Abstract
Background
Near-infrared spectroscopy (NIRS) has the potential to continuously and noninvasively monitor intestinal function. This technology may be valuable because among neonates, intestinal maturity is highly variable and difficult to assess based solely on clinical signs. The aim of this study was to determine if there is an association between NIRS-based StO2 measurements and peristaltic activity assessed by transabdominal ultrasonography (US).
Material/Methods
Nineteen neonates of gestational age >32 weeks were categorized according to “no/low” versus “normal/hyperactive” motility levels, based on blinded US scan results. StO2 was recorded every 2 s for 24 h, following the ultrasound recording. Differences between the resulting estimates of average StO2 (bias of fits) and goodness-of-fit (residuals) were evaluated.
Results
Newborns with normal/hyperactive motility had higher mean StO2 than newborns with no/low motility (72.3±4.4 vs. 65.5±7.9, p<0.05, F=5.65). Residual errors were not significantly different between the 2 groups (p=0.213, F=0.213). A multivariate linear regression model using the means, residuals, and pairwise products of both, demonstrated more significant separation (0.47±0.26 vs. −0.24±0.33, p<0.01, F=27.4). A non-linear variant of the multivariate linear regression model demonstrated greatest separation (0.68±0.24 vs. −0.49±0.53, p<0.01, F=41.9).
Conclusions
This is the first study to demonstrate an association between NIRS-based StO2 measurements and peristaltic activity visualized by ultrasound imaging. NIRS may offer a continuous, noninvasive method to assess motility. This may have significant implications in premature infants at risk for feeding intolerance or necrotizing enterocolitis.
MeSH Keywords: Gastrointestinal Motility; Infant, Newborn; Spectroscopy, Near-Infrared; Ultrasonography
Background
After birth, the neonate must transition to the gastrointestinal system as the primary source of nutrition. In premature newborns, the gastrointestinal system and other organ systems in the body are underdeveloped. This is a challenge because the neonatologist must assess the function of the intestine for management of enteral feeding. Much of the knowledge about the physiology of the intestines in neonates is derived and subsequently inferred from experimental animal models [1,2]. Clinical methods to assess intestinal function include abdominal distension, aspiration of gastric residuals (GR), bowel auscultation, and abdominal x-ray [3,4]. However, reliability of these techniques has been questioned [3,5–7]. However, recently two noninvasive technologies have demonstrated potential to assess intestinal function in the Neonatal Intensive Care Unit (NICU): transabdominal ultrasonography (US) and Near-Infrared Spectroscopy (NIRS).
Different modalities of transabdominal US have been utilized by some investigators for assessment of necrotizing enterocolitis (NEC) [8–13]. For example, studies have found that US is more sensitive than x-rays in detecting intramural gas, portal venous gas, free peritoneal gas and free peritoneal fluid [13–17]. Color Doppler US of the abdominal wall is more accurate than clinical examination and x-ray in detecting bowel necrosis [13,14].
In principle, transabdominal US allows the visualization of peristaltic movements. The bowel walls are visible as multiple white echoic lines in a darker hypoechoic background of peritoneum. Peristaltic contractