Abstract
Supplemental Digital Content is available in the text.
Published ahead of print March 5, 2015
The measurement of hemoglobin concentration in the blood (Hb) plays a central role in the detection, evaluation, and management of chronic and acute anemia. The gold standard for laboratory determination of Hb is hemoglobin cyanide (HiCN).1 HiCN testing is not routinely used in hospitals due to its complexity, so cyanide-free central laboratory hematology analyzers (e.g., Coulter, Sysmex) have become the clinical standard.2 It is tempting to assume that satellite CO-Oximeters (e.g., ABL, Radiometer, Denmark; Nova, Nova Biomedical, Waltham, MA) used for arterial blood gas measurement in the operating room or critical care unit are interchangeable with hematology analyzers, but in fact they are not.
Pulse CO-Oximetry is the multiwavelength technology contained in the first devices to have received Food and Drug Administration 510(k) clearance for the continuous, noninvasive monitoring of total hemoglobin (SpHb; Masimo, Irvine, CA). Generally speaking, SpHb monitoring is not yet as accurate as laboratory hemoglobin (lab-Hb), and it is therefore not intended today as a replacement for lab-Hb. The focus should instead be on the value-added benefits of supplementing intermittent, delayed lab-Hb values with continuous, real-time visibility of whether Hb is stable, increasing, or decreasing.
The purpose of this article is to provide a perspective on the appropriate role and evaluation of SpHb and the value-added benefits of continuous Hb monitoring. We offer an alternative viewpoint to balance the 3 separate but similar opinions published earlier in Anesthesia & Analgesia by Drs. Rice, Gravenstein, and Morey.3–5 These authors propose “what is required of a noninvasive hemoglobin monitor and whether the conventional statistics adequately answer our questions about clinical accuracy.” In doing so, Rice et al. concluded that the accuracy of SpHb monitoring “is not good enough to make the (a) transfusion decision.” In the present article, clinical advisors to Masimo Corporation respond to these evaluations with a measured perspective on the value-added clinical decision process that this technology will bring to patient management and safety.
It is also time to review and reassess the fundamental assumptions regarding lab-Hb and its use in making clinical decisions. Given that it is noninvasive and its ability to provide continuous, real-time data that can be correlated at bedside with events happening to the patient, SpHb monitoring offers a new paradigm and opens up new possibilities for improved patient care.
LESSONS FROM PULSE OXIMETRY
Pulse oximetry, invented by Takuo Aoyagi of Nihon Kohden in 1974, was originally limited in clinical adoption because of concerns about accuracy in oxygen saturation (Spo2).6 Early studies during normoxia showed Spo2 differences of ≥6% compared with arterial blood analyzed on CO-Oximeters (Sao2),7 differences of 10% to 20% during hypoxia,8 and known signal dropouts and inaccuracy during low perfusion and motion. Despite these accuracy limitations, pulse oximetry became a “standard of care” by the late 1980s. This was partly due to the fact that even when pulse oximeter Spo2 measurements varied widely from the CO-Oximeter values, changes in Spo2 “appeared to reflect changes in saturation accurately in the same patient.”9 In their 1989 review article, Tremper and Barker10 noted that the pulse oximeter was “one of the most important advances in noninvasive monitoring because it provides a means of continuously and quickly assessing oxygen saturation.” More than 20 years later, Severinghaus6 noted that the “Introduction of pulse oximetry coincided with a 90% reduction in anesthesia-related fatalities.”
The contribution of pulse oximetry to improvements in patient safety was not because its noninvasive measurements replaced invasive laboratory measurements. Rather, as was emphasized back in 1989, it was the continuous and real-time properties of pulse oximetry that were so central to this new paradigm of patient monitoring. With continuous Spo2 monitoring, clinicians became aware that oxygen saturation was decreasing when the patient otherwise appeared to be well oxygenated. Continuous Spo2 monitoring also provided noninvasive reassurance that arterial blood was oxygen saturated when other means of clinical assessment were not helpful. Using this monitor as part of routine patient care allowed the clinician to focus on other aspects of management. However, clinical studies did not actually demonstrate significant improvements in patient outcomes due to pulse oximetry until recently.11,12 Earlier studies in the operating room showed no effect of pulse oximetry upon gross patient outcomes, such as death or myocardial infarction.13 Nevertheless, no anesthesiologist would have provided anesthesia without pulse oximetry since the early 1980s, despite this lack of outcomes evidence.
One can certainly argue that acute changes in Hb do not generally occur as rapidly as changes in Sao2 in clinical practice, but that is not our point. Spo2 changes can occur within seconds, whereas SpHb changes are more likely to occur over a few minutes. Nevertheless, the SpHb changes during acute surgical hemorrhage are still much too rapid to track effectively with intermittent and delayed lab-Hb measurements. We illustrate this point with clinical examples below.
KEY TENETS IN THE ROLE AND EVALUATION OF SpHb MONITORING
Spo2 monitoring by pulse oximetry offers lessons for the new technology of SpHb monitoring through Pulse CO-Oximetry. Like Spo2, SpHb monitoring provides continuous, real-time information. Also like pulse oximetry, the largest benefits of SpHb monitoring come from providing continuous Hb values to indicate reassurance or an unexpected status change, rather than from replacing lab-Hb. As a randomized controlled trial, a prospective cohort study, and case studies have shown us (refs. shown below), SpHb monitoring will help optimize transfusion decisions and aid in the detection of occult bleeding. In the pursuit of these high-impact benefits, when evaluating SpHb monitoring clinicians should consider the following tenets:
