 For several decades the Clinical Decision Making group at Tufts has supported a consultation service that has provided such personalized decision analyses.11 Rather than providing generic information regarding a particular set of treatments for any given clinical problem, as is frequently the case with many decision aids, decision analysis can support an individualized treatment recommendation based on both patient-specific risks and individual patient values and preferences for health outcomes.12–17 Indeed, several prior decision aids have been developed using decision analysis to guide patients in the choice of anti-thrombotic therapy for AF.18–22
Realizing that guidelines generated by expert panels are static documents, our goal was to develop tools to enhance guidelines so that the values and preferences of individual patients for treatments and health outcomes can be easily incorporated in discussions clinicians have with their patients. In a qualitative study examining this issue, Van der Weijden and colleagues have suggested several approaches, including the development of patient versions of clinical practice guidelines and wording in the guideline itself that stresses the importance of incorporating patient participation in the decision-making process.23,24 A nice example that complements a clinical practice guideline with a patient version is the recently published AF guideline by the UK National Institute for Health and Clinical Excellence (NICE) in which a patient decision aid has been published alongside the clinician guideline.25 Thus, the enhanced guidelines can be used to facilitate a shared decision making process. To accomplish this, we developed the Atrial Fibrillation Guideline Support Tool (AFGuST).
Methods
Development of decision analytic model
In order to develop patient-specific recommendations that could be used as an adjunct to AF guidelines, we first developed decision analytical models that consider various antithrombotic therapies for patients with AF. We did not model treatment with any of the novel oral anticoagulants (NOACs) that have recently been approved for use in the United States, Canada, and Europe. Anticoagulant therapy with warfarin is used as a proxy for more generalized oral anticoagulant therapy. Since this makes the decision support tool’s recommendation for anticoagulant therapy more conservative, use of any of the NOACs would be reasonable when oral anticoagulation with warfarin is recommended (as supported by the most recent ACCP guidelines). We used a standard computer program (Decision Maker, Boston, Massachusetts) to build the model, analyze results, and perform sensitivity analyses. During each monthly cycle, patients face a chance of stroke and hemorrhage, either of which may lead to death, significant neurological sequelae or symptom resolution. The simulation is run for the entire life expectancy of the hypothetical cohort of similar patients. Base case values for model parameters are summarized in Table 1 and the decision tree figure and modeling details are provided in appendix figure 1 and figure 2 and accompanying text. Patient-specific stroke risk was based upon the CHA2DS2VASc (Congestive heart failure, Hypertension,
Table 1.
Data Required in the Analysis: Probabilities, Rates, and Quality of Life.
| Parameter | Value | ||||
|---|---|---|---|---|---|
| Annual Rate of Ischemic Stroke (untreated) |
Based upon CHA2DS2VASc score [see Appendix Table 2] 26 | ||||
| Efficacy of treatment with warfarin - |
0.68 8 | ||||
| aspirin – | at age 50 | 0.60 27 | |||
| at age 77 | 0.00 27 | ||||
| Probable outcome of Ischemic Stroke: |
|||||
| Death - | 0.16 28 | ||||
| Permanent sequelae : | 0.44 8 29 | ||||
| with severe disability - | 0.69 8 29 | ||||
| with mild disability - | 0.31 8 29 | ||||
| Good recovery - | 0.40 8 29 | ||||
| Annual Rate of extracranial bleeding event: (warfarin) - |
Based upon HAS-BLED score [see Table 3] 30 | ||||
| (untreated) – | (HAS-BLED bleeding rate)/2.4 31 | ||||
| (aspirin) - | (Bleeding rate in untreated) * 1.08 31 | ||||
| Annual rate ICH low risk referent group (untreated) |
0.0004 30 | ||||
| Multivariate Hazard Ratios for ICH (untreated) |
30 | ||||
| Age < 65 | 1.0 | ||||
| Age 65 – 74 | 1.97 | ||||
| Age ≥ 75 | 2.43 | ||||
| Female | 0.7 | ||||
| Prior Ischemic Stroke | 1.21 | ||||
| Hx of ICH | 8.92 | ||||
| Hx of Severe Bleed | 3.1 | ||||
| Hx of Myocardial Infarction | 0.82 | ||||
| Hx of Ischemic Heart Disease | 0.81 | ||||
| Hx of Poorly Controlled HTN † | 1.32 | ||||
| Annual rate Subdural Hematoma (untreated) |
0.00027 8 32 33 | ||||
| Location of hemorrhage | Lobar ICH | Deep ICH | Subdural hematoma |
Extracranial | |
| Relative hazard of bleeding (vs. no treatment) |
|||||
| warfarin - | 4.1 33,34 | 4.1 33,34 | 5.5 33,38,39 | 2.4 41 | |
| aspirin - | 1.84 35–37 | 1.84 35–37 | 2.0 40 | 1.08 41 | |
| Probable outcome from bleed (without warfarin/with warfarin)* |
42 | 42 | |||
| Death – | 0.19 / 0.38 | 0.21 / 0.41 | 0.26737 43 | 0.024/0.051 | |
| Severe long-term disability - | 0.43 / 0.43 | 0.44 / 0.42 | 0.07/0.09 44 | 37,43 | |
| Mild long-term disability - | 0.20 / 0.11 | 0.19 / 0.10 | 0.40/0.50 44 | ||
| Good recovery - | 0.19 / 0.08 | 0.17 / 0.07 | 0.263/0.143 | ||
| Long-term symptoms | Base-Case Value of Quality of Life | ||||
| Well | 1.0 | ||||
| Well while receiving anticoagulant therapy |
0.99 45 | ||||
| Severe long-term disability | 0.11 45 | ||||
| Mild long-term disability | 0.76 45 | ||||
| Death | 0.0 | ||||
| Short-term symptoms | |||||
| ICH‡ | 0.79 | ||||
| Ischemic stroke‡ | 0.79 | ||||
| Extracranial bleed ξ | 0.84 | ||||
| Base-Case Value of Age-Adjusted Annual Excess Mortality |
|||||
| Stroke with long-term disability | 0.08 46 | ||||
Poorly controlled hypertension – systolic BP ≥ 160 mmHG.
Assume outcomes of bleeding events for aspirin-treated patients are the same as for untreated patients.
Assume quality of life is 0 for duration of hospitalization. Length of stay for specific cerebrovascular disorders except transient ischemic attack (diagnosis-related group, 14) is 6.4 days.
Length of stay for gastrointestinal hemorrhage (diagnosis-related group, 174) is 4.9 days. Duration of short-term utility loss for major extracranial bleed is 12 months.
Age ≥75 years [double weight], Diabetes, previous Stroke [double weight], Vascular disease, Age 65–74 years, female Sex category)47 (Appendix Table 1), while patient-specific risk of major extracranial bleeding was based upon the HAS-BLED score (Hypertension, Abnormal renal or liver function, Stroke history, Bleeding History, Labile INR, elderly - Age ≥65 years, Drugs – non-steroidal anti-inflammatory drugs or alcohol) (Appendix Table 2).48 Patient-specific annual rates of ICH were calculated separately using a multivariable regression model (see hazard ratios in Table 1).30
Development of patient-specific guideline support
Our goal was to develop tools that can be used to: 1) quickly and easily obtain patient utilities for health outcomes and treatments, and 2) facilitate shared decision making by showing patients and clinicians how those patient-specific values and preferences impact the optimal treatment decision. Using steps described to facilitate the development of web-based decision support tools49, we first specified and developed consensus regarding the necessary clinical content. The synthesis of evidence was facilitated by the PI’s (MHE) participation as a member of the American College of Chest Physician’s 2012 guideline development for antithrombotic therapy in patients with AF 6 In the early design phase (“sandpit testing”) we experimented with many alternative graphical approaches for presenting data to patients (e.g., numeric tables, graphs, pictograms). We tested prototypes of the AFGuST through individual meetings with clinicians (general internists, cardiologists, and neurologists) and a series of patient focus groups. The director of our primary care network along with 2 other general internists (ME, DS, NW), 3 cardiologists (GL, AC, FK), and 3 stroke neurologists (MF, DK, BK) are members of our project team. We conducted a series of 4 patient focus groups and iterated on patient pamphlet and video design after each focus group. Between 2 and 5 patients attended each focus group. Other focus group attendees included the PI (MHE), study coordinator (RW), qualitative researcher (LA), and 2 graphic designers (RW, KN). To avoid ethical issues regarding treatment recommendations that may have been at odds with current therapy, focus groups were comprised of patients who did not have AF. We sought patients between the ages of 60 and 85 years as the prevalence of AF increases substantially with increasing age. The average age of AF patients in clinical trials is 69 years.8 We also sought patients who had at least one significant non-AF diagnosis. Study protocols for focus groups were approved by the University of Cincinnati Institutional Review board (IRB).
We developed a 25-minute video that patients can view prior to their office visit that provides some clinical background about the risk of stroke from AF, the efficacy of anticoagulation therapy and the tradeoffs between the risk of stroke and the risk of bleeding from anticoagulant therapy. The video also helps patients to understand the standard gamble 50,51 technique that we use in the personalized patient pamphlet to assess their individual values for relevant health outcomes. We felt this was particularly important in light of difficulties described in other studies using a standard gamble utility assessment approach within a decision aid.52 The patient pamphlet was reviewed and edited by our organization’s PR department to make sure the language was understandable at a 5th grade level.
Using an iterative process49, we presented the video and personalized pamphlet to focus groups and clinicians, determined what they had difficulty understanding and obtained their feedback about what we could improve or add. We then updated and improved both the pamphlet and the video and met again with focus groups.
Results
Obtaining patients’ values and preferences for key health states
A particular challenge in this project was determining an efficient and understandable method to obtain patient values and preferences (i.e., utilities) for health outcomes in order to incorporate them into the decision-making process. The decision analytic model (see appendix) contains a number of different health states for which patient-specific utilities could be assigned. From a purely practical perspective, attempting to perform utility assessments for all health states would take a prohibitive amount of time. The major trade-off that patients need to consider is the risk of major bleeding events (increased by anticoagulant therapy) versus the risk of AF-related stroke (prevented to some degree by anticoagulant therapy). The vast majority of major hemorrhages are gastrointestinal bleeds. 40,53 In addition, the quality adjustment factors used for stoke in our model, are based upon the degree of neurological deficit and not the cause of the stroke (i.e., ischemic vs. hemorrhagic). We performed comprehensive sensitivity analyses on the values (utilities) of all health outcomes within clinically plausible ranges to see which had the greatest impact on the decision. We found that the values assigned to major gastrointestinal hemorrhage and stroke (AF-related or hemorrhagic) with severe long-term neurological sequelae had the greatest impact on the result of the decision analysis. Thus, to simplify the process of personalized utility assessment we focused only on patients’ values for these two health outcomes.
Multiple techniques exist to assess utilities for hypothetical health states (i.e., those not yet experienced by patients). These include visual analog scales, standard gambles, and time tradeoffs.50,54,55 The standard gamble, which determines the risk of a bad outcome, such as death, that a patient would be willing to take to avoid the outcome for which the utility is being assessed (e.g., stroke with severe long-term neurological sequelae) and the time tradeoff, which involves giving up future years of life in a less than perfect state of health in exchange for a shorter life expectancy in a good state of health, are difficult to use for the assessment of temporary health states.56–58 This is because few patients are willing to take a risk of death or tradeoff life expectancy to avoid a health outcome that is only transient. Visual analog scales, frequently called feeling thermometers are simple and easy to administer. Thus, we settled on using a visual analog scale to obtain quality of life for the temporary health state of a hypothetical gastrointestinal bleed, and a standard gamble for long-term sequelae following a hypothetical severe stroke.
The pamphlet provides a detailed scenario description of a major gastrointestinal bleed which we adapted from Devereaux et al.59 This description includes physical symptoms, treatment and expected recovery (see figure 1 - left panel). We use a visual analog scale to obtain a rating of their quality of life in the immediate period following a hypothetical major gastrointestinal bleed (see figure 1 - right panel).
Figure 1. Your Values for: Major Bleeding While Taking Blood Thinning Treatment.
The panel to the left describes Physical Symptoms, Treatment, and Recovery that can be expected following a major gastrointestinal bleed. The panel to the right shows a visual analog rating scale used to assess a patient’s quality of life following a hypothetical major bleed. The top portion of the panel demonstrates an example, while the bottom portion of the panel is used to obtain a patient’s personalized assessment for quality of life following a major bleed. In order to assist with numeracy, emoticons are also used to describe the zero to one hundred numerical scale.
