Graphical abstract
Keywords: Plasmodium falciparum, Mefloquine, GeLC–MS/MS label-free quantification, Proteomics, Pgh1
Highlights
-
•
We studied protein expression in mefloquine-sensitive and -resistant P. falciparum.
-
•
Congenic mefloquine-sensitive and -resistant Thai TM036 lines were used.
-
•
The protein profiles of both lines were determined by GeLC–MS/MS-based proteomics.
-
•
The mefloquine-treated resistant line had 36 up- and 34 down-regulated proteins.
-
•
The functions of the proteins identified herein warrant further investigation.
Abstract
Malaria is a mosquito borne infectious disease caused by protozoa of genus Plasmodium. There are five species of Plasmodium that are found to infect humans. Plasmodium falciparum can cause severe malaria leading to higher morbidity and mortality of malaria than the other four species. Antimalarial resistance is the major obstacle to control malaria. Mefloquine was used in combination with Artesunate for uncomplicated P. falciparum in South East Asia and it has developed and established mefloquine resistance in this region. Here, gel-enhanced liquid chromatography/tandem mass spectrometry (GeLC–MS/MS)-based proteomics and label-free quantification were used to explore the protein profiles of mefloquine-sensitive and -induced resistant P. falciparum. A Thai P. falciparum isolate (S066) was used as a model in this research. Our data revealed for the first time that 69 proteins exhibited at least 2-fold differences in their expression levels between the two parasite lines. Of these, 36 were up-regulated and 33 were down-regulated in the mefloquine-resistant line compared with the mefloquine-sensitive line. These findings are consistent with those of past studies, where the multidrug resistance protein Pgh1 showed an up-regulation pattern consistent with that expected from its average 3-copy pfmdr1 gene number. Pgh1 and eight other up-regulated proteins (i.e., histo-aspartyl protease protein, exportin 1, eukaryotic translation initiation factor 3 subunit 8, peptidyl-prolyl cis-trans isomerase, serine rich protein homologue, exported protein 1, ATP synthase beta chain and phospholipid scramblase 1) were further validated for their expression levels using reverse transcriptase quantitative real-time PCR. The data support the up-regulation status in the mefloquine-resistant parasite line of all the candidate genes referred to above. Therefore, GeLC–MS/MS-based proteomics combined with label-free quantification is a reliable approach for exploring mefloquine resistance biomarkers in P. falciparum. Identification of these proteins leads to better understanding of mefloquine resistant mechanisms in malaria parasites.
1. Introduction
Malaria is a life-threatening disease caused by protozoa called Plasmodium. The Plasmodium transmits to human by the infected female Anopheles mosquitoes. According to WHO report in 2012, malaria infects 207 million people each year around the world, causing more than 627,000 deaths, especially in children under five years old in sub-Saharan Africa [1]. Despite the numerous published studies that have reported effective approaches for malaria diagnosis and treatment, parasite resistance to multiple drugs has ensured that malaria remains a global health problem. Research has shown that most drug-resistant Plasmodium falciparum parasites originated in Southeast Asia [2]. Mefloquine was introduced as a first-line malaria treatment in Thailand in 1984. Unfortunately, mefloquine-resistant parasites developed within 6 years of its use. The combination therapy of artemisinin derivatives and mefloquine has been recommended to improve the efficacy of anti-malarial treatment in South East Asia [3], [4]. Although this drug combination has proved to be highly effective for malaria treatment, the molecular mechanisms of mefloquine resistance in malaria parasites remain unclear.
A point mutation in the P-glycoprotein gene homologue (pfmdr1) at codon 86 (Asn to Tyr) was reported to effect chloroquine and mefloquine susceptibility in P. falciparum in vitro [5], [6]. However, amplification and overexpression of pfmdr1 provided stronger evidence regarding the association with mefloquine resistance from both in vitro culture and parasites from patients [7], [8]. Pfmdr1 is a homologue of the human P-glycoprotein, an adenosine triphosphate (ATP)-Binding Cassette (ABC) transporter family member. ABC transporters are transmembrane proteins that translocate compounds across membranes using the energy from ATP. An increased mefloquine efflux was observed in mefloquine-resistant P. falciparum resulting in alteration of the mefloquine accumulated in the parasite cell [9]. Nonylphenol ethoxylate (NP30) is a potential P. falciparum P-glycoprotein substrate and drug efflux inhibitor. According to in vitro assay results, NP30 was able to sensitize mefloquine resistance in more than 80% of the P. falciparum parasites that were tested. This finding refers to the mefloquine efflux activity related to P-glycoprotein [10]. Several studies suggested that the increase in pfmdr1 copy number is associated with mefloquine resistance. Moreover, the evidences in vitro and in vivo have shown that the pfmdr1 gene amplification has a major role in the development of mefloquine resistance [8], [11], [12]. pfmdr1 gene amplification is better predictor for mefloquine resistance than pfmdr1 polymorphisms. Therefore, the mechanisms of mefloquine resistance could be multifactorial traits that act together and result in expression of the resistant phenotype. However, only 58% in vitro and 63% in vivo of mefloquine resistant P. falciparum showed the association with the increase of gene copy number [8]. Therefore, there are other factors involved in mechanisms of mefloquine resistance.
Accordingly, this study attempted to explore other molecular markers of mefloquine resistance using mass spectrometry-based proteomics, with the aim of obtaining a better understanding of mefloquine resistant mechanisms in P. falciparum parasites. To accomplish this aim, the Thai P. falciparum isolate S066 was used as a model parasite for this research. In 2009, the resistant line was developed by exposure of the culture parasites to stepwise increasing in mefloquine concentration. The sensitive line was also maintained in continuous culture in the same condition with resistant line. Genotyping with available genetic markers including msp1, msp2, glurp, and ten microsatellite markers revealed that these two lines contained the same single genotype. This culture-adapted mefloquine resistant P. falciparum line has an amplified pfmdr1 locus with an average 3 copies of pfmdr1 [11]. The above study [11] was based on mefloquine-sensitive and -resistant P. falciparum that originated from the same strain, meaning there should be minimal background genetic variation between these two lines. Recently, several techniques have become available for performing proteomic experiments. Gel-enhanced liquid chromatography/tandem mass spectrometry (GeLC–MS/MS) is an uncomplicated and powerful proteomic technique used for studying biological samples. In GeLC–MS/MS, a protein lysate from a biological sample is separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and each gel lane is sliced into small pieces, in-gel digestion is performed, and the treated sample is analyzed by liquid chromatography (LC–MS/MS). The database search results for all the slices are combined yielding global protein identification and quantification. GeLC–MS/MS can be used in combination with a label-free approach to obtain protein quantification information. The label-free method involves straightforward sample preparation with no extra costs and the technique is not time consuming. Additionally, label-free methods can be multiplexed to a higher degree than unlabeled methods, and can even be used for data that has already been acquired. The exponentially modified protein abundance index (emPAI) approach is a spectral counting label-free quantification approach. It was developed by Ishihama and co-workers [13], [14] to measure the protein composition of sample solutions. Label-free emPAI quantitative proteomics has been proved successful for quantification of Aspergillus fumigatus secretomes at different temperatures [15], and quantification of mannose-binding proteins from normal donor and hepatocellular carcinoma patient sera [16]. Hence, the goal of this research was to conduct a comparative proteomic analysis of mefloquine-sensitive and -resistant P. falciparum parasites to gain better insight into mefloquine resistant mechanisms using the GeLC–MS/MS approach combined with emPAI label-free protein quantitation.
2. Materials and methods
2.1. P. falciparum culture
Mefloquine-sensitive or -resistant P. falciparum isolate S066 parasites were grown at 37 °C in O+ erythrocytes washed in RPMI 1640 medium (Invitrogen, MA, USA). Media were supplemented with 0.5% Albumax II (Gibco, New Zealand), 5 μg/mL of hypoxanthine (Sigma, USA) and 50 μg/mL of gentamicin sulphate (Government Pharmaceutical Organization, Thailand). Parasites were continuously cultured in 5% CO2 to obtain a high proportion of ring-forms, after which the medium was discarded. Infected red blood cells were resuspended in 5% sorbitol solution (Sigma, USA) and incubated for 20 min at 37 °C. The cells were then washed in RPMI medium and further cultured. The sorbitol synchronization was repeated again after 32 h to ensure a high level of synchrony in the culture. Parasitaemias and culture progression were monitored every 24 h by Giemsa staining (Sigma, USA). When the percentage parasitaemia reached 5% and schizont-stage parasites were present, the culture was harvested.
2.2. Parasite protein preparation
To reduce contamination from red blood cell proteins, P. falciparum parasites were released from infected erythrocytes by lysis in 0.05% saponin (Sigma, USA) in phosphate-buffered saline (Sigma, USA) for 5 min at 4 °C, followed by centrifugation at 1000 × g at 4 °C for 5 min. Parasite pellets were washed with PBS three times. Pellets were snap-frozen in liquid nitrogen and stored at −80 °C until use. Each parasite pellet was resuspended in 100 μL lysis buffer (1% SDS, 1% Triton X, 0.5% NaCl), a protease cocktail inhibitor (Sigma, USA) was added, and the preparation was sonicated on ice with four 10-s pulses (Cole-Parmer, IL, USA). The protein concentration of the lysate was 14 μg/μL using the Bradford protein assay with BSA as a standard (Thermo, USA).
2.3. SDS-PAGE
The 30 μL of protein from the mefloquine-sensitive and -resistant P. falciparum lines was denatured by heating for 5 min at 95 °C in sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol and bromophenol blue) (GE Healthcare, UK). Both of the denatured protein samples were loaded onto 10% SDS-polyacrylamide gels. A constant electric current was applied to separate the proteins until the blue dye front reached the bottom of the gel. To detect the protein bands, the SDS-polyacrylamide gels were stained with Coomassie blue G-250 (Biorad, CA, USA). After that, the gel was de-stained in 45% methanol and 10% acetic acid in deionized water, until clear bands were visible. Each gel lane was trimmed into 28 pieces and kept at −20 °C until use.
2.4. In-gel digestion
Gel pieces were de-stained prior to tryptic digestion. Acetonitrile (50%) was added to the gel followed by incubation for 15 min. The colourless gel pieces were then dehydrated by acetonitrile and allowed to dry completely in a fume hood. To rehydrate the gel pieces, trypsin solution at a concentration of 0.02 mg/mL in 50 mM ammonium bicarbonate (Sigma, USA) was added to the tubes and each gel piece was incubated at 37 °C overnight. Peptides were extracted by addition of acetonitrile and the solution was shaken for 15 min. The supernatant was collected and the peptide mixtures were completely dried by a speed-vac (Eppendorf, Hamburg, Germany). The samples were stored at −20 °C prior to mass spectrometric analysis.
2.5. NanoLC–MS/MS analysis
Each tryptic-digested fraction was resuspended in 0.1% formic acid containing 2% acetonitrile and then introduced to an UltiMate 3000 nano-LC system (Dionex, Surrey, UK) coupled with a micrOTOF-Q (Bruker Daltonics, Bremen, Germany). Separation was done on a 58 min gradient, with a flow rate of 200 nL/min. Mobile phase A was 2% acetonitrile and 0.1% formic acid in HPLC grade water and mobile phase B was 0.1% formic acid in HPLC grade acetonitrile. Data acquisitions were controlled using Hystar software (Bruker Daltonics, Bremen, Germany). MS and MS/MS spectra covered the mass range of m/z 400–2000 and m/z 50–1500, respectively.
2.6. Data analysis
LC–MS/MS data files were converted to a mascot generic file (.mgf) format using DataAnalysis™ software, version 3.4. The .mgf files were merged using Mascot daemon 2.4 software and searched using Mascot version 2.4.1 (Matrix Science, London, UK) against the NCBInr database (16 April 2015), which contained 66,387,522 sequences entries. P. falciparum was set as the taxonomy filter. Missed cleavage was set to 1, the peptide tolerance was set to 1.2 Da, and the tandem MS tolerance was set to 0.6 Da. Variable modifications were set to include methionine oxidation and cysteine carbamidomethylation. We searched each identified peptide against the NCBI database. Quantification was performed using emPAI provided by Mascot [13]. The emPAI values in this report were the mean of three biological replications. Hits with a minimum of at least two peptides and a minimum mascot ion score of 20 were chosen as true identification for further analysis. PANTHER software was used to classify the proteins identified according to gene ontology [17].
2.7. RNA extraction and cDNA synthesis
Total RNA was isolated from mefloquine-sensitive and -resistant P. falciparum using TRI-reagent (Ambion Inc., Austin, TX, USA), following the manufacturer's protocol. Briefly, after red blood cell release, parasite pellets were suspended in 1 mL of TRI-reagent and then incubated at room temperature for 15 min. Nucleic acids were recovered from the lysates by adding 200 μL of chloroform (Sigma, MO, USA) followed by centrifugation at 12,000 × g for 15 min at 4 °C. The aqueous layer was transferred to a clean microcentrifuge tube and isopropanol was added to precipitate the nucleic acids. The pellet was collected by centrifugation (12,000 × g, 10 min, 4 °C), washed with 75% ethanol, air dried, and resuspended in 50 μL of RNase-free water. To remove contaminating DNA, DNase I (Qiagen, Netherlands) was added to the RNA solution and it was incubated at 37 °C for 1 h. Addition of 50 mM of EDTA, followed by incubation at 65 °C for 10 min was done to inactivate DNase I. The 260/280 nm absorbance ratio was used to assess the purity of the DNA and RNA using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Thermo Fischer Scientific Inc., USA) was used for cDNA synthesis. RNA from the mefloquine-sensitive and -resistant P. falciparum, an oligo(dT)18 primer, dNTPs, 10× first strand buffer and RevertAid™ Reverse Transcriptase were mixed and incubated at 42 °C for 1 h. For inactivation, the reaction was heated to 70 °C for 5 min. The cDNA was kept at −20 °C until use.
2.8. Quantitative real-time PCR (qPCR)
A SYBR-Green fluorescence-based qPCR assay was used to determine the expression levels of nine candidate markers in the mefloquine-sensitive and -resistant P. falciparum lines using an ABI Prism 7500 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). The relevant mRNA sequences were retrieved from the NCBI database. Gene-specific primers were designed using Primer Express software (Applied Biosystems). The following primers were synthesized for the nine candidate markers: multidrug resistance protein Pgh1, histo-aspartyl protease (HAP) protein, exportin 1, eukaryotic translation initiation factor 3 subunit 8, peptidyl-prolyl cis-trans isomerase, serine rich protein (SERP) homologue, exported protein 1 (Exp-1), ATP synthase beta chain and phospholipid scramblase 1. For the real-time qPCR assays, 5 μL of 2× Power SYBR® Green PCR Master Mix (Applied Biosystems), 10 nM of each primer, and 0.5 μL of cDNA were prepared to achieve a 10 μL final reaction volume. The PCR conditions are shown in Supplemental Table 4. In every experiment, the beta-tubulin reference gene was also amplified. The average CT value was calculated. Relative quantification analysis used the comparative CT (2−ΔΔCT) method [18] to determine the mRNA expression level. The qRT-PCR analyses comprised three separate biological replicates.
The expression levels of the nine candidate markers were examined by culturing the mefloquine-sensitive and -resistant P. falciparum under a sublethal mefloquine dose. During in vitro culture of P. falciparum, a mefloquine concentration at a sublethal dose (IC5) was added to the culture media. When the parasitaemia reached 5% and schizont-stage parasites were present, the culture was harvested. RNA extraction, cDNA synthesis, and reverse transcriptase qPCR were performed as described above.
3. Results and discussion
3.1. Proteomic profiling of mefloquine-sensitive and -resistant P. falciparum using GeLC–MS/MS
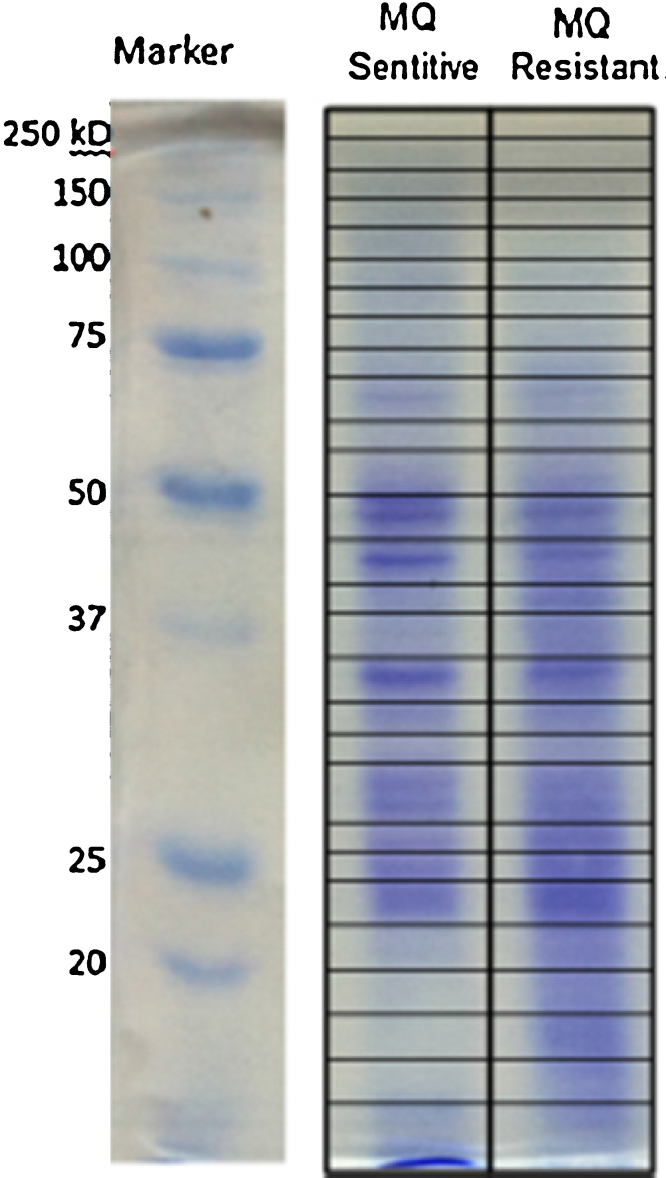
According to in vitro culture of malaria parasite, mefloquine-resistant P. falciparum exhibited delay of stage development compared to sensitive parasite. The resistant line took approximately 3 h slower than the sensitive line to complete erythrocytic cycle. The overall parasite morphology including amount of hemozoin in those two lines was not significant difference. Process of freezing and thawing from liquid nitrogen were not affect to the resistant characteristic of the mefloquine-resistant P. falciparum because IC50 of the resistant line was remained 10-fold higher than the sensitive line and the resistant line still carried 3 copies of pfmdr1 gene. The schizont proteomes of the mefloquine-sensitive and -resistant P. falciparum were separated by SDS-PAGE. A Coomassie blue stained gel of such a preparation is shown in Fig. 1.
Fig. 1.
Coomassie blue stained gels of mefloquine-sensitive and mefloquine-resistant P. falciparum proteins separated by SDS-PAGE.
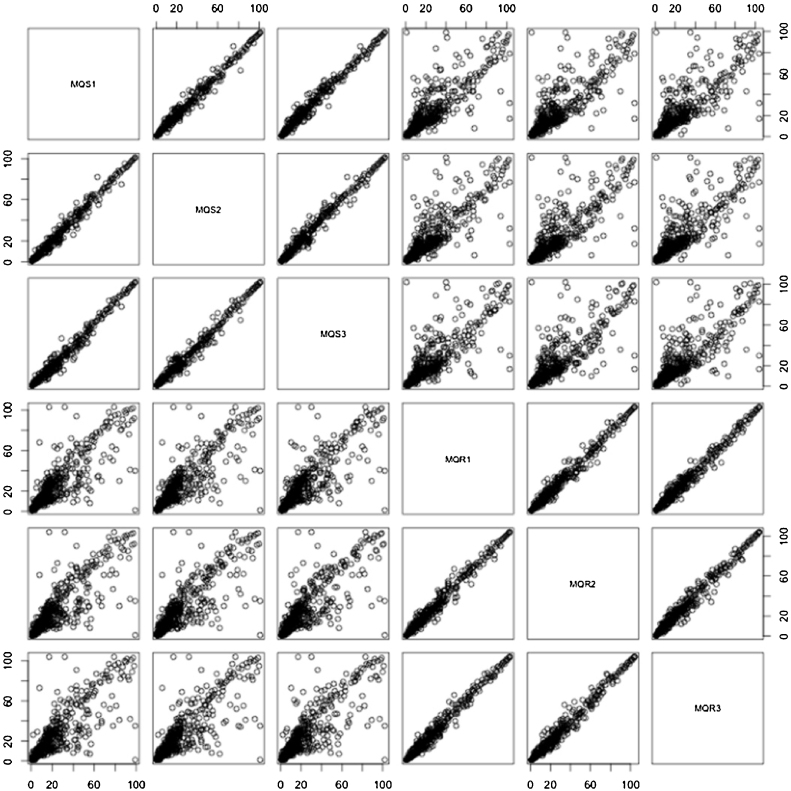
Each lane from a gel was cut into 28 pieces and each piece was subject to mass spectrometry. SDS-PAGE of three biological replicates is presented in Supplemental Figure 1. Protein identification of three biological replicates is presented in Supplemental Table 1. Total 469 proteins were similarly found in all three biological replicates. The emPAI values were applied for protein quantification. A matrix plot of emPAI values of three biological replicates is demonstrated in Fig. 2.
Fig. 2.
A matrix plot of emPAI values of three biological replicates. MQS1, MQS2, MQS3, MQR1, MQR2 and MQR3 are mefloquine-sensitive P. falciparum 1, mefloquine-sensitive P. falciparum 2, mefloquine-sensitive P. falciparum 3, mefloquine-resistant P. falciparum 1, mefloquine-resistant P. falciparum 2 and mefloquine-resistant P. falciparum 3, respectively.
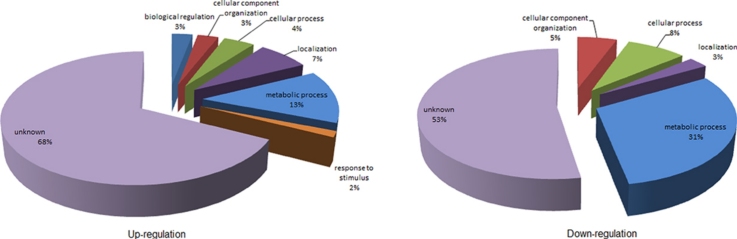
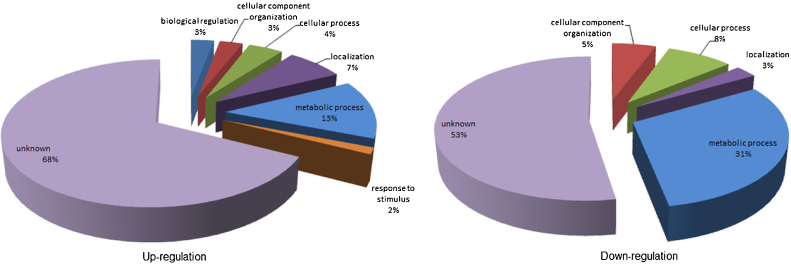
Based on the matrix plot, all three biological replicates of mefloquine-sensitive P. falciparum (MQS1, MQS2 and MQS3) and mefloquine-resistant P. falciparum (MQR1, MQR2 and MQR3) demonstrated the correlation among their groups. When comparing emPAI between mefloquine-sensitive and mefloquine-resistant P. falciparum, there was deviation of emPAI value from the linearity corresponded to the differences of protein expression between both parasites. Protein quantification and Mascot information of three biological replicates are shown in Supplemental Tables 2 and 3, respectively. Due to protein quantification, a total of 400 proteins (86%) were common to both mefloquine-sensitive and -resistant P. falciparum. The common proteins identified between the mefloquine-sensitive and -resistant P. falciparum were mostly housekeeping proteins (e.g., transcription factors, histones and cytoskeletal proteins). Nevertheless, 69 proteins had at least 2-fold differences in their expression levels according to the emPAI values and 36 proteins were up-regulated and 33 were down-regulated in the mefloquine-resistant parasites compared to their mefloquine-sensitive counterparts. Gene ontology cluster analysis in the PANTHER database was used to examine the 36 up-regulated and 33 down-regulated proteins according to their biological processes (Fig. 3).
Fig. 3.
Classification of differentially expressed proteins according to their biological processes. Left pie chart represents the gene ontology categories for genes up-regulated in mefloquine-resistant P. falciparum. Right pie chart represents the gene ontology categories for genes down-regulated in mefloquine-resistant P. falciparum.
The up-regulated proteins were classified into the following seven groups: 3% biological regulation, 3% cellular component organization, 4% cellular process, 7% localization, 13% metabolic process, 2% in response to stimulus, and 68% were of unknown function. When considering the “in response to stimulus” group proteins, mefloquine is potentially the stimulating factor in the culture-adapted mefloquine-resistant parasites because such parasites possibly respond to the stress conditions generated by mefloquine. In the mefloquine-resistant strain, proteins in the “biological regulation” group were also up-regulated. Alterations in biological regulation processes may be important for modulating the resistance phenotype of the malaria parasite. In contrast, classification of the down-regulated proteins sorted them into the five following groups: 5% cellular component organization, 8% cellular process, 3% localization, 31% metabolic process, and 53% were of unknown function. The “metabolic process” and “cellular process” group proteins are involved in resource management and general functions of the parasite. Both groups had lower expression levels in mefloquine-resistant P. falciparum. Interestingly, examination of the in vitro culture showed that the mefloquine-resistant parasite line developed blood stages slower than the mefloquine-sensitive line. This finding might result from down-regulation of metabolic and cellular processes. Moreover, down-regulation of these general processes is consistent with the many scientific publications on the reduced fitness of drug-resistant malaria parasites. Drug-resistant parasite genotypes are likely to be less fit than their wild-type counterparts (or congenic lines) and lower fitness puts such parasites at a survival disadvantage [19], [20], [21] under conditions of reduced drug pressure. The lower fitness of drug-resistant parasites probably results from the reduced activities of general cellular processes. Parasite proteins with unknown functions were the major group in both the up-regulated and down-regulated proteins.
Data for the up-regulated and down-regulated proteins from mefloquine-resistant P. falciparum are provided in Table 1, Table 2. Based on the up-regulated proteins illustrated in Table 1, the multidrug resistance protein Pgh1 is up-regulated 8.5-fold; this result is consistent with the existence of three copies of the pfmdr1 gene in the mefloquine-resistant parasite line.
Table 1.
Up-regulated proteins in mefloquine-sensitive and mefloquine-resistant P. falciparum.
| Accession | Protein | Mass | pI | emPAI value |
Fold change | |
|---|---|---|---|---|---|---|
| MQ sensitive | MQ resistant | |||||
| gi|124513520 | Protein kinase | 111,269 | 5.98 | 0.24 ± 0.02 | 0.86 ± 0.15 | 3.58 |
| gi|3023709 | Enolase | 48,673 | 6.21 | 0.08 ± 0.02 | 0.81 ± 0.10 | 10.13 |
| gi|8050813 | Multidrug resistance protein Pgh1 | 161,695 | 8.94 | 0.02 ± 0.00 | 0.17 ± 0.08 | 8.50 |
| gi|124802886 | tRNA pseudouridine synthase D | 116,647 | 5.96 | 0.42 ± 0.04 | 1.75 ± 0.23 | 4.17 |
| gi|385236 | Rhoptry protein | 104,789 | 6.25 | 0.11 ± 0.05 | 0.27 ± 0.09 | 2.45 |
| gi|4699811 | Chain A, chloroquine binds in the cofactor binding site of Plasmodium falciparum lactate dehydrogenase | 34,102 | 7.12 | NDa | 0.37 ± 0.04 | NDa |
| gi|237665424 | Rhoptry-associated protein 2 | 46,709 | 8.90 | 0.12 ± 0.05 | 0.26 ± 0.05 | 2.17 |
| gi|37777722 | Exported protein 1 | 13,399 | 9.66 | 0.24 ± 0.09 | 0.68 ± 0.13 | 2.83 |
| gi|86171188 | Histone h2a | 14,114 | 10.29 | 1.09 ± 0.11 | 2.41 ± 0.27 | 2.21 |
| gi|1172530 | Plasmepsin-2 | 51,457 | 5.42 | 0.09 ± 0.02 | 0.23 ± 0.07 | 2.56 |
| gi|124513738 | Conserved Plasmodium protein, unknown function | 186,204 | 6.29 | 0.04 ± 0.01 | 0.18 ± 0.06 | 4.50 |
| gi|124808181 | HAP protein | 51,661 | 8.04 | 0.23 ± 0.06 | 0.52 ± 0.11 | 2.26 |
| gi|124806537 | ATP synthase beta chain, mitochondrial precursor | 58,357 | 6.01 | 0.06 ± 0.02 | 0.13 ± 0.05 | 2.17 |
| gi|124504723 | Exportin 1 | 147,843 | 5.69 | 0.20 ± 0.04 | 0.73 ± 0.07 | 3.65 |
| gi|86170934 | Plasmodium falciparum membrane protein pf12 precursor | 39,409 | 8.69 | 0.10 ± 0.03 | 0.20 ± 0.02 | 2.00 |
| gi|124802544 | Phospholipid scramblase 1 | 32,997 | 8.10 | 0.05 ± 0.01 | 0.24 ± 0.09 | 2.80 |
| gi|124512958 | Hypothetical protein | 16,515 | 4.08 | 0.23 ± 0.08 | 0.52 ± 0.13 | 2.26 |
| gi|124806675 | Conserved Plasmodium protein | 690,984 | 5.59 | NDa | 0.01 ± 0.00 | NDa |
| gi|1262910 | Cytidine triphosphate synthetase | 98,786 | 6.22 | 0.01 ± 0.00 | 0.04 ± 0.01 | 4.00 |
| gi|124805548 | Eukaryotic translation initiation factor 3 subunit 8 | 115,941 | 5.26 | 0.03 ± 0.00 | 0.08 ± 0.03 | 2.67 |
| gi|124803860 | Peptidyl-prolyl cis-trans isomerase | 21,717 | 7.10 | 0.38 ± 0.02 | 0.91 ± 0.05 | 2.39 |
| gi|237664980 | Pf38-like hypothetical protein | 35,052 | 8.18 | 0.05 ± 0.01 | 0.11 ± 0.00 | 2.20 |
| gi|124808108 | Conserved Plasmodium protein, unknown function | 338,172 | 5.10 | 0.01 ± 0.00 | 0.06 ± 0.02 | 6.00 |
| gi|296005506 | Hypothetical protein | 696,344 | 9.21 | 0.01 ± 0.00 | 0.03 ± 0.00 | 3.00 |
| gi|124512392 | Hypothetical protein | 32,145 | 5.22 | 0.12 ± 0.00 | 0.25 ± 0.05 | 2.08 |
| gi|124506149 | RNA pseudouridylate synthase | 1,186,842 | 8.59 | 0.03 ± 0.01 | 0.08 ± 0.02 | 2.67 |
| gi|124801199 | Peptide chain release factor subunit 1 | 48,034 | 6.59 | 0.02 ± 0.00 | 0.08 ± 0.03 | 4.00 |
| gi|124512284 | Hypothetical protein | 117,007 | 8.86 | 0.01 ± 0.00 | 0.03 ± 0.00 | 3.00 |
| gi|160687 | Serine rich protein homologue | 110,410 | 5.93 | 0.03 ± 0.01 | 0.07 ± 0.02 | 2.33 |
| gi|124804472 | Conserved Plasmodium protein | 244,889 | 5.54 | 0.01 ± 0.00 | 0.02 ± 0.00 | 2.00 |
| gi|124015257 | Erythrocyte membrane protein 1 | 252,432 | 5.22 | 0.01 ± 0.00 | 0.03 ± 0.01 | 3.00 |
| gi|258597217 | Conserved Plasmodium protein | 176,010 | 9.50 | 0.01 ± 0.00 | 0.02 ± 0.00 | 2.00 |
| gi|124513598 | DNA helicase | 169,009 | 8.32 | 0.01 ± 0.00 | 0.02 ± 0.00 | 2.00 |
| gi|124512436 | Hypothetical protein | 84,324 | 9.47 | 0.01 ± 0.00 | 0.04 ± 0.01 | 4.00 |
| gi|124505687 | Erythrocyte binding antigen-181 | 180,922 | 6.16 | 0.04 ± 0.01 | 0.09 ± 0.02 | 2.25 |
| gi|124804753 | Conserved Plasmodium protein | 82,452 | 9.03 | 0.02 ± 0.00 | 0.04 ± 0.01 | 2.00 |
ND, not detectable.
Table 2.