1. The value of SpHb monitoring should not be based on the premise that it replaces lab-Hb.
Because lab-Hb has been traditionally used as the main indicator for red blood cell (RBC) transfusion, a simplistic view of the purpose of SpHb monitoring would be as a replacement of lab-Hb, where the clinician must choose to measure either lab-Hb or SpHb. However, this perspective offers a false choice. Although some clinicians may wish to use SpHb to replace lab-Hb, continuous SpHb derives its primary clinical (and financial) value from continuous, real-time measurement and trending. Instead of replacement, the true value of SpHb monitoring is as a supplement to lab-Hb by providing real-time visibility of hemoglobin changes, or lack thereof, in the periods between invasive blood sampling and lab-Hb analysis.14
As discussed in Subsection 4, Trend Accuracy, below and based on the described studies on trending accuracy of SpHb versus sporadic laboratory Hb measurements, SpHb monitoring can help clinicians: (a) detect decreasing Hb when it is assumed to be stable; (b) identify stable Hb values when they are assumed to be decreasing; and (c) identify increases in Hb when they are assumed not to be increasing. Thus, a downward SpHb trend may alert clinicians to order a blood sample sooner than they would otherwise have done, thus facilitating an earlier needed RBC transfusion or other intervention. Likewise, a stable or increasing SpHb trend, together with other aspects of the patient assessment, can prevent an unnecessary RBC transfusion from occurring.
2. Lab-Hb variability should be considered when comparing SpHb to lab-Hb.
In single-point comparisons between SpHb and lab-Hb, some clinicians assume that lab-Hb is a perfectly accurate gold standard. In fact, there are significant differences in Hb measurements within and between various lab-Hb devices. All lab-Hb methods have significant variability and the actual gold standard for Hb measurement is HiCN, as defined by the International Committee for Standardization in Hematology.
Bland and Altman15 have pointed out that all reference devices have inherent errors. Reference devices that analyze blood not only have measurement variation due to technical factors but they have even more variability due to the sampling technique and handling.16 Therefore, a scientific and objective evaluation of SpHb accuracy should consider the intramethod and intermethod variability of hematology analyzers, CO-Oximeters, and point-of-care Hb methods (e.g., HemoCue, HemoCue America, Brea, CA; i-Stat, Abbott Point of Care, Princeton, NJ).17 Due to their limitations in accuracy, point-of-care Hb methods such as SpHb should be considered to supplement rather than replace lab-Hb.18
Many do not consider Hb variability in their assessment of SpHb monitoring. In their publication, Rice et al.3 assert that: “Thus, between 6 and 10 g/dL is where an operating room noninvasive Hb device needs to be accurate within 1 g/dL.” The flaw in this assertion is that lab-Hb devices often vary >1 g/dL. The lack of awareness in Hb variability may be rooted in lab-Hb specifications and hospital laboratory calibration techniques, which emphasize repeatability using controlled reference samples instead of the variation between different devices using clinical blood samples. Use of clinical blood samples induces another “real-world” variation19 when compared using the same model lab device (intramethod variability) and even more when compared using separate model lab devices (intermethod variability).
Gehring et al.20 evaluated intradevice variability and showed that the same blood sample analyzed on 2 identical models from 5 different CO-Oximeter manufacturers had an average SD of 0.5 g/dL and the highest SD of 1.2 g/dL. This means that when compared with an identical model of the same device, the average CO-Oximeter variation was within 0.5 g/dL 68% of the time and within 1.0 g/dL 95% of the time. Clinicians who would strictly transfuse at an Hb of 7.5 g/dL but not at 8.5 g/dL might be surprised to learn this.
Interdevice measurements vary even more significantly. In a study of 50 postsurgical patients, the CO-Oximeter varied as much as 0.73 g/dL and the hematology analyzer varied as much as 0.62 g/dL, when compared with gold-standard HiCN.21 In 471 consecutive samples analyzed on both a CO-Oximeter and a hematology analyzer, Torp et al. showed that the CO-Oximeter had a bias of −0.6 g/dL and an SD (also called “precision”) of 0.9 g/dL.a In a similar study design, also comparing with a hematology analyzer, Frasca et al.,22 showed a bias ± SD of 0.6 ± 0.9 g/dL for the CO-Oximeter, 0.3 ± 1.3 g/dL for the HemoCue point-of-care device, and 0.0 ± 1.0 g/dL for SpHb. Therefore, a CO-Oximeter with an expected SD of 0.7 g/dL would only be within 1.4 g/dL compared with a hematology analyzer, when 95% of measurements are considered. If a CO-Oximeter is not within 1 g/dL of a hematology analyzer 95% of the time, it is obviously inappropriate to expect SpHb to meet this same standard. Another very recent study compared Hb determined by CO-Oximetry and by Coulter Counter (Beckman-Coulter, Brea, CA) in patients undergoing spinal fusion.23 These authors found a mean difference between paired measurements of 0.4 g/dL, with 95% confidence limits of −0.7 to +1.47 g/dL. They conclude that these common laboratory gold standards are not interchangeable, and their variability must be considered in comparisons with “novel point-of-care and continuous hemoglobin monitoring technology.”
These and other studies show that measured Hb values will consistently differ in every device. This calls into question the use of the terms “inaccurate” or “erroneous” when comparing Hb from different methods as well as the use of a specific lab-Hb value as a fixed threshold for transfusion.
3. The intermittent and delayed nature of lab-Hb should be considered, especially for those clinical situations where transfusion decisions must be made in real time.
By focusing exclusively on whether SpHb is a suitable replacement for lab-Hb measurements, it is in part assumed that lab-Hb is always available and expedient. In fact, lab-Hb is intermittent, delayed by variable amounts of time, and often not available to support the transfusion decision in the needed timeframe. A recent study showed that 31% of transfusions during surgery were made without any preceding lab-Hb value due to its unavailability in a timely manner.24
Inadequate measurement is associated with inappropriate transfusion decisions.25 The following situational factors can help us further understand how lab-Hb, or lack thereof, affects RBC transfusion decision making.
Lab-Hb is not available when the transfusion decision is made (often for multiunit transfusions).
Lab-Hb is available, but the Hb threshold is not evidence based (often when Hb is perceived to be decreasing).
Lab-Hb is available, the Hb threshold is evidence-based, but transfusion may not be required if the patient is not actively bleeding or is without signs/symptoms (often when lab-Hb turnaround time is long).
Hb in values can change in minutes. Without SpHb monitoring, in a vast majority of cases both during and after surgery, Hb status is based on dated lab-Hb measurements combined with clinical estimates of blood loss, which are also known to be consistently inaccurate.b An alternative approach would be to increase the frequency of blood draws for lab-Hb measurement, but this approach is unlikely to be clinically acceptable in most cases because excessive diagnostic blood draws can be a significant source of blood loss and cause more problems for an already anemic patient.26 Thus, whenever lab-Hb is not available, SpHb monitoring can provide the only indication of Hb concentration, and it does so continuously.
4. The trend accuracy (precision) of continuous SpHb monitoring should be the primary consideration and accuracy an important but secondary consideration.
The word monitor comes from the Latin word monere, which means to warn. So as stated above, it is important to evaluate SpHb, like any monitor, with its proper purpose in mind. As a real-time monitor, the primary accuracy consideration for SpHb should be its trend accuracy (precision). Many investigators and opinion writers have not considered trending, trend accuracy, or the potential clinical benefits of Hb trends in supporting transfusion and other clinical decisions. Even when studies evaluate SpHb trend accuracy with positive conclusions, they can be ignored. Such was the case in 3 of the studies cited by Rice et al.3 who did not mention trend accuracy or the clinical application of the trend anywhere in their article.
As shown in Figure 1, Frasca et al.22 demonstrated in the surgical intensive care unit that when compared with Hb changes by a hematology analyzer, changes in SpHb had a similar correlation (0.64) to changes in Hb from the CO-Oximeter (0.60) and a higher correlation than changes in Hb from the HemoCue invasive point-of-care device (0.39). It must be emphasized that all Hb methods have variability and even extreme outliers. This means that a 2 g/dL decrease in the CO-Oximeter does not mean that the hematology analyzer will also decrease by 2 g/dL. It should be noted that adequate training and proper use of measurement methods are key to obtaining accurate results, a fact that must be considered when interpreting these results.
Figure 1.
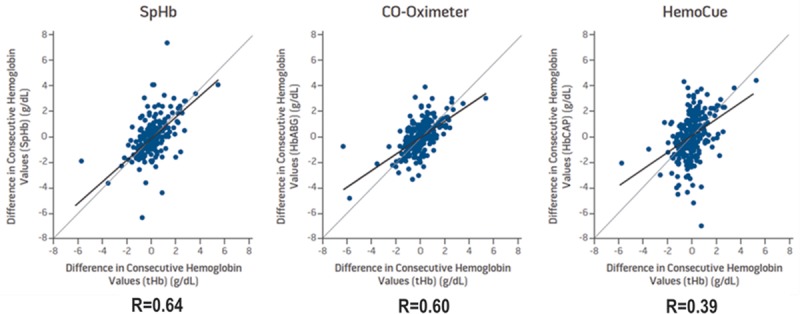
Changes in hemoglobin from a hematology analyzer compared with changes in SpHb, CO-Oximeter, and HemoCue (N = 471 measurements in 62 surgical intensive care subjects). Adapted from Frasca et al. 2011.22
Even when trend accuracy (precision) is statistically analyzed, as in Figure 1, this can be misleading because it compares lab-Hb measurements from static blood samples with SpHb values that are averaged over several minutes but recorded as snapshot values. Figure 2 illustrates a plot of continuous SpHb (which was blinded to the investigators) and sequential blood draws subsequently analyzed on both a hematology analyzer and a CO-Oximeter. If only 1 blood sample for the lab-Hb method was collected in this case, an observer might conclude at time point (A) that the SpHb difference from the CO-Oximeter is >1 g/dL lab-Hb and SpHb is not accurate enough. Likewise, when multiple SpHb differences from lab-Hb are examined as a simple list of numbers (e.g., 1, 0, 2 g/dL, etc.), or expressed statistically as bias and SD, some authors conclude that SpHb is not accurate enough. Yet, if the variability between 2 lab-Hb devices is also examined at the same time as it is in time point (A), the assessment of SpHb accuracy changes considerably. During rapid changes in Hb, shown in time points (B) and (C), a snapshot comparison of lab-Hb and SpHb can be misleading because SpHb is averaged over several minutes and needs some time to “catch up” to the lab-Hb reading. However, when the rapid changes are viewed in a trend plot, SpHb is clearly providing an indication of the changes in Hb that are occurring (and in fact, is doing so earlier than the lab-Hb because the results are often significantly delayed from time of the blood draw). Even if SpHb trend accuracy is being considered, Figure 2 also illustrates the pitfalls of comparing changes in 2 lab-Hb values and 2 SpHb values, due to the changing bias between the 2 lab-Hb measurements at multiple time points. Acceptable SpHb trend accuracy analysis does not require identical SpHb changes compared with lab-Hb changes but should instead seek directional SpHb agreement with larger directional lab-Hb changes. Further trend plot examples are provided in the Supplemental Digital Content (Figure A1–A6, http://links.lww.com/AA/B62; and Figure A7–A12, http://links.lww.com/AA/B63) to facilitate additional qualitative assessment. More studies are needed to better evaluate the SpHb trend accuracy and its role in clinical practice.
Figure 2.
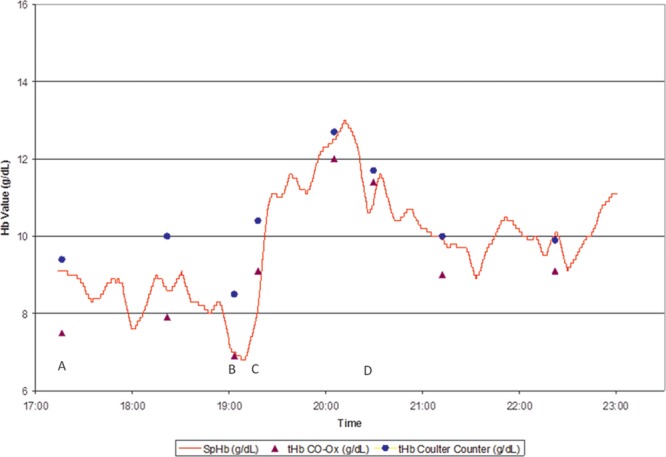
Trend plot of a surgical case in which blinded SpHb values (red line) are shown with lab-Hb measurements from both a CO-Oximeter (blue circles) and hematology analyzer (purple triangles). Note in (A) the larger-than-expected difference between lab-Hb values from 2 different devices. B and C, How differences between lab-Hb and single SpHb values during a time of rapidly changing hemoglobin can be misleading. D, The variation between 2 lab-Hb devices can change. Actual patient trend plot courtesy of Masimo.
5. All SpHb studies should be considered and study limitations should be noted.
Over 130 studies on SpHb accuracy have been presented at scientific meetings or published in scientific journals. All of these are listed on Masimo’s website, whether or not the manufacturer agrees with the study methods or conclusions.c The majority of these studies drew positive conclusions regarding SpHb accuracy. Unfortunately, only a small minority of SpHb studies have evaluated trend accuracy or compared another method along with SpHb to a hematology analyzer.
It is important to consider the entire body of evidence on SpHb accuracy, while also understanding the limitations of each study. Multiple positive studies on SpHb accuracy were not referenced in the review by Rice et al.,3 including 2 studies in which SpHb accuracy in point-to-point comparisons was found to be similar to, or better than, point-of-care Hb measurements (HemoCue).27,28 In addition, one of the SpHb studies featured by Rice et al. was conducted without following manufacturer’s directions for use.29 Not surprisingly, this resulted in the largest variability from lab-Hb seen in any published study. The specific errors in the methods of this study were described in a Letter to the Editor,30 yet these limitations were not acknowledged. In addition, a later study by the same investigators with more careful adherence to the directions for use was not cited, even though it demonstrated a 53% narrower confidence interval (lower variation) than the first study.31
We believe that investigations of SpHb monitoring accuracy are most useful when: (1) trend accuracy (precision) is evaluated; (2) all directions for use are followed; (3) SpHb is compared with HiCN or the clinical standard, a calibrated hematology analyzer; (4) arterial or venous blood samples are obtained using proper technique, with sampling and mixing carefully controlled; (5) enough subjects are enrolled to ensure the statistical relevance and coverage of a wide range of Hb values with expected changes in Hb values; and (6) ≥1 additional Hb methods (CO-Oximeter and/or point-of-care device) are also compared with the hematology analyzer.
6. The latest SpHb sensor offers important enhancements from earlier versions.
Some investigators have found that using previous versions of SpHb software in patients with wide shifts in peripheral perfusion yielded a greater degree of variation in both absolute and trend accuracy.32 Other investigators have noted a negative effect of high inspired oxygen fraction values upon SpHb accuracy.33 Masimo has recently addressed these conditions, and the same investigators have reported notable improvements with the latest version “Rev-K” SpHb sensor.d An example of the performance during variable and low perfusion with the Rev-K sensor compared with previous versions is shown in Figure 3.
Figure 3.
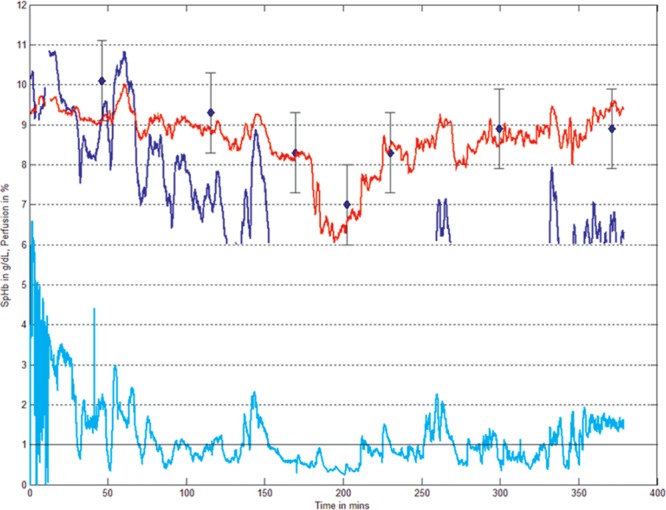
Revision “K” SpHb sensors (shown in red) have less variability than earlier revision SpHb sensors (shown in dark blue) during side perfusion variation and fewer dropouts during low perfusion (0.3%–1% Perfusion Index, shown in cyan), demonstrating improved trending accuracy compared with lab-Hb (shown in blue diamonds). Actual patient trend plot courtesy of Masimo.
As with Spo2 measurements, SpHb bias can vary among patients as well as the reference device to which it is being compared. To address this, Masimo has introduced a new SpHb software feature called In-Vivo Adjustment, which is available in the European and other markets as it awaits Food and Drug Administration 510(k) clearance. In-Vivo Adjustment allows the clinician to adjust the initial SpHb measurement for a given patient to their lab-Hb reference. A recent study conducted in surgical patients showed that after In-Vivo Adjustment, both the subsequent bias and SD of SpHb were reduced by 0.5 g/dL when compared with the lab reference device.34
7. The appropriate indications for transfusion, risks of transfusion, and limitations of using a single hemoglobin measurement to transfuse should be considered.