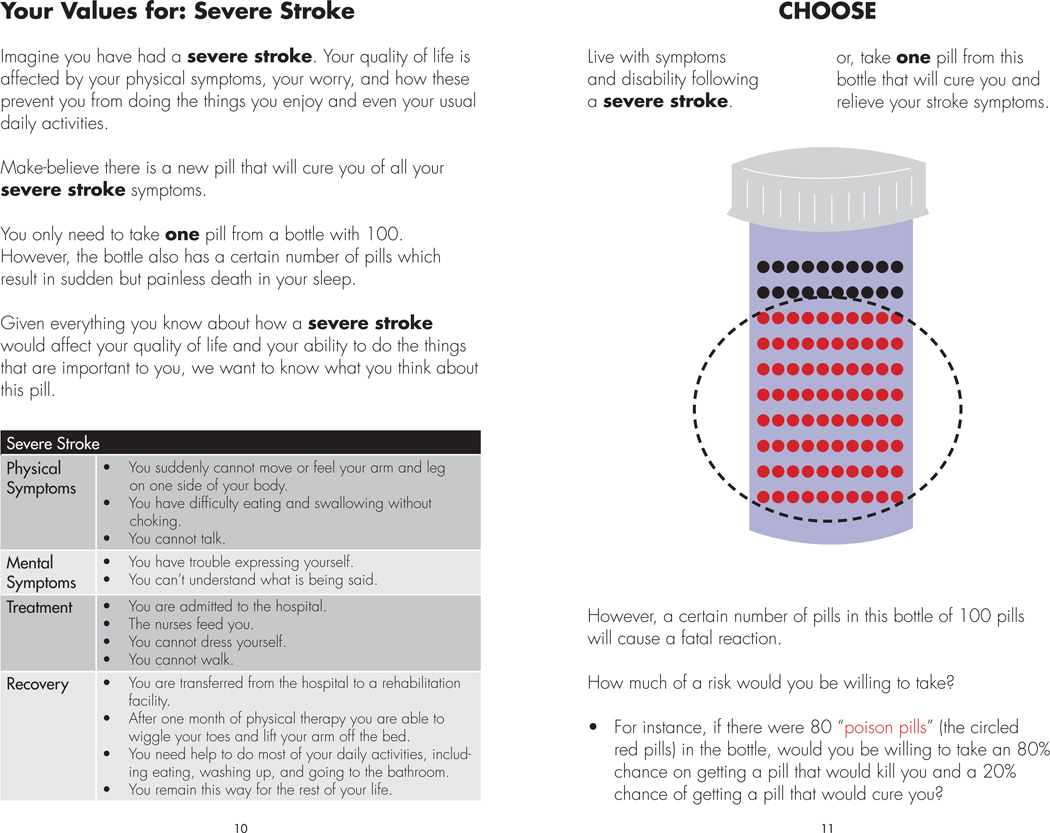
We next provide a detailed scenario description of stroke with long-term major neurological sequelae, again adapted from Devereaux et al. (see figure 2 – left panel).59 For the standard gamble, we use an approach which to our knowledge has not been described in the literature. Using an illustration of a bottle containing 100 pills patients are told that they can take a single pill from this bottle and it will relieve them of their stroke symptoms. They also are told that a certain number of pills in this bottle will cause a painless but fatal reaction and they will not wake up from their sleep if they received such a “poison pill.” We next ask them to draw a circle around the largest number of “poison pills” they would tolerate being in the bottle while still being willing to take a chance on the curative medicine (see figure 2 – right panel).
Figure 2. Your Values for: Severe Stroke.
The panel to the left describes Physical Symptoms, Mental Symptoms, Treatment, and Recovery that can be expected following a severe stroke. The panel to the right uses a pill bottle motif to perform a standard gamble, assessing how large a risk of painless death a patient would be willing to take to avoid living with the long-term sequelae of a severe stroke. The next page in the pamphlet (not shown) is used to obtain the patient’s own value of quality of life for this health outcome.
While the poison pill analogy has been used before60, the standard gamble typically requires a time consuming iterative ping-pong approach to zero in on the patient’s indifference point. By providing a demonstration of the standard gamble in the video, patients in the focus groups understood the concept well enough to be able to simply circle the number of poison pills in the bottle they would be willing to tolerate in the gamble.
Decision Analyses and Generation of Patient-Specific Templates –
While much of the patient pamphlet is generic, we insert a personalized template which we generate from the decision analytic model based upon each patient’s individual risk of stroke and major bleeding, calculated using the CHA2DS2VASc 47 and HAS-BLED 48 scores, and their risk of intracerebral hemorrhage.30
We use this template to help clinicians discuss the anticoagulation decision with their patients while also incorporating their patient’s values for health outcomes (obtained in the steps described above) including major extracranial bleeds and stroke with severe neurological sequelae. Patients are shown several examples and then asked to map out their own results. Figure 3 shows one of the examples.
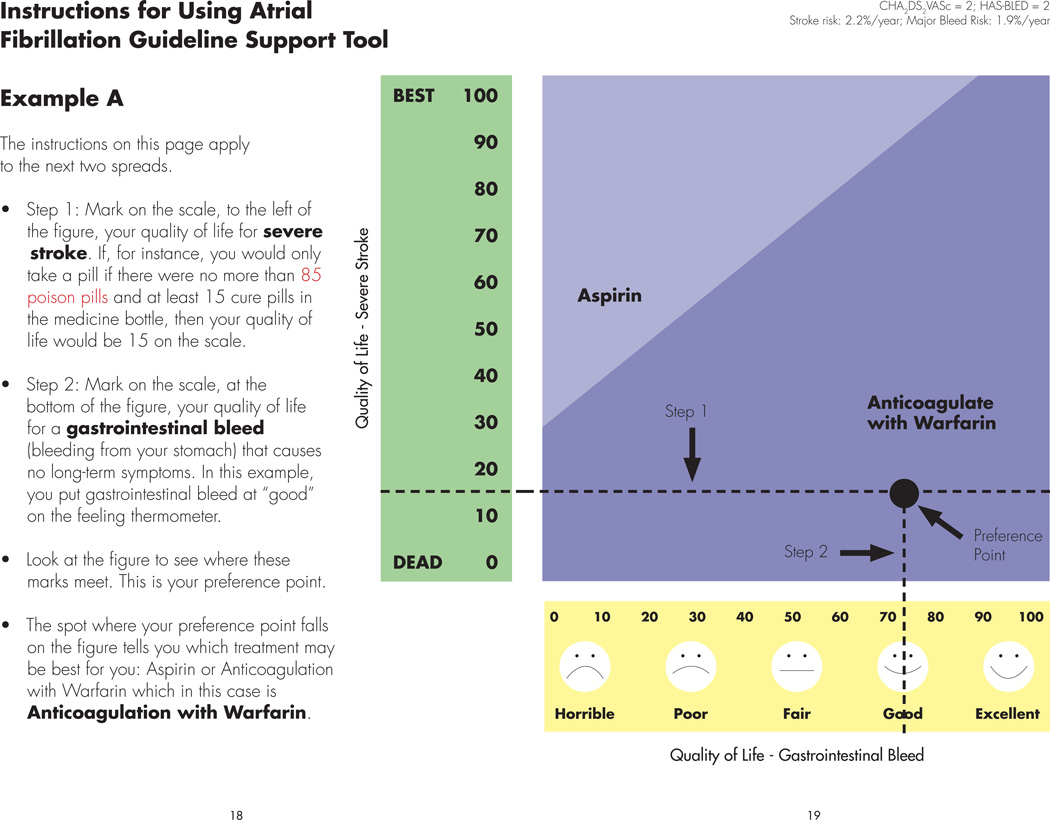
Figure 3. Instructions for Using Atrial Fibrillation Guideline Support Tool.
This figure demonstrates an example for patients of how they can use the AFGuST to find their “optimal” choice of antithrombotic therapy, based upon their personalized risk of stroke, major bleeding while receiving aspirin or oral anticoagulant therapy and their values for the health outcomes of major gastrointestinal bleed and severe stroke. Instructions are provided to the left of the figure. The horizontal, x-axis represents an individual patient’s values and preferences (quality of life) for a gastrointestinal bleed, while the vertical, y-axis represents a patient’s quality of life following a stroke with severe neurological sequelae. There are two treatment regions in the figure representing the optimal strategy for a patient with this particular combination of stroke and bleeding risk. Patients mark the values (utilities) they provided previously for quality of life following a severe stroke (step 1) and quality of life in the early aftermath of a gastrointestinal bleed (step 2). They then draw a line from each of these points on the y- and x-axes and mark the point where these two lines intersect. This is called their “preference point.” An example is shown for a patient with a CHA2DS2VASc of 2 (stroke risk – 2.9%/year) and a HAS-BLED of 2 (major bleeding risk – 1.9%/year), who believes their quality of life would be low following a stroke with severe neurological consequences (~ 15, on a zero to one hundred scale), and higher following a significant gastrointestinal bleed (~73, on a zero to one hundred scale). The “preference point” for such a patient falls in the region to the lower right where anticoagulation with warfarin is best. Alternatively, for a patient who felt that their quality of life following a major bleed and following a stroke would be modestly decreased (not shown), aspirin would be the best treatment choice, since their decision point would fall above the threshold line dividing the two treatment regions. The patient is asked to find their own “preference point” using their personalized health values on a following page of the pamphlet.
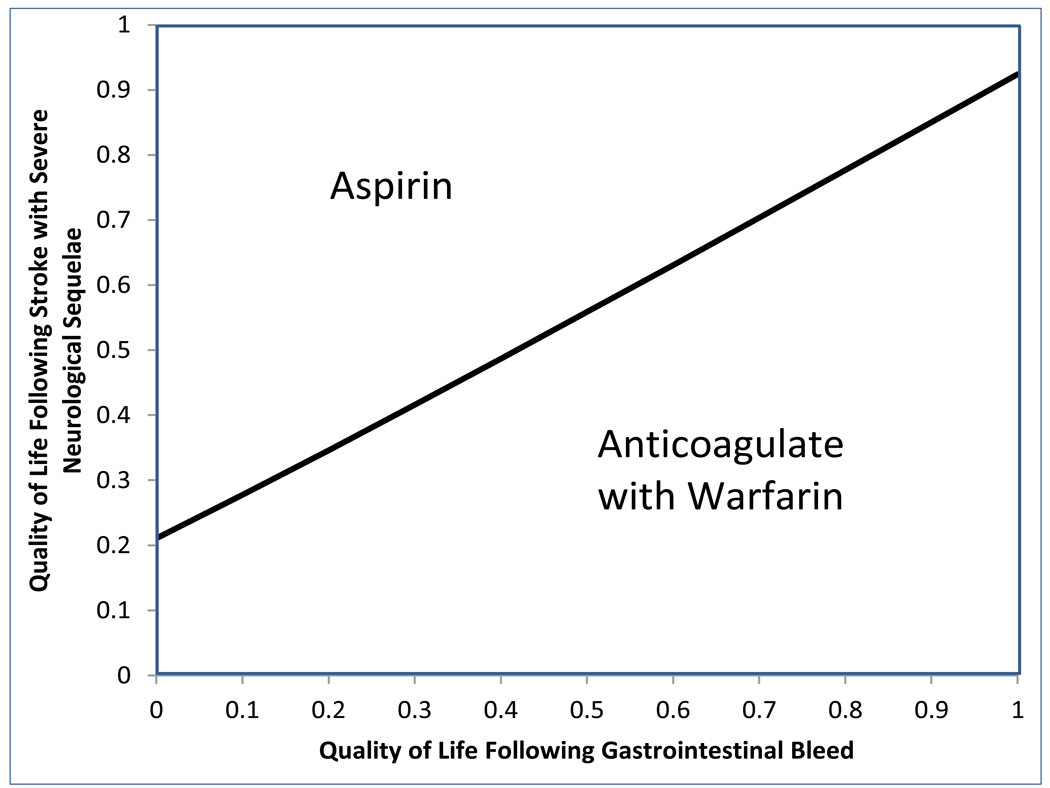
In order to create these personalized templates, we analyzed the decision model for a large number of scenarios consisting of different combinations of demographic and clinical parameters. For many scenarios the best choice of treatment was insensitive to patient values and preferences for the two health states assessed. For instance, for a 64 year-old woman with a CHA2DS2VASc of 1 and a HAS-BLED of 1, 2, 3, or even 4 (but no history of prior ICH), aspirin is always the optimal choice, independent of a patient’s preferences for the two health outcomes assessed. On the other hand for a 74 year-old woman with a CHA2DS2VASc of 2 and a HAS-BLED of 1, oral anticoagulation therapy is always best. However, there are numerous scenarios in which patient preferences may drive the optimal choice of treatment. Figure 4 and Figure 5 demonstrate examples of two such patient scenarios in which preferences are important - a 74 year-old woman with a history of a prior bleed and heavy alcohol use (CHA2DS2VASc of 2, HAS-BLED of 3) and 60 year-old man with a history of diabetes mellitus, hypertension, alcohol use, NSAID use, and abnormal liver function and renal function (CHA2DS2VASc of 2, HAS-BLED of 4).
Figures 4 and 5.
provide examples of different patient-specific templates that could be inserted into the AFGuST pamphlet for a 74 year-old woman (figure 4) with a history of a prior bleed and heavy alcohol use (CHA2DS2VASc of 2, HAS-BLED of 3) and 60 year-old man (figure 5) with a history of diabetes mellitus, hypertension, alcohol use, NSAID use, and abnormal liver function and renal function (CHA2DS2VASc of 2, HAS-BLED of 4). The region in which Anticoagulation with Warfarin is the best choice is larger in Figure 4 for the patient with a lower risk of major hemorrhage.