Down-regulated proteins in mefloquine-sensitive and mefloquine-resistant P. falciparum.
| Accession | Protein | Mass | pI | emPAI value |
Fold change | |
|---|---|---|---|---|---|---|
| MQ sensitive | MQ resistant | |||||
| gi|124513762 | Thioredoxin-related protein | 23,972 | 9.44 | 1.42 ± 0.06 | 0.34 ± 0.07 | −4.18 |
| gi|124810210 | 40S ribosomal protein S3 | 24,652 | 10.2 | 0.78 ± 0.03 | 0.33 ± 0.05 | −2.36 |
| gi|124513590 | Phosphoethanolamine N-methyltransferase | 31,024 | 5.43 | 2.55 ± 0.08 | 0.41 ± 0.03 | −6.22 |
| gi|124506992 | S-adenosylmethionine synthetase | 44,816 | 6.28 | 0.62 ± 0.03 | 0.08 ± 0.01 | −7.75 |
| gi|124511826 | Histone H2B | 13,755 | 10.27 | 7.20 ± 0.37 | 1.86 ± 0.12 | −3.87 |
| gi|124803615 | Casein kinase II, alpha subunit | 39,865 | 8.9 | 0.31 ± 0.09 | 0.09 ± 0.01 | −3.44 |
| gi|296005005 | Hypothetical protein | 37,477 | 4.13 | 0.46 ± 0.04 | 0.21 ± 0.06 | −2.19 |
| gi|124801997 | 60S ribosomal protein L13 | 23,739 | 10.19 | 0.35 ± 0.01 | 0.16 ± 0.03 | −2.19 |
| gi|124511970 | 40S ribosomal protein S5 | 21,849 | 9.67 | 0.62 ± 0.03 | 0.18 ± 0.02 | −3.44 |
| gi|3242984 | Merozoite capping protein-1 | 38,293 | 9.33 | 0.21 ± 0.01 | 0.10 ± 0.02 | −2.10 |
| gi|124505943 | Hypothetical protein | 30,696 | 9.47 | 0.59 ± 0.03 | 0.26 ± 0.04 | −2.27 |
| gi|86171362 | Pyridoxine biosynthetic enzyme pdx1 | 32,992 | 6.76 | 0.54 ± 0.02 | 0.11 ± 0.02 | −4.91 |
| gi|124513178 | Small GTPase Rab11 | 24,737 | 8.97 | 0.33 ± 0.01 | 0.15 ± 0.03 | −2.20 |
| gi|829215 | Unnamed protein product | 10,747 | 5.04 | 0.88 ± 0.00 | 0.37 ± 0.05 | −2.38 |
| gi|124512798 | 40S ribosomal protein S7 | 22,467 | 9.81 | 0.37 ± 0.00 | 0.17 ± 0.03 | −2.18 |
| gi|82541094 | Histone 3 | 15,437 | 11.14 | 0.57 ± 0.03 | 0.25 ± 0.01 | −2.28 |
| gi|124810024 | Conserved Plasmodium protein, unknown function | 40,043 | 9.6 | 0.31 ± 0.01 | NDa | NDa |
| gi|86171008 | Organic anion transporter | 96,918 | 5.59 | 5.13 ± 0.33 | 1.48 ± 0.07 | −3.47 |
| gi|124513666 | Hypothetical protein | 42,133 | 7.14 | 0.41 ± 0.10 | 0.19 ± 0.01 | −2.16 |
| gi|124504989 | Formate-nitrate transporter | 34,436 | 8.74 | 0.23 ± 0.03 | 0.11 ± 0.01 | −2.09 |
| gi|124513774 | Hypothetical protein | 32,763 | 9.22 | 0.24 ± 0.06 | 0.12 ± 0.02 | 2.00 |
| gi|124505615 | Plasmodium exported protein (PHISTb) | 35,939 | 8.75 | 0.22 ± 0.04 | 0.10 ± 0.02 | −2.20 |
| gi|21591743 | Rhoptry-associated protein 3 | 46,947 | 8.52 | 0.17 ± 0.00 | NDa | NDa |
| gi|124505467 | Small GTP-binding protein sar1 | 22,006 | 6.76 | 0.38 ± 0.09 | 0.17 ± 0.04 | −2.24 |
| gi|124805752 | Glutathione peroxidase | 23,937 | 8.99 | 0.34 ± 0.03 | 0.16 ± 0.05 | −2.13 |
| gi|552237 | Ubiquitin | 8659 | 6.79 | 1.16 ± 0.05 | NDa | NDa |
| gi|124504725 | N-ethylmaleimide-sensitive fusion protein | 89,078 | 6.31 | 0.04 ± 0.00 | 0.02 ± 0.00 | −2.00 |
| gi|124507271 | Hypothetical protein | 45,472 | 9.71 | 0.48 ± 0.03 | 0.17 ± 0.03 | −2.82 |
| gi|124512998 | Hypothetical protein | 277,526 | 7.79 | 0.04 ± 0.00 | 0.01 ± 0.00 | −4.00 |
| gi|309688 | PfsXLX | 377,125 | 5.71 | 0.03 ± 0.00 | 0.01 ± 0.00 | −3.00 |
| gi|467989 | Adenine nucleotide translocase | 33,691 | 9.68 | 0.23 ± 0.04 | 0.11 ± 0.03 | −2.09 |
| gi|124514090 | Hypothetical protein | 62,438 | 9.23 | 0.14 ± 0.02 | 0.06 ± 0.01 | −2.33 |
| gi|258597207 | Conserved Plasmodium protein | 30,641 | 4.98 | 0.42 ± 0.03 | 0.12 ± 0.02 | −3.50 |
ND, not detectable.
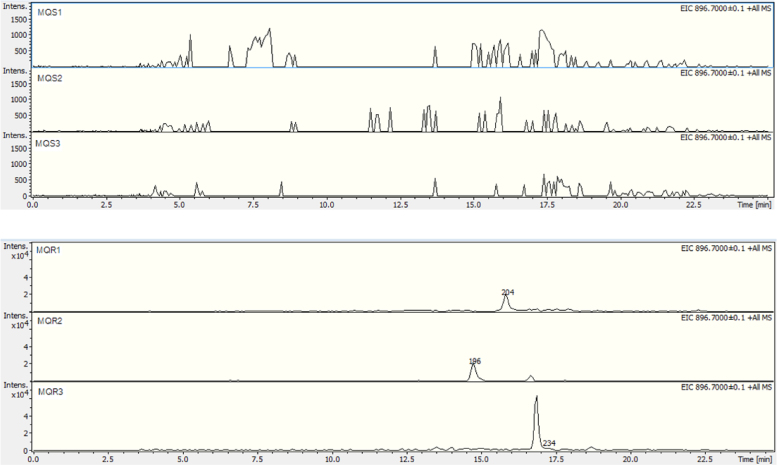
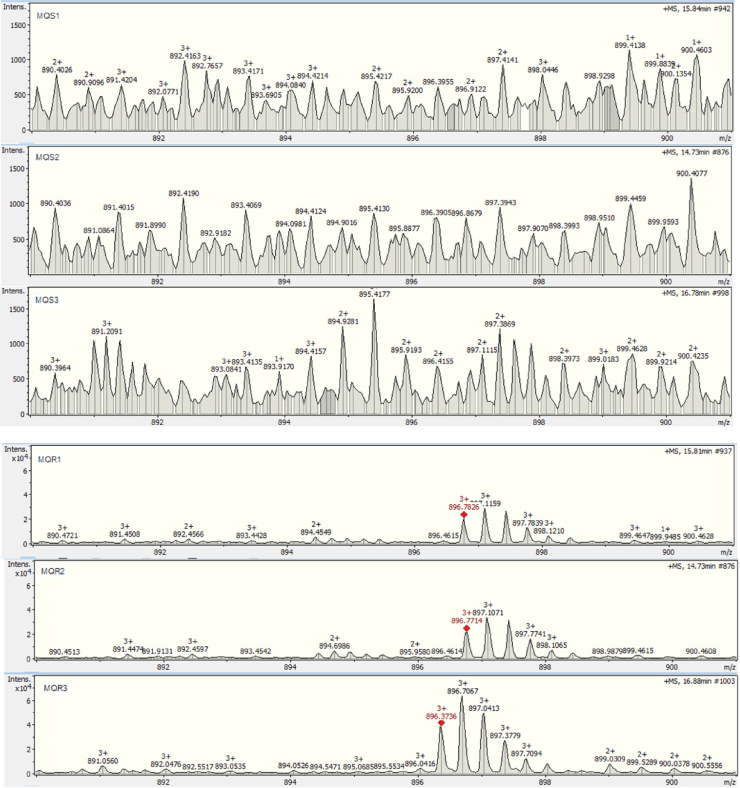
This result also shows how effective GeLC–MS/MS-based proteomics are when combined with emPAI label-free quantification at identifying and quantifying proteins from complex samples. In addition, extracted ion chromatograms of a precursor ion of a pfmdr1 peptide (FAAIDEFIESLPNKYDTNVGPYGK) at m/z 896.7 from three biological replicates are demonstrated in Fig. 4. Based on the correlated retention times in all chromatograms, mefloquine-resistant parasite showed peak of pfmdr1 peptide while this peak was absent in the mefloquine-sensitive parasite. This result corresponded to the up-regulation of pfmdr1. Comparison of intensities of precursor ions of pfmdr1 peptide at m/z 896.7 between mefloquine-sensitive and mefloquine-resistant parasites was also presented in Fig. 5. The intensity of m/z 896.7 precursor ions in mefloquine-resistant parasite revealed higher abundance than in mefloquine-sensitive parasite. This finding also referred to the up-regulation of pfmdr1. The extracted ion chromatogram and precursor ion intensity supported the finding from label-free quantification.
Fig. 4.
Extracted ion chromatograms of a pfmdr1 peptide (FAAIDEFIESLPNKYDTNVGPYGK) at m/z 896.7 from three biological replicates. MQS1, MQS2, MQS3, MQR1, MQR2 and MQR3 are mefloquine-sensitive P. falciparum 1, mefloquine-sensitive P. falciparum 2, mefloquine-sensitive P. falciparum 3, mefloquine-resistant P. falciparum 1, mefloquine-resistant P. falciparum 2 and mefloquine-resistant P. falciparum 3, respectively.
Fig. 5.
Precursor ion intensities of a pfmdr1 peptide (FAAIDEFIESLPNKYDTNVGPYGK) at m/z 896.7 from three biological replicates. MQS1, MQS2, MQS3, MQR1, MQR2 and MQR3 are mefloquine-sensitive P. falciparum 1, mefloquine-sensitive P. falciparum 2, mefloquine-sensitive P. falciparum 3, mefloquine-resistant P. falciparum 1, mefloquine-resistant P. falciparum 2 and mefloquine-resistant P. falciparum 3, respectively.
The up- and down-regulation data from label-free quantification also revealed several interesting protein markers for mefloquine resistance. Some of them have known relationships with drug resistance in other organisms. For example, HAP, which has an aspartic-type endopeptidase activity, was up-regulated in the drug-resistant parasite. This enzyme is one of four essential proteases in the P. falciparum food vacuole [22]. Plasmepsin I, II, and IV and HAP are involved in haemoglobin degradation, a major nutrient source for malaria parasites. Within the food vacuole, haemoglobin is degraded to peptides and is then exported to the cytoplasm for degradation [23]. However, HAP cannot initiate the degradation of native haemoglobin. Rather, it requires plasmepsin I, II, and IV for digestion initiation. These four proteases have been continually researched as potential targets for developing novel drugs against malaria parasites [24]. Although, the association between mefloquine resistance and overexpression of HAP is not clearly understood, this protein is interesting in that it is located in the food vacuole, which is also the site of mefloquine action, and plays a crucial role in parasite survival. Our results have also revealed that exportin 1, a nuclease transporter, was up-regulated in the mefloquine-resistant line. In many eukaryotes, this protein is an essential mediator for nuclear protein, mRNA, and drug export [25]. CRM1/exportin 1 inhibition was able to sensitize drug-resistant human myeloma cell lines and patient myeloma cells to cancer chemotherapy drugs such as doxorubicin, bortezomib, and carfilzomib. These findings support an association between exportin 1 and drug resistance in cancer [26]. P. falciparum exportin 1 probably controls the mefloquine resistance phenotype via nucleocytoplasmic export, which affects subcellular localization, cell cycle regulation and transcription. This suggests that eukaryotic translation initiation factor 3 (eIF-3), which plays a role in protein synthesis in eukaryotes, is up-regulated in mefloquine-resistant parasites. In a quantitative proteomic analysis, eIF-3 showed higher expression levels in amphotericin-B-resistant Leishmania infantum [27]. Additionally, eLF-3 was shown to be up-regulated in human lymphoblastic leukaemia cells exhibiting cross-resistance to prednisolone, vincristine, asparaginase and daunorubicin by a genome-wide screening approach [28]. Correlating to the role of eIF-3 in other organisms, mefloquine-resistant P. falciparum may change its regulation of protein synthesis through eIF-3. Peptidyl-prolyl cis-trans isomerase, which accelerates the folding of other proteins, was an up-regulated protein found in this study. Peptidyl-prolyl cis-trans isomerase in Saccharomyces cerevisiae mediates sensitivity to the macrolide antifungal agent, rapamycin [29]. Up-regulation of peptidyl-prolyl cis-trans isomerase has also been seen in gemcitabine-resistant human pancreatic cancer cells [30]. Overexpression of peptidyl-prolyl cis-trans isomerase induced chemoresistance to cisplatin; however, when it was knocked down cisplatin sensitivity increased [31]. As the above studies indicate, several attempts have been made to explain the crucial role played by this protein in drug resistance; hence, there is now interest in studying its potential role in drug resistance in P. falciparum. SERP, which contains a cysteine-type peptidase motif, was also overexpressed in mefloquine-resistant parasites. In S. cerevisiae, the MLF3 gene encoding a SERP conferred leflunomide resistance when its copy number increased [32]. As indicated by its role in drug resistance in yeast, SERP may also have an important molecular function involving mefloquine resistance in P. falciparum. From the proteomics result, it appears that Exp-1 was also overexpressed in the mefloquine-resistant parasites. A transmembrane protein and P. falciparum antigen, Exp-1 is transported to the parasitophorous vacuole membrane and to red blood cell (RBC) cytoplasm [33]. Generally, the mechanical properties of RBC membranes are altered during infection with malaria parasites, thereby enabling the transport of a range of nutrients and small molecules. This process is established through the insertion of parasite exported proteins into the RBC membrane [34]. There has been little discussion about the function of Exp-1 in malaria parasites. However, it is possibly involved in mefloquine transportation by mediating RBC membrane permeability. Our proteomic findings have also provided evidence that the ATP synthase beta chain is up-regulated in mefloquine resistant parasites. Despite this protein being a mitochondrial precursor in P. falciparum, it has also been identified in the food vacuole of the parasite [35]. ATP synthase beta chain assembles with other subunits to form a large complex that is essential for parasite survival [36]. Commonly, ATP synthase is an ATP binding cassette protein member involved in mediating substrate transport across membranes against a concentration gradient. This protein may confer in the parasite the ability to transport mefloquine. Proteomics showed that phospholipid scramblase 1 was also overexpressed. However, thus far very little attention has been paid to phospholipid scramblase 1 of P. falciparum. Phospholipid scramblase 1 of other organisms mediates the translocation of phospholipids upon binding of calcium ions to membranes. Human phospholipid scramblase 1 can modulate the response to arsenic trioxide (As2O3) during ovarian cancer treatment [37]. In malaria parasites, phospholipid scramblase 1 might play a role in modulating the mefloquine response. Enolase, a glycolytic enzyme, was also up-regulated in mefloquine resistant line. In lymphoma cell, enolase was found to participate in the drug resistance. Inhibition of enolase expression is a new strategy for reducing drug resistance [38]. Enolase of P. falciparum might involve in mefloquine resistant mechanisms. In case of this protein might not involve in mefloquine resistance. It is possible to play a role in balancing general cellular function to maintain parasite survival.
Based on the data we obtained on protein function and our literature reviews, we selected nine up-regulated proteins (Pgh1, HAP, exportin 1, eukaryotic translation initiation factor 3, peptidyl-prolyl cis-trans isomerase, SERP, Exp-1, ATP synthase beta chain and phospholipid scramblase 1) for further validation using reverse transcriptase qPCR.
3.2. Relative gene expression levels in mefloquine-sensitive and -resistant P. falciparum using reverse transcriptase qPCR
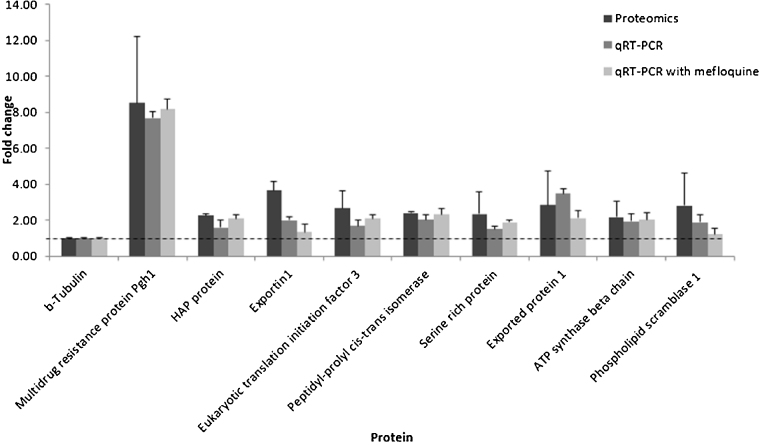
To confirm the LC–MS/MS results, qRT-PCR was used to examine gene transcription. Nine up-regulated expressed genes from mefloquine-sensitive and -resistant P. falciparum were selected according to their potentially interesting functions. All primers used for these experiments were designed from the sequences available at NCBI GenBank. The gene-specific primers are listed in Supplementary Table 4. Target genes were amplified in triplicate and beta-tubulin was the reference gene. As shown by the data in Table 1 and confirmed by the data in Fig. 6, the multidrug resistance protein Pgh1 was upregulated in the mefloquine-resistant parasites. The data show that pfmdr1 has an average of 3 copy numbers related to its transcription and translation, while the proteomics and RT-qPCR data showed 8.5-fold (±3.69) and 7.67-fold (±0.34) increases, respectively. The other eight genes also showed evidence of higher expression levels in the mefloquine-resistant parasites. In the presence of mefloquine, the expression levels of the nine candidate markers increased further. Therefore, GeLC–MS/MS based proteomics combined with emPAI label-free quantification was able to identify and quantify the biological samples accurately. Hence, this technique can generate a marker library for mefloquine resistance. While some marker proteins may not be involved in the mefloquine resistance mechanism directly, they are probably related to survival processes in the parasite. Mefloquine resistance protein markers can be uncharacterized proteins with no known function, making the resistance mechanisms more difficult to understand. However, candidate markers from proteomic libraries provide a fascinating avenue for exploration of their relationships with mefloquine resistance mechanisms in P. falciparum.
Fig. 6.
Differences in the expression levels of nine marker genes in mefloquine-sensitive and -resistant P. falciparum quantified using proteomics and qRT-PCR techniques.
4. Conclusion
In this investigation, the aim was to assess the mefloquine resistance mechanisms of P. falciparum using GeLC–MS/MS-based proteomics combined with emPAI label-free quantification. The findings suggest that mefloquine resistance might stem from multifactorial traits involving up- and down-regulation of proteins with functions in transportation and gene regulation processes. The multidrug resistance protein Pgh1 was substantially up-regulated in the mefloquine-resistant parasite line, confirming that it may play an important role in mefloquine resistance mechanisms. However, several other proteins identified for the first time in the present study also have the potential to enhance the mefloquine resistance phenotype. Therefore, further investigation and experimentation with proteins differentially expressed between mefloquine-sensitive and -resistant parasites are strongly recommended.
Conflict of interest
There is no conflict of interest to declare.
Acknowledgements
We thank Dr. Charlie Woodrow for his help. This work is supported by grant from Faculty of Tropical Medicine, Mahidol University and National Research Council of Thailand.
Funding was also obtained from Mahidol University, and the Wellcome Trust of Great Britain.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijms.2015.09.009.
Contributor Information
Onrapak Reamtong, Email: onrapak.rea@mahidol.ac.th.
Mallika Imwong, Email: mallika.imw@mahidol.ac.th.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Coomassie blue stained gels of mefloquine-sensitive and mefloquine-resistant P. falciparum proteins from three biological replicates separated by SDS-PAGE. MQS1, MQS2, MQS3, MQR1, MQR2 and MQR3 are mefloquine-sensitive P. falciparum 1, mefloquine-sensitive P. falciparum 2, mefloquine-sensitive P. falciparum 3, mefloquine-resistant P. falciparum 1, mefloquine-resistant P. falciparum 2 and mefloquine-resistant P. falciparum 3, respectively.
Summary of protein identification in 3 biological replicates.
The emPAI of identified proteins in 3 biological replicates.
Mascot information of identified proteins in first biological replicate of mefloquine sensitive parasite.
Mascot information of identified proteins in second biological replicate of mefloquine sensitive parasite.
Mascot information of identified proteins in third biological replicate of mefloquine sensitive parasite.
Mascot information of identified proteins in first biological replicate of mefloquine resistant parasite.
Mascot information of identified proteins in second biological replicate of mefloquine resistant parasite.
Mascot information of identified proteins in third biological replicate of mefloquine resistant parasite.
List of primers and optimal PCR amplification conditions used for the quantitative real-time RT-PCR.
References
- 1.WHO Malaria Policy Advisory Committee and Secretariat Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of March 2013 meeting. Malar. J. 2013;12:213. doi: 10.1186/1475-2875-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White N.J., Nosten F., Looareesuwan S., Watkins W.M., Marsh K., Snow R.W., Kokwaro G., Ouma J., Hien T.T., Molyneux M.E., Taylor T.E., Newbold C.I., Ruebush T.K., 2nd, Danis M., Greenwood B.M., Anderson R.M., Olliaro P. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 3.Wongsrichanalai C., Lin K., Pang L.W., Faiz M.A., Noedl H., Wimonwattrawatee T., Laoboonchai A., Kawamoto F. In vitro susceptibility of Plasmodium falciparum isolates from Myanmar to antimalarial drugs. Am. J. Trop. Med. Hyg. 2001;65:450–455. doi: 10.4269/ajtmh.2001.65.450. [DOI] [PubMed] [Google Scholar]
- 4.Nosten F., van Vugt M., Price R., Luxemburger C., Thway K.L., Brockman A., McGready R., ter Kuile F., Looareesuwan S., White N.J. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 5.Peel S.A., Bright P., Yount B., Handy J., Baric R.S. A strong association between mefloquine and halofantrine resistance and amplification, overexpression, and mutation in the P-glycoprotein gene homolog (pfmdr) of Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1994;51:648–658. doi: 10.4269/ajtmh.1994.51.648. [DOI] [PubMed] [Google Scholar]
- 6.Price R.N., Cassar C., Brockman A., Duraisingh M., van Vugt M., White N.J., Nosten F., Krishna S. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob. Agents Chemother. 1999;43:2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peel S.A., Merritt S.C., Handy J., Baric R.S. Derivation of highly mefloquine-resistant lines from Plasmodium falciparum in vitro. Am. J. Trop. Med. Hyg. 1993;48:385–397. doi: 10.4269/ajtmh.1993.48.385. [DOI] [PubMed] [Google Scholar]
- 8.Price R.N., Uhlemann A.-C., Brockman A., McGready R., Ashley E., Phaipun L., Patel R., Laing K., Looareesuwan S., White N.J., Nosten F., Krishna S. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na-Bangchang K., Bray P.G., Ward S.A. Study on the biochemical basis of mefloquine resistant Plasmodium falciparum. Exp. Parasitol. 2007;117:141–148. doi: 10.1016/j.exppara.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Ciach M., Zong K., Kain K.C., Crandall I. Reversal of mefloquine and quinine resistance in Plasmodium falciparum with NP30. Antimicrob. Agents Chemother. 2003;47:2393–2396. doi: 10.1128/AAC.47.8.2393-2396.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preechapornkul P., Imwong M., Chotivanich K., Pongtavornpinyo W., Dondorp A.M., Day N.P.J., White N.J., Pukrittayakamee S. Plasmodium falciparum pfmdr1 amplification, mefloquine resistance, and parasite fitness. Antimicrob. Agents Chemother. 2009;53:1509–1515. doi: 10.1128/AAC.00241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowman A.F., Galatis D., Thompson J.K. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl. Acad. Sci. U.S.A. 1994;91(3):1143–1147. doi: 10.1073/pnas.91.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Shinoda K., Tomita M., Ishihama Y. emPAI Calc – for the estimation of protein abundance from large-scale identification data by liquid chromatography–tandem mass spectrometry. Bioinformatics. 2010;26:576–577. doi: 10.1093/bioinformatics/btp700. [DOI] [PubMed] [Google Scholar]