Those that presume SpHb is intended to replace lab-Hb often make the point that a single Hb value varying >1 g/dL from the “real” Hb value may result in an incorrect transfusion decision. This assertion is in contrast to established best transfusion practices that stipulate that the decision to transfuse should consider a variety of factors, including whether (1) the patient is actively bleeding, (2) signs and symptoms of anemia are present, (3) absolute Hb level, (4) whether Hb is changing or stable, (5) patient health and adaptive cardiovascular response ability, and (6) possibilities for increasing oxygen delivery without a transfusion.35–37
RBC transfusion is one of the most frequent procedures performed in United States hospitals, occurring in 1 in 10 inpatients.38 Meta-analysis from risk-adjusted observational studies shows that RBC transfusions are associated with a 69% increase in mortality, an 88% increase in infection, and a 250% increase in the incidence of adult respiratory distress syndrome.39 An analysis of 941,496 operations involving 15,186 transfused patients revealed a dose-dependent association of intraoperative RBC transfusion with increased risk of mortality, wound problems, pulmonary complications, postoperative renal dysfunction, systemic sepsis, composite morbidity, and postoperative length of stay when compared with propensity-matched not-transfused patients.40 Restrictive transfusion practices, in which RBC transfusions are given at lower-than-usual Hb threshold, have been shown to be safe in multiple randomized controlled trials, and meta-analysis indicates they result in a 23% lower in-hospital mortality.41
In a systematic evaluation of 450 studies and 494 clinical scenarios, investigators reported that: “Decisions to transfuse RBCs are often based on unsubstantiated Hb level or hematocrit ‘triggers’.” The authors concluded that 59% of transfusions actually reduced “well-being” defined as the health of the transfusion recipient (and therefore were considered inappropriate) and 29% had no benefit.42
Clinicians are even less aware of the costs of blood transfusions than the potential risks. Although the acquisition cost of a unit of blood varies between $200 and $250 in most hospitals, activity-based costing that considers all of the other costs associated with administering blood reveals a total cost between $522 and $1,183 per unit and probably more when adjusted for inflation and other factors.43
Given the risks and costs of RBC transfusions, there is a growing recognition of the need for strategies to reduce unnecessary transfusions. The American Medical Association’s Physician Consortium for Performance Improvement and the Joint Commission, with participation of the Centers for Medicare and Medicaid Services, recently identified RBC transfusions as 1 of the top 5 overused procedures in medicine.44 The Joint Commission has also introduced measures that track the appropriateness of RBC transfusion as a continuous quality indicator.e
Even in the presence of compelling evidence, practice patterns may take a long time to change. This is evidenced by the fact that RBC transfusion practices remain highly variable by institution, procedure, and physician, with the majority of transfusions occurring at a higher lab-Hb trigger than the most current evidence-based guidelines would suggest.24
8. The impact of using SpHb monitoring to supplement lab-Hb and optimize transfusion decisions should be considered.
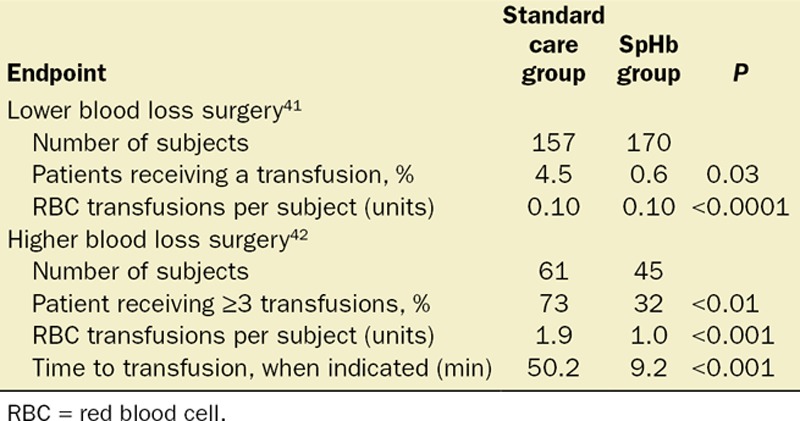
It is important to note that accuracy studies and conceptual acceptability charts as proposed by Drs. Rice, Gravenstein, and Morey to evaluate SpHb accuracy cannot assess the clinical and financial value of tracking the Hb continuously and in real time. Although many focus on the value of SpHb in making the decision to transfuse, it is equally important to focus on the value of SpHb when it helps us with the decision not to transfuse. Some of the authors who criticize SpHb for its inability to replace lab-Hb do not reference several studies that have shown how SpHb monitoring actually affects transfusion behavior. The clinical application of SpHb monitoring to optimize blood transfusions has been evaluated in 2 prospective studies (published in abstract form as of this writing), which are summarized in Table 1. Both of these studies, one in lower blood loss orthopedic surgeryf and the other in higher blood loss neurosurgery,g demonstrate that SpHb monitoring helps anesthesiologists reduce unnecessary RBC transfusions. In the lower blood loss orthopedic surgery, the SpHb monitoring reduced the number of patients who received any transfusion. In the higher blood loss neurosurgery, SpHb monitoring reduced the total number of units given in multiunit transfusions. Interestingly, there were no differences in the frequency of lab-Hb testing between the 2 groups in the orthopedic surgery study, illustrating again the fact that SpHb supplements but does not replace lab-Hb testing. In addition, SpHb monitoring was shown to help speed the transfusion decision, when transfusion was actually indicated.
Table 1.