Using the AFGuST in Clinical Practice
A decision aid such as the AFGuST could be used in a variety of clinical settings. One could provide decision support for patients with newly incident AF during their initial hospitalization. The guideline support tool could be used retrospectively as a quality assurance tool to flag patients who may not be receiving optimal antithrombotic therapy. Utilizing the AFGuST in the ambulatory care setting to provide decision support for patients with prevalent AF is particularly appealing, being both practical and clinically rational. First, it is important to recognize that treatment decisions about antithrombotic therapy are not static and must be continually revisited. Clinical events that alter the risk factor profile for either thromboembolism or major bleeding may follow the initial decision regarding antithrombotic therapy. Furthermore, numerous studies have demonstrated that both under use and inappropriate use of anticoagulant therapy for patients with AF is common.61–63 Therefore, a strategy employing the integration of the AFGuST into an ambulatory care environment for patients with AF of undetermined duration makes sense.
In preparation for an ambulatory visit during which the anticoagulation decision would be discussed or revisited, we would envision giving patients a personalized pamphlet (based on their age, gender, CHA2DS2VASc and HAS-BLED score) and either a DVD or web-link to the video that they can review while they read their pamphlet at home prior to their next office visit. Thus, time will be saved and they can come to their office visit activated and prepared to have a shared decision making discussion with their clinician.
Discussion
The recognition of stroke risk from AF and its prevention have become high profile issues for a number of organizations. The American College of Chest Physicians Foundation and the American Heart Association have developed standardized patient educational tools and booklets.64 The Alliance for Aging Research recently convened a roundtable and developed a consensus document “Assessing Stroke and Bleeding Risk in Atrial Fibrillation,”65 while a United States congressional resolution introduced into the House of Representatives in 2011 focused on encouraging programs that increase public and clinician awareness of AF, including risk assessment, treatment, and appropriate clinical management.66
As such, decision aids have been developed to provide AF patients with general information about the underlying stroke risks of AF and the benefits of antithrombotic therapy.64,67,68 In some cases patient-specific risk projections for stroke have been presented. However, most have not also presented patient-specific risk projections for major bleeding. Decision aids that have presented individualized risks of stroke and major bleeding have left it to the patient and their physician to decide whether a given change in risk/benefit with versus without treatment is worth taking.25 Using graphical techniques (e.g., pictograms) such an aid might report for a given patient: with no medication 13 people out of 100 like you will have a stroke over 5 years while 2 in 100 will have a bleed. With coumadin, 5 people in 100 will have a stroke over 5 years and 9 in 100 will have a bleed.25,69 This is a cognitively complex task that requires a high level of numeracy. Using a decision analytical approach and multi-attribute outcome metrics allows us to decompose the cognitive problem into several simpler tasks by first assessing patient’s values and preferences for stroke and major hemorrhage, and then projecting a single outcome, in quality-adjusted life years, for each strategy. Thus, the comparison the patient and their clinician must make is simply which strategy provides the largest quality-adjusted life expectancy.
Other studies have explored using a decision analytic approach to augment decision aids for the antithrombotic therapy decision in patients with AF.70,71 The Decision Analysis in Routine Treatment Study (DARTS) team has examined the feasibility of a shared decision-making tool for patients in the United Kingdom (UK) with AF.72,73 Using stroke prediction models from the Framingham study74 and a large series of look-up tables representing results of a decision model, they developed patient-specific guidelines for warfarin therapy in patients with AF.72 Through focus groups with general practitioners in the United Kingdom, they described uncertainty about the appropriate usage of warfarin in patients with AF. Furthermore, they found that “readily accessible information on the evidence base would generally be welcomed.”73 The DARTS model differs from the AFGuST in a number of ways. There are profound differences in the calculation of patient risk for stroke and bleeding and in the probabilistic events considered in the decision models. DARTS uses a variant of the Framingham stroke risk equation which is not specific for patients with atrial fibrillation, or for cardioembolic stroke, whereas the AFGuST uses the CHA2DS2VASC score47, developed specifically on patients with AF. Bleeding risk for the DARTS analysis only considers gastrointestinal bleeding, whereas the AFGuST also considers the far more devastating central nervous system bleeds. In a more recent clinical trial (DARTSII) examining the efficacy of this computerized decision aid compared with a paper-based guideline tool, Thomson and colleagues found that use of the computerized decision aid significantly lowered decisional conflict and improved patients’ sense of being well informed.22 Of particular interest, they discontinued the arm of their study that used a standard gamble approach to assessing utilities for the personalized decision analysis.52 This was done as a result of a qualitative analysis using videotaped transcripts of clinician-patient interactions that suggested the standard gamble values elicitation exercise was causing confusion. Indeed, this is one of the reasons we demonstrated a sample standard gamble exercise in the video that accompanies the AFGuST.
Finally, it should be realized that the decision regarding antithrombotic therapy for patients with an elevated risk of both stroke and major bleeding is preference sensitive; meaning there isn’t a RIGHT decision for every patient. Thus, the true goal for these patients is that the decision making process be the best it can be. To achieve that, the delivery of patient-centered care requires an active role for the patient, and the communication of understandable and relevant, patient-specific information by healthcare professionals to patients.75,76 Therefore, the AFGuST has been designed to inform and activate patients and to prompt physicians to discuss the anticoagulation decision with their patients, hopefully resulting in “better” decisions, measured by increased patient knowledge, and improved confidence and satisfaction with the decision-making process.77,78
The AFGuST has several limitations. In order to calculate stroke risk we have used the CHA2DS2VASC47, which is similar to the CHADS2 79, but provides additional discrimination for age (65 to 75), female gender, and the presence of concomitant vascular disease. Studies have shown their receiver operator curve areas to be comparable.47 While the European Society of Cardiology’s guidelines uses both CHA2DS2VASc and HAS-BLED, the most recent version of the ACCP guidelines published in 2012 still used the CHADS2. In addition, guidelines do not explicitly integrate a quantitative assessment of bleeding risk in their recommendations. Therefore, it is possible or even likely that for some patients the ACCP guideline will make a different recommendation (based on the CHADS2 score) than the AFGuST. Finally, if evidence continues to accumulate that stroke risk is lower in non-clinical trial settings, 33,80 this will expand the size of the population for whom anticoagulant therapy is a preference-sensitive decision, making a tool like the AFGuST helpful for an even larger group of patients facing this difficult decision.
Despite their continued development, current risk prediction models for major hemorrhage, such as HEMORR2HAGES and HAS-BLED, do not incorporate psychosocial and socio-demographic information or fall risk that may bear on the risk of bleeding with anticoagulant therapy.48,81 Therefore, the recommendation of the AFGuST cannot be interpreted as a mandate that replaces clinical judgment. Rather, it must be interpreted holistically within the broader clinical context of the whole patient. We must make sure to appropriately communicate these limitations to the clinicians using such decision support tools.