- 15.Adav S.S., Ravindran A., Sze S.K. Proteomic analysis of temperature dependent extracellular proteins from Aspergillus fumigatus grown under solid-state culture condition. J. Proteome Res. 2013;12:2715–2731. doi: 10.1021/pr4000762. [DOI] [PubMed] [Google Scholar]
- 16.Yang G., Chu W., Zhang H., Sun X., Cai T., Dang L., Wang Q., Yu H., Zhong Y., Chen Z., Yang F., Li Z. Isolation and identification of mannose-binding proteins and estimation of their abundance in sera from hepatocellular carcinoma patients. Proteomics. 2013;13:878–892. doi: 10.1002/pmic.201200018. [DOI] [PubMed] [Google Scholar]
- 17.Mi H., Muruganujan A., Thomas P.D. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013;41:D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan V., Yip B., Lam Y.H., Tse H.Y., Wong H.S., Chan T.K. Quantitative polymerase chain reaction for the rapid prenatal diagnosis of homozygous alpha-thalassaemia (Hb Barts hydrops fetalis) Br. J. Haematol. 2001;115:341–346. doi: 10.1046/j.1365-2141.2001.03112.x. [DOI] [PubMed] [Google Scholar]
- 19.Babiker H.A., Hastings I.M., Swedberg G. Impaired fitness of drug-resistant malaria parasites: evidence and implication on drug-deployment policies. Expert Rev. Anti Infect. Ther. 2009;7:581–593. doi: 10.1586/eri.09.29. [DOI] [PubMed] [Google Scholar]
- 20.Andersson D.I. The biological cost of mutational antibiotic resistance: any practical conclusions? Curr. Opin. Microbiol. 2006;9:461–465. doi: 10.1016/j.mib.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Andersson D.I., Levin B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 22.Egan T.J. Four aspartic proteases occur in the Plasmodium falciparum food vacuole. Trends Parasitol. 2002;18:150. doi: 10.1016/s1471-4922(02)02265-1. [DOI] [PubMed] [Google Scholar]
- 23.Kolakovich K.A., Gluzman I.Y., Duffin K.L., Goldberg D.E. Generation of hemoglobin peptides in the acidic digestive vacuole of Plasmodium falciparum implicates peptide transport in amino acid production. Mol. Biochem. Parasitol. 1997;87:123–135. doi: 10.1016/s0166-6851(97)00062-5. [DOI] [PubMed] [Google Scholar]
- 24.Coombs G.H., Goldberg D.E., Klemba M., Berry C., Kay J., Mottram J.C. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 2001;17:532–537. doi: 10.1016/s1471-4922(01)02037-2. [DOI] [PubMed] [Google Scholar]
- 25.Stade K., Ford C.S., Guthrie C., Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 26.Turner J.G., Dawson J., Emmons M.F., Cubitt C.L., Kauffman M., Shacham S., Hazlehurst L.A., Sullivan D.M. CRM1 inhibition sensitizes drug resistant human myeloma cells to topoisomerase II and proteasome inhibitors both in vitro and ex vivo. J. Cancer. 2013;4:614–625. doi: 10.7150/jca.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brotherton M.-C., Bourassa S., Légaré D., Poirier G.G., Droit A., Ouellette M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014;4:126–132. doi: 10.1016/j.ijpddr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lugthart S., Cheok M.H., den Boer M.L., Yang W., Holleman A., Cheng C., Pui C.H., Relling M.V., Janka-Schaub G.E., Pieters R., Evans W.E. Identification of genes associated with chemotherapy crossresistance and treatment response in childhood acute lymphoblastic leukemia. Cancer Cell. 2005;7:375–386. doi: 10.1016/j.ccr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Koltin Y., Faucette L., Bergsma D.J., Levy M.A., Cafferkey R., Koser P.L., Johnson R.K., Livi G.P. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol. Cell. Biol. 1991;11:1718–1723. doi: 10.1128/mcb.11.3.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuramitsu Y., Taba K., Ryozawa S., Yoshida K., Zhang X., Tanaka T., Maehara S., Maehara Y., Sakaida I., Nakamura K. Identification of up- and down-regulated proteins in gemcitabine-resistant pancreatic cancer cells using two-dimensional gel electrophoresis and mass spectrometry. Anticancer Res. 2010;30:3367–3372. [PubMed] [Google Scholar]
- 31.Choi K.J., Piao Y.J., Lim M.J., Kim J.H., Ha J., Choe W., Kim S.S. Overexpressed cyclophilin A in cancer cells renders resistance to hypoxia- and cisplatin-induced cell death. Cancer Res. 2007;67:3654–3662. doi: 10.1158/0008-5472.CAN-06-1759. [DOI] [PubMed] [Google Scholar]
- 32.Fujimura H.A. Saccharomyces cerevisiae MLF3/YNL074C gene, encoding a serine-rich protein of unknown function, determines the level of resistance to the novel immunosuppressive drug leflunomide. Biochim. Biophys. Acta. 1998;1442:415–418. doi: 10.1016/s0167-4781(98)00190-0. [DOI] [PubMed] [Google Scholar]
- 33.Gunther K., Tummler M., Arnold H.H., Ridley R., Goman M., Scaife J.G., Lingelbach K. An exported protein of Plasmodium falciparum is synthesized as an integral membrane protein. Mol. Biochem. Parasitol. 1991;46:149–157. doi: 10.1016/0166-6851(91)90208-n. [DOI] [PubMed] [Google Scholar]
- 34.Miller L.H., Chien S., Usami S. Decreased deformability of Plasmodium coatneyi-infected red cells and its possible relation to cerebral malaria. Am. J. Trop. Med. Hyg. 1972;21:133–137. doi: 10.4269/ajtmh.1972.21.133. [DOI] [PubMed] [Google Scholar]
- 35.Lamarque M., Tastet C., Poncet J., Demettre E., Jouin P., Vial H., Dubremetz J.F. Food vacuole proteome of the malarial parasite Plasmodium falciparum. Proteomics Clin. Appl. 2008;2:1361–1374. doi: 10.1002/prca.200700112. [DOI] [PubMed] [Google Scholar]
- 36.Balabaskaran Nina P., Morrisey J.M., Ganesan S.M., Ke H., Pershing A.M., Mather M.W., Vaidya A.B. ATP synthase complex of Plasmodium falciparum: dimeric assembly in mitochondrial membranes and resistance to genetic disruption. J. Biol. Chem. 2011;286:41312–41322. doi: 10.1074/jbc.M111.290973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodigepalli K.M., Anur P., Spellman P., Sims P.J., Nanjundan M. Phospholipid scramblase 1, an interferon-regulated gene located at 3q23, is regulated by SnoN/SkiL in ovarian cancer cells. Mol. Cancer. 2013;12:32. doi: 10.1186/1476-4598-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X., Miao X., Wu Y., Li C., Guo Y., Liu Y., Chen Y., Lu X., Wang Y., He S. ENO1 promotes tumor proliferation and cell adhesion mediated drug resistance (CAM-DR) in non-Hodgkin's lymphomas. Exp. Cell Res. 2015;335:216–223. doi: 10.1016/j.yexcr.2015.05.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coomassie blue stained gels of mefloquine-sensitive and mefloquine-resistant P. falciparum proteins from three biological replicates separated by SDS-PAGE. MQS1, MQS2, MQS3, MQR1, MQR2 and MQR3 are mefloquine-sensitive P. falciparum 1, mefloquine-sensitive P. falciparum 2, mefloquine-sensitive P. falciparum 3, mefloquine-resistant P. falciparum 1, mefloquine-resistant P. falciparum 2 and mefloquine-resistant P. falciparum 3, respectively.
Summary of protein identification in 3 biological replicates.
The emPAI of identified proteins in 3 biological replicates.
Mascot information of identified proteins in first biological replicate of mefloquine sensitive parasite.
Mascot information of identified proteins in second biological replicate of mefloquine sensitive parasite.
Mascot information of identified proteins in third biological replicate of mefloquine sensitive parasite.
Mascot information of identified proteins in first biological replicate of mefloquine resistant parasite.
Mascot information of identified proteins in second biological replicate of mefloquine resistant parasite.
Mascot information of identified proteins in third biological replicate of mefloquine resistant parasite.
List of primers and optimal PCR amplification conditions used for the quantitative real-time RT-PCR.