Study Summary: SpHb Monitoring Impact on RBC Transfusion Decision Making
9. The impact of using SpHb monitoring to supplement lab-Hb in the detection of occult bleeding should be considered.
Delayed detection of internal bleeding occurs in every hospital, at times resulting in injury or death. Late detection of bleeding increases both risk and cost.45 Vital signs alone are not specific enough to reliably diagnose occult bleeding. Decreasing Hb can identify internal bleeding,46 but lab measurements are often infrequent and delayed, and a blood sample taken during the early phases of an acute blood loss may not be indicative of the overall Hb level. SpHb monitoring has demonstrated its lifesaving potential to help clinicians detect occult bleeding in the operating room, intensive care units, and labor and delivery wards.22,47 Figure 4 illustrates the impact of SpHb in detection of occult bleeding during surgery.
Figure 4.
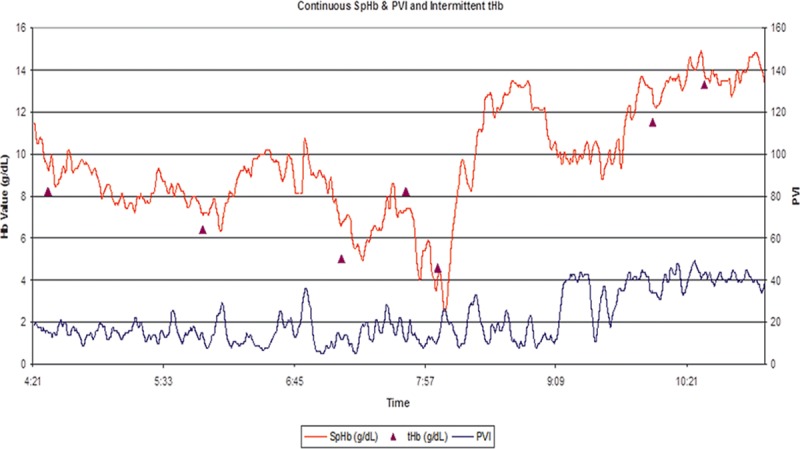
Trend plot of a liver transplant surgery in which visible SpHb values (red line) are shown with lab-Hb measurements (purple triangles). The last lab-Hb value before the time marked as 7:57 was >8 g/dL. As the surgeon was closing and with no significant change in vital signs, SpHb values began to drop while pleth variability index (PVI) began to rise, which is associated with reduced intravascular volume status. Due to the changing SpHb values, another blood sample was taken, later revealing a lab-Hb <5 g/dL. The dropping SpHb values caused the surgeon to stop wound closure and re-explore the abdomen. A large volume of blood was found behind the liver and bleeding vessel repaired. The SpHb values decreased <3 g/dL, and RBC transfusion was initiated as hemodynamic instability occurred. The aggressive transfusion that followed resulted in an over transfusion as the SpHb reached 14 g/dL. Actual patient trend plot courtesy of Masimo.
CONCLUSIONS
SpHb accuracy may eventually improve to the point where it can actually replace invasive blood sampling and lab analysis (lab-Hb). In the meantime, SpHb monitoring can and will supplement lab-Hb continuously between individual lab-Hb measurements. SpHb monitoring is a technological breakthrough because it allows real-time, continuous evaluation of changes (or absence of changes) of Hb levels. There are already thousands of SpHb devices in clinical use around the world. Although the technology and its accuracy are still improving, SpHb has repeatedly demonstrated clinically usable accuracy in head-to-head comparisons with lab-Hb and similar trend accuracy (precision) as invasive methods. Most importantly, because of its continuous, noninvasive trending ability, SpHb monitoring has been shown to help clinicians reduce RBC transfusions and initiate more timely RBC transfusions when they are needed. In occult bleeding patients, SpHb has helped save lives and enhance the quality of care.
Clinicians should carefully consider the appropriate role for SpHb monitoring in their practice and thereby establish appropriate criteria for performance. Twenty years from now, we will hopefully look back on the advent of SpHb monitoring and marvel at the impact it has had on patient safety, quality, and cost of care, just like we do today with pulse oximetry.
DISCLOSURES
Name: Steven J. Barker, PhD, MD.
Contribution: This author wrote the manuscript and reviewed the final draft before submission.
Attestation: Steven J. Barker approved the final manuscript.
Conflicts of Interest: Board of Directors, Masimo; Chair, Scientific Advisory Board, Masimo; paid consultant, Masimo.
Name: Aryeh Shander, MD.
Contribution: This author reviewed the initial and final draft before submission and contributed to the content.
Conflicts of Interest: Consultant, Speaker, Research for Masimo.
Name: Michael A. Ramsay, MD
Contribution: This author reviewed the initial and final draft before submission and contributed to the content.
Attestation: Michael A. Ramsay approved the final manuscript.
Conflicts of Interest: Consultant, Speaker, Research for Masimo.
This manuscript was handled by: Maxime Cannesson, MD, PhD.
Supplementary Material
FOOTNOTES
Torp KD, MD, Shine TSJ, MD, Aniskevich S, MD, Peiris P, MD, Shapiro DP, MD. Comparison of coulter counter and Co-Oximeter (pHOx) measurements for hemoglobin. American Society of Anesthesiologists Annual Conference. New Orleans, LA, 2009:A937.
Hill S, Broomer B, Stover J, White W. Accuracy of estimated blood loss in spine surgery. American Society of Anesthesiologists Annual Conference. San Diego, CA, 2011: A054.
Masimo.com. Available at: www.masimo.com/cpub/clinical-hemoglobin-htm. Accessed October 24, 2013.
Applegate R, Collier C, Macknet M, Hassanian M, Andrews G, Um M. Continued improvement in absolute and trend accuracy of noninvasive and continuous hemoglobin monitoring American Society of Anesthesiologists. San Francisco, CA, 2013: A2287.