Over the past 2 years, several novel anticoagulants have come on the scene. Four, dabigatran, rivaroxaban, apixaban and edoxaban have received approval for use in patients with AF. At this time, knowledge regarding the efficacy and safety of these novel agents is limited to a small number of studies. Uncommon but serious adverse events may emerge with larger-scale use of these agents. Thus, decisions among anticoagulant agents are complex and a bit premature, and the benefits and circumstances in which one agent may be better than another for an individual patient remain unclear. Furthermore, the most recent guidelines from the ACCP focus on the decision to use anticoagulant therapy rather than specifying a particular anticoagulant. Therefore, our guideline decision support tool does not address choices among competing anticoagulants. The guideline support tool is sufficiently flexible to incorporate new data at an appropriate future date to either substitute a newer agent in the place of warfarin, or possibly consider choices among anticoagulants.
Finally, shared decision making represents a significant opportunity to operationalize the goals of patient-centered care featured in the new Health Care Affordability Act. The final rule for Medicare accountable care organizations requires that delivery systems engage in shared decision making to qualify for participation in the Medicare Shared Savings Program.82,83 In order to operationalize this mandate, tools such as the AFGuST will need to be developed, refined, tested and used in clinical settings.
Conclusion
Using an iterative design process that included clinicians and patient focus groups, we developed patient-tailored booklets and an informational video that could be used to facilitate a shared decision making experience for patients and their clinicians considering alternate treatments to prevent stroke due to AF. The AFGuST could facilitate a patient-centered discussion that incorporates patient-specific information regarding stroke and bleeding risk as well as individual patient’s values and preferences for relevant health outcomes.
Practice Implications
As suggested by the International Patient Decision Aids Standards (IPDAS) collaboration84, having developed a stable prototype, we are currently pilot testing the AFGuST to evaluate its usability and understandability in a small clinical study of patients with AF. Our next goal is to perform a cluster-randomized clinical trial to evaluate the impact of the AFGuST on the decision-making process and various measures of decision quality. If effective, the AFGuST could be used as an adjunct to published guidelines to enhance patient-centered conversations about the anticoagulation management of patients with atrial fibrillation. Although we have developed an application for patients with AF, the conceptual model of patient-centered decision-making can be extended to other patient populations, and the general tools and paradigm developed to support these activities (e.g., patient-specific decision models, and decision analysis results reporting modules) can be used to address other clinical decisions. Mindful of the increasing use of electronic health records (EHR), we develop these tools with the eventual goal of integrating them into comprehensive health information systems (e.g., through patient portals and as point-of-care decision support for clinicians).
Supplementary Material
Acknowledgments
Declaration of funding:
Support for this study came from the Informed Medical Decisions Foundation, Pfizer Educational Group, and NIH/NCATS Grant Number 8UL1TR000077-05. The funding sources had no role in the planning, design, or conduct of this study or the writing of this report. The findings and conclusions in this manuscript do not necessarily reflect the view of the Informed Medical Decisions Foundation.
M.H. Eckman has received research support from the Pfizer Medical Education Group. G.Y.H. Lip has served as a consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Medtronic, Portola and Boehringer Ingelheim and has been on the speakers bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic and Sanofi Aventis.
M. Flaherty has received research support from the Pfizer Medical Education Group, and has served as a consultant to Boehringer Ingelheim, and has served on an advisory board for, as a consultant to, and on a speaker’s program for CSL Behring. R. E. Wise has received research support from the Pfizer Medical Education Group. L. Arduser has received research support from the Pfizer Medical Education Group. D. Kleindorfer has received research support from the Pfizer Medical Education Group. B Kissela has received research support from the Pfizer Medical Education Group. J. Kues and A. Costea have received research support from the Pfizer Medical Education Group.
Footnotes
Declaration of financial/other relationships:
K. Naylor and F. Khan have no relevant financial relationships to disclose. CMRO Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Contributor Information
Mark H. Eckman, Division of General Internal Medicine and the Center for Clinical Effectiveness, University of Cincinnati, Cincinnati, OH, USA
Ruth E. Wise, Division of General Internal Medicine and the Center for Clinical Effectiveness, University of Cincinnati, Cincinnati, OH, USA
Katherine Naylor, Department of Architecture and Planning University of Cincinnati, Cincinnati, OH, USA.
Lora Arduser, Department of English, University of Cincinnati, Cincinnati, OH, USA.
Gregory Y.H. Lip, University of Birmingham Centre for Cardiovascular Sciences, Birmingham, UK
Brett Kissela, Department of Neurology, University of Cincinnati, Cincinnati, OH, USA.
Matthew Flaherty, Department of Neurology, University of Cincinnati, Cincinnati, OH, USA.
Dawn Kleindorfer, Department of Neurology, University of Cincinnati, Cincinnati, OH, USA.
Faisal Khan, Division of Cardiology, University of Cincinnati, Cincinnati, OH, USA.
Daniel P. Schauer, Division of General Internal Medicine and the Center for Clinical Effectiveness, University of Cincinnati, Cincinnati, OH, USA
John Kues, Department of Family and Community Medicine, University of Cincinnati, Cincinnati, OH, USA.
Alexandru Costea, Division of Cardiology, University of Cincinnati, Cincinnati, OH, USA.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of Diagnosed Atrial Fibrillation in Adults: National Implications for Rhythm Management and Stroke Prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. Jama. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Weisbord SD, Whittle J, Brooks RC. Is warfarin really underused in patients with atrial fibrillation? J Gen Intern Med. 2001;16(11):743–749. doi: 10.1111/j.1525-1497.2001.10432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen N, Almoznino-Sarafian D, Alon I, Gorelik O, Koopfer M, Chachashvily S, et al. Warfarin for stroke prevention still underused in atrial fibrillation: patterns of omission. Stroke. 2000;31(6):1217–1222. doi: 10.1161/01.str.31.6.1217. [DOI] [PubMed] [Google Scholar]
- 4.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 5.Eckman MH. Patient-centered decision making: a view of the past and a look toward the future. Med Decis Making. 2001;21(3):241–247. doi: 10.1177/0272989X0102100309. [DOI] [PubMed] [Google Scholar]
- 6.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeil BJ, Pauker SG, Sox JHC, Tversky A. On the elicitation of preferences for alternative therapies. N Engl J Med. 1982;306:1259–1262. doi: 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
- 8.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–1457. [PubMed] [Google Scholar]
- 9.Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87(2):144–152. doi: 10.1016/s0002-9343(89)80689-8. [DOI] [PubMed] [Google Scholar]
- 10.Elwyn G, Edwards A, Eccles M, Rovner D. Decision analysis in patient care. Lancet. 2001;358(9281):571–574. doi: 10.1016/S0140-6736(01)05709-9. [DOI] [PubMed] [Google Scholar]
- 11.Plante DA, Kassirer JP, Zarin DA, Pauker SG. Clinical decision consultation service. Am J Med. 1986;80:1169–1176. doi: 10.1016/0002-9343(86)90680-7. [DOI] [PubMed] [Google Scholar]
- 12.Pell I, Dowie J, Clarke A, Kennedy A, Bhavnani V. Development and preliminary evaluation of a clinical guidance programme for the decision about prophylactic oophorectomy in women undergoing a hysterectomy. Qual Saf Health Care. 2002;11(1):32–38. doi: 10.1136/qhc.11.1.32. discussion 38-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson A, Thomson RG. The potential use of decision analysis to support shared decision making in the face of uncertainty: the example of atrial fibrillation and warfarin anticoagulation. Qual Health Care. 2000;9(4):238–244. doi: 10.1136/qhc.9.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Short D, Frischer M, Bashford J. The development and evaluation of a computerised decision support system for primary care based upon ‘patient profile decision analysis’. Inform Prim Care. 2003;11(4):195–202. doi: 10.14236/jhi.v11i4.567. [DOI] [PubMed] [Google Scholar]
- 15.Brothers TE, Robison JG, Elliott BM. Prospective decision analysis for peripheral vascular disease predicts future quality of life. J Vasc Surg. 2007;46(4):701–708. doi: 10.1016/j.jvs.2007.05.045. discussion 708. [DOI] [PubMed] [Google Scholar]
- 16.Dowie J, Wildman M. Choosing the surgical mortality threshold for high risk patients with stage Ia non-small cell lung cancer: insights from decision analysis. Thorax. 2002;57(1):7–10. doi: 10.1136/thorax.57.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery AA, Harding J, Fahey T. Shared decision making in hypertension: the impact of patient preferences on treatment choice. Fam Pract. 2001;18(3):309–313. doi: 10.1093/fampra/18.3.309. [DOI] [PubMed] [Google Scholar]
- 18.14th Annual Meeting of the International Society for Technology Assessment in Health Care. Ottawa, Ontario, Canada: 1998. A randomized trial of an audiobooklet decision aid in patients with atrial fibrillation. [Google Scholar]
- 19.Man-Son-Hing M, O’Connor AM, Drake E, Biggs J, Hum V, Laupacis A. The effect of qualitative vs quantitative presentation of probability estimates on patient decision-making: a randomized trial. Health Expect. 2002;5(3):246–255. doi: 10.1046/j.1369-6513.2002.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Protheroe J, Fahey T, Montgomery AA, Peters TJ. Effects of patients’ preferences on the treatment of atrial fibrillation: observational study of patient-based decision analysis. West J Med. 2001;174(5):311–315. doi: 10.1136/ewjm.174.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson A, Thomson R, Parkin D, Sudlow M, Eccles M. How patients with atrial fibrillation value different health outcomes: a standard gamble study. J Health Serv Res Policy. 2001;6(2):92–98. doi: 10.1258/1355819011927288. [DOI] [PubMed] [Google Scholar]
- 22.Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual Saf Health Care. 2007;16(3):216–223. doi: 10.1136/qshc.2006.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Weijden T, Legare F, Boivin A, Burgers JS, van Veenendaal H, Stiggelbout AM, et al. How to integrate individual patient values and preferences in clinical practice guidelines? A research protocol. Implement Sci. 2010;5:10. doi: 10.1186/1748-5908-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Weijden T, Pieterse AH, Koelewijn-van Loon MS, Knaapen L, Legare F, Boivin A, et al. How can clinical practice guidelines be adapted to facilitate shared decision making? A qualitative key-informant study. BMJ Qual Saf. 2013;22(10):855–863. doi: 10.1136/bmjqs-2012-001502. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Healthcare Excellence. Atrial fibrillation: the management of atrial fibrillation. 2014 [Google Scholar]
- 26.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Walraven C, Hart RG, Connolly S, Austin PC, Mant J, Hobbs FD, et al. Effect of Age on Stroke Prevention Therapy in Patients With Atrial Fibrillation. The Atrial Fibrillation Investigators. Stroke. 2009 doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 28.Sherman DG, Kim SG, Boop BS, Corley SD, Dimarco JP, Hart RG, et al. Occurrence and characteristics of stroke events in the Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) study. Arch Intern Med. 2005;165(10):1185–1191. doi: 10.1001/archinte.165.10.1185. [DOI] [PubMed] [Google Scholar]
- 29.Penado S, Cano M, Acha O, Hernandez JL, Riancho JA. Stroke severity in patients with atrial fibrillation. Am J Med. 2002;112(7):572–574. doi: 10.1016/s0002-9343(02)01063-x. [DOI] [PubMed] [Google Scholar]
- 30.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 31.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 32.Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39(2):69–72. doi: 10.1001/archneur.1982.00510140003001. [DOI] [PubMed] [Google Scholar]
- 33.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomernacki NK, Udaltsova N, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang MC, Go AS, Hylek EM, Chang Y, Henault LE, Jensvold NG, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54(8):1231–1236. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36(8):1801–1807. doi: 10.1161/01.STR.0000174189.81153.85. [DOI] [PubMed] [Google Scholar]
- 36.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280(22):1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 37.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288(19):2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 38.Wintzen AR, de Jonge H, Loeliger EA, Bots GT. The risk of intracerebral hemorrhage during oral anticoagulant treatment: a population study. Ann Neurol. 1984;16(5):553–558. doi: 10.1002/ana.410160505. [DOI] [PubMed] [Google Scholar]
- 39.Hart RG, Boop BS, Anderson DC Oral anticoagulants intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26(8):1471–1477. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 40.Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch Intern Med. 1996;156(4):409–416. [PubMed] [Google Scholar]
- 41.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164(8):880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 43.Fang MC, Go AS, Chang Y, Hylek EM, Henault LE, Jensvold NG, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120(8):700–705. doi: 10.1016/j.amjmed.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156(16):1829–1836. [PubMed] [Google Scholar]