Implementation guide for the Joint Commission patient blood management performance measures: The Joint Commission, 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed November 18, 2013.
Ehrenfeld JM, Henneman JP. Impact of continuous and noninvasive hemoglobin monitoring on intraoperative blood transfusions. Proceedings of the 2010 Annual Meeting of the American Society Anesthesiologists. San Diego, CA, 2010:Abstract #LB05.
Awada WFN, Maher F. Reduction in red blood cell transfusions during neurosurgery with noninvasive and continuous hemoglobin monitoring Proceeding of the Society for Technology in Anesthesia Annual Meeting, 2013:51.
Accepted for publication September 18, 2014.
Published ahead of print March 5, 2015
Funding: None.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Davis BH, Jungerius B International Council for the Standardization of Haematology (ICSH) International Council for Standardization in Haematology technical report 1—2009: new reference material for haemiglobincyanide for use in standardization of blood haemoglobin measurements. Int J Lab Hematol. 2010;32:139–41. doi: 10.1111/j.1751-553X.2009.01196.x. [DOI] [PubMed] [Google Scholar]
- 2.Zwart A, van Assendelft OW, Bull BS, England JM, Lewis SM, Zijlstra WG. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition). J Clin Pathol. 1996;49:271–4. doi: 10.1136/jcp.49.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice MJ, Gravenstein N, Morey TE. Noninvasive hemoglobin monitoring: how accurate is enough? Anesth Analg. 2013;117:902–7. doi: 10.1213/ANE.0b013e31829483fb. [DOI] [PubMed] [Google Scholar]
- 4.Morey TE, Gravenstein N, Rice MJ. Let’s think clinically instead of mathematically about device accuracy. Anesth Analg. 2011;113:89–91. doi: 10.1213/ANE.0b013e318219a290. [DOI] [PubMed] [Google Scholar]
- 5.Morey TE, Gravenstein N, Rice MJ. Assessing point-of-care hemoglobin measurement: be careful we don’t bias with bias. Anesth Analg. 2011;113:1289–91. doi: 10.1213/ANE.0b013e31822906b2. [DOI] [PubMed] [Google Scholar]
- 6.Severinghaus JW. Takuo Aoyagi: discovery of pulse oximetry. Anesth Analg. 2007;105:S1–4. doi: 10.1213/01.ane.0000269514.31660.09. [DOI] [PubMed] [Google Scholar]
- 7.Cecil WT, Thorpe KJ, Fibuch EE, Tuohy GF. A clinical evaluation of the accuracy of the Nellcor N-100 and Ohmeda 3700 pulse oximeters. J Clin Monit. 1988;4:31–6. doi: 10.1007/BF01618105. [DOI] [PubMed] [Google Scholar]
- 8.Severinghaus JW, Naifeh KH. Accuracy of response of six pulse oximeters to profound hypoxia. Anesthesiology. 1987;67:551–8. doi: 10.1097/00000542-198710000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Ridley SA. A comparison of two pulse oximeters. Assessment of accuracy at low arterial saturation in paediatric surgical patients. Anaesthesia. 1988;43:136–40. [PubMed] [Google Scholar]
- 10.Tremper KK, Barker SJ. Pulse oximetry. Anesthesiology. 1989;70:98–108. doi: 10.1097/00000542-198901000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Castillo A, Deulofeut R, Critz A, Sola A. Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 technology. Acta Paediatr. 2011;100:188–92. doi: 10.1111/j.1651-2227.2010.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010;112:282–7. doi: 10.1097/ALN.0b013e3181ca7a9b. [DOI] [PubMed] [Google Scholar]
- 13.Moller JT, Pedersen T, Rasmussen LS, Jensen PF, Pedersen BD, Ravlo O, Rasmussen NH, Espersen K, Johannessen NW, Cooper JB. Randomized evaluation of pulse oximetry in 20,802 patients: I. Design, demography, pulse oximetry failure rate, and overall complication rate. Anesthesiology. 1993;78:436–44. doi: 10.1097/00000542-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix J, Tucci M. Noninvasive or invasive hemoglobin measurement? Crit Care Med. 2012;40:2715–6. doi: 10.1097/CCM.0b013e31825bc975. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 16.Kalra J. Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem. 2004;37:1052–62. doi: 10.1016/j.clinbiochem.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27:499–508. doi: 10.1007/s10877-013-9456-3. [DOI] [PubMed] [Google Scholar]
- 18.Seguin P, Kleiber A, Chanavaz C, Morcet J, Mallédant Y. Determination of capillary hemoglobin levels using the HemoCue system in intensive care patients. J Crit Care. 2011;26:423–7. doi: 10.1016/j.jcrc.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–65. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 20.Gehring H, Duembgen L, Peterlein M, Hagelberg S, Dibbelt L. Hemoximetry as the “gold standard”? Error assessment based on differences among identical blood gas analyzer devices of five manufacturers. Anesth Analg. 2007;105:S24–30. doi: 10.1213/01.ane.0000268713.58174.cc. [DOI] [PubMed] [Google Scholar]
- 21.Gehring H, Hornberger C, Dibbelt L, Rothsigkeit A, Gerlach K, Schumacher J, Schmucker P. Accuracy of point-of-care-testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol Scand. 2002;46:980–6. doi: 10.1034/j.1399-6576.2002.460809.x. [DOI] [PubMed] [Google Scholar]
- 22.Frasca D, Dahyot-Fizelier C, Catherine K, Levrat Q, Debaene B, Mimoz O. Accuracy of a continuous noninvasive hemoglobin monitor in intensive care unit patients. Crit Care Med. 2011;39:2277–82. doi: 10.1097/CCM.0b013e3182227e2d. [DOI] [PubMed] [Google Scholar]