- 46.Lai SM, Alter M, Friday G, Sobel E. Prognosis for survival after an initial stroke. Stroke. 1995;26(11):2011–2015. doi: 10.1161/01.str.26.11.2011. [DOI] [PubMed] [Google Scholar]
- 47.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 48.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2011;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 49.Elwyn G, Kreuwel I, Durand MA, Sivell S, Joseph-Williams N, Evans R, et al. How to develop web-based decision support interventions for patients: a process map. Patient Educ Couns. 2010;82(2):260–265. doi: 10.1016/j.pec.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 50.Martin AJ, Glasziou PP, Simes RJ, Lumley T. A comparison of standard gamble, time trade-off, and adjusted time trade-off scores. Int J Technol Assess Health Care. 2000;16(1):137–147. doi: 10.1017/s0266462300161124. [DOI] [PubMed] [Google Scholar]
- 51.Gambles Littenberg B. In: Encyclopedia of Medical Decision Making. Kattan M, editor. Los Angeles: Sage Reference Publications; 2009. pp. 527–529. [Google Scholar]
- 52.Murtagh MJ, Thomson RG, May CR, Rapley T, Heaven BR, Graham RH, et al. Qualitative methods in a randomised controlled trial: the role of an integrated qualitative process evaluation in providing evidence to discontinue the intervention in one arm of a trial of a decision support tool. Qual Saf Health Care. 2007;16(3):224–229. doi: 10.1136/qshc.2006.018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D’Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort prospective collaborative study (ISCOAT)Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348(9025):423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 54.Green C, Brazier J, Deverill M. Valuing health-related quality of life A review of health state valuation techniques. Pharmacoeconomics. 2000;17(2):151–165. doi: 10.2165/00019053-200017020-00004. [DOI] [PubMed] [Google Scholar]
- 55.Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ. 1986;5(1):1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 56.Jansen SJ, Stiggelbout AM, Wakker PP, Vliet Vlieland TP, Leer JW, Nooy MA, et al. Patients’ utilities for cancer treatments: a study of the chained procedure for the standard gamble and time tradeoff. Med Decis Making. 1998;18(4):391–399. doi: 10.1177/0272989X9801800406. [DOI] [PubMed] [Google Scholar]
- 57.Lalonde L, Clarke AE, Joseph L, Grover SA Conventional chained standard gambles in the assessment of coronary heart disease prevention and treatment. Canadian Collaborative Cardiac Assessment Group. Med Decis Making. 1999;19(2):149–156. doi: 10.1177/0272989X9901900205. [DOI] [PubMed] [Google Scholar]
- 58.Wright DR, Wittenberg E, Swan JS, Miksad RA, Prosser LA. Methods for measuring temporary health States for cost-utility analyses. Pharmacoeconomics. 2009;27(9):713–723. doi: 10.2165/11317060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 59.Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. Bmj. 2001;323(7323):1218–1222. doi: 10.1136/bmj.323.7323.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzales E, Eckman M, Pauker S“Gambler”. A computer workstation for patient utility assessment. Med Decis Making. 1992;12:350. [Google Scholar]
- 61.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 62.Monette J, Gurwitz JH, Rochon PA, Avorn J. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: results of a survey of long-term care practitioners [see comments] J Am Geriatr Soc. 1997;45(9):1060–1065. doi: 10.1111/j.1532-5415.1997.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 63.Lip GY, Zarifis J, Watson RD, Beevers DG. Physician variation in the management of patients with atrial fibrillation. Heart. 1996;75(2):200–205. doi: 10.1136/hrt.75.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.American College of Chest Physicians Foundation. Afib - What You and Your Family Should Know: American College of Chest Physicians Foundation. 2011 [Google Scholar]
- 65.Alberts MJ. Assessing Stroke and Bleeding Risk in Atrial Fibrillation - consensus statement on appropriate anticoagulant use: The Alliance for Aging Research. 2012 [Google Scholar]
- 66.H.Res. In: 295 (112th): Promoting increased awareness, diagnosis, and treatment of atrial fibrillation. Representatives Ho., editor. Washington, DC: 2011. [Google Scholar]
- 67.Man-Son-Hing M, Gage BF, Montgomery AA, Howitt A, Thomson R, Devereaux PJ, et al. Preference-based antithrombotic therapy in atrial fibrillation: implications for clinical decision making. Med Decis Making. 2005;25(5):548–559. doi: 10.1177/0272989X05280558. [DOI] [PubMed] [Google Scholar]
- 68.Man-Son-Hing M, Laupacis A, O’Connor AM, Biggs J, Drake E, Yetisir E, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. Jama. 1999;282(8):737–743. doi: 10.1001/jama.282.8.737. [DOI] [PubMed] [Google Scholar]
- 69.Fraenkel L, Street RL, Jr, Fried TR. Development of a tool to improve the quality of decision making in atrial fibrillation. BMC Med Inform Decis Mak. 2012;11:59. doi: 10.1186/1472-6947-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howitt A, Armstrong D. Implementing evidence based medicine in general practice: audit and qualitative study of antithrombotic treatment for atrial fibrillation. Bmj. 1999;318(7194):1324–1327. doi: 10.1136/bmj.318.7194.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Protheroe J, Fahey T, Montgomery AA, Peters TJ. The impact of patients’ preferences on the treatment of atrial fibrillation: observational study of patient based decision analysis. Bmj. 2000;320(7246):1380–1384. doi: 10.1136/bmj.320.7246.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomson R, Parkin D, Eccles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet. 2000;355:956–962. doi: 10.1016/S0140-6736(00)90012-6. [DOI] [PubMed] [Google Scholar]
- 73.Thomson R, Robinson A, Greenaway J, Lowe P. Development and description of a decision analysis based decision support tool for stroke prevention in atrial fibrillation. Qual Health Care. 2002;11(1):25–31. doi: 10.1136/qhc.11.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 75.Rimer BK, Halabi S, Sugg Skinner C, Kaplan EB, Crawford Y, Samsa GP, et al. The short-term impact of tailored mammography decision-making interventions. Patient Educ Couns. 2001;43(3):269–285. doi: 10.1016/s0738-3991(00)00172-5. [DOI] [PubMed] [Google Scholar]
- 76.O’Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas H, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. Bmj. 1999;319(7212):731–734. doi: 10.1136/bmj.319.7212.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ende J, Kazis L, Ash A, Moskowitz MA. Measuring patients’ desire for autonomy: decision making and information-seeking preferences among medical patients. J Gen Intern Med. 1989;4(1):23–30. doi: 10.1007/BF02596485. [DOI] [PubMed] [Google Scholar]
- 78.Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making. 1996;16(1):58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 79.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. Jama. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 80.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the Tipping Point: The Decision to Anticoagulate Patients With Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2011;(4):14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 82.Grossman EG. PATIENT PROTECTION AND AFFORDABLE CARE ACT HEALTH-RELATED PORTIONS OF THE HEALTH CARE AND EDUCATION RECONCILIATION ACT OF 2010. Washington, DC: 2010. [Google Scholar]
- 83.Centers for Medicare and Medicaid Services. Medicare Shared Savings Program: accountable care organizations. 2011:67802–67990. [PubMed] [Google Scholar]
- 84.Elwyn G, O’Connor AM, Bennett C, Newcombe RG, Politi M, Durand MA, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi) PLoS One. 2009;4(3):e4705. doi: 10.1371/journal.pone.0004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.