- 23.Carabini LM, Navarre WJ, Ault ML, Bebawy JF, Gupta DK. A comparison of hemoglobin measured by co-oximetry and central laboratory during major spine fusion surgery. Anesth Analg. 2015;120:60–5 doi: 10.1213/ANE.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 24.Frank SM, Savage WJ, Rothschild JA, Rivers RJ, Ness PM, Paul SL, Ulatowski JA. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology. 2012;117:99–106. doi: 10.1097/ALN.0b013e318255e550. [DOI] [PubMed] [Google Scholar]
- 25.Friedman MT, Ebrahim A. Adequacy of physician documentation of red blood cell transfusion and correlation with assessment of transfusion appropriateness. Arch Pathol Lab Med. 2006;130:474–9. doi: 10.5858/2006-130-474-AOPDOR. [DOI] [PubMed] [Google Scholar]
- 26.Salisbury AC, Reid KJ, Alexander KP, Masoudi FA, Lai SM, Chan PS, Bach RG, Wang TY, Spertus JA, Kosiborod M. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–53. doi: 10.1001/archinternmed.2011.361. [DOI] [PubMed] [Google Scholar]
- 27.Raikhel M. Accuracy of noninvasive and invasive point-of-care total blood hemoglobin measurement in an outpatient setting. Postgrad Med. 2012;124:250–5. doi: 10.3810/pgm.2012.07.2584. [DOI] [PubMed] [Google Scholar]
- 28.Shah N, Martinez GJ, Osea EA. Accuracy and precision of the Pronto-7 for noninvasive hemoglobin testing in an outpatient setting. Blood. 2011;118:3147. [Google Scholar]
- 29.Gayat E, Bodin A, Sportiello C, Boisson M, Dreyfus JF, Mathieu E, Fischler M. Performance evaluation of a noninvasive hemoglobin monitoring device. Ann Emerg Med. 2011;57:330–3. doi: 10.1016/j.annemergmed.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 30.O’Reilly M. Response to Gayat et al. Ann Emerg Med. 2011;58:106–7. doi: 10.1016/j.annemergmed.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Gayat E, Aulagnier J, Matthieu E, Boisson M, Fischler M. Non-invasive measurement of hemoglobin: assessment of two different point-of-care technologies. PLoS One. 2012;7:e30065. doi: 10.1371/journal.pone.0030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Applegate RL, II, Barr SJ, Collier CE, Rook JL, Mangus DB, Allard MW. Evaluation of pulse cooximetry in patients undergoing abdominal or pelvic surgery. Anesthesiology. 2012;116:65–72. doi: 10.1097/ALN.0b013e31823d774f. [DOI] [PubMed] [Google Scholar]
- 33.Gayat E, Bodin A, Fischler M. Instability in non-invasive haemoglobin measurement: a possible influence of oxygen administration. Acta Anaesthesiol Scand. 2011;55:902. doi: 10.1111/j.1399-6576.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 34.Isosu T, Obara S, Hosono A, Ohashi S, Nakano Y, Imaizumi T, Mogami M, Murakawa M. Validation of continuous and noninvasive hemoglobin monitoring by pulse CO-oximetry in Japanese surgical patients. J Clin Monit Comput. 2013;27:55–60. doi: 10.1007/s10877-012-9397-2. [DOI] [PubMed] [Google Scholar]
- 35.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 36.Hare GM, Tsui AK, Ozawa S, Shander A. Anaemia: can we define haemoglobin thresholds for impaired oxygen homeostasis and suggest new strategies for treatment? Best Pract Res Clin Anaesthesiol. 2013;27:85–98. doi: 10.1016/j.bpa.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Shander A, Gross I, Hill S, Javidroozi M, Sledge S College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus. 2013;11:193–202. doi: 10.2450/2012.0195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levit K, Wier L, Stranges E, Ryan K, Elixhauser A. HCUP Facts and Figures: Statistics on Hospital-Based Care in the United States, 2007. Rockville, MD: Agency for Healthcare Research and Quality; 2009. [PubMed] [Google Scholar]
- 39.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 40.Ferraris VA, Davenport DL, Saha SP, Austin PC, Zwischenberger JB. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147:49–55. doi: 10.1001/archsurg.2011.790. [DOI] [PubMed] [Google Scholar]
- 41.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012:CD002042. doi: 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shander A, Fink A, Javidroozi M, Erhard J, Farmer SL, Corwin H, Goodnough LT, Hofmann A, Isbister J, Ozawa S, Spahn DR International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232–246.e53. doi: 10.1016/j.tmrv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 44.The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32:4, 8. [PubMed] [Google Scholar]
- 45.Herwaldt LA, Swartzendruber SK, Zimmerman MB, Scholz DA, Franklin JA, Caldarone CA. Hemorrhage after coronary artery bypass graft procedures. Infect Control Hosp Epidemiol. 2003;24:44–50. doi: 10.1086/502114. [DOI] [PubMed] [Google Scholar]
- 46.Bruns B, Lindsey M, Rowe K, Brown S, Minei JP, Gentilello LM, Shafi S. Hemoglobin drops within minutes of injuries and predicts need for an intervention to stop hemorrhage. J Trauma. 2007;63:312–5. doi: 10.1097/TA.0b013e31812389d6. [DOI] [PubMed] [Google Scholar]
- 47.Butwick AJ, Hilton G, Riley ET, Carvalho B. Non-invasive measurement of hemoglobin during cesarean hysterectomy: a case series. Int J Obstet Anesth. 2011;20:240–5. doi: 10.1016/j.ijoa.2011.03.009. [DOI] [PubMed] [Google Scholar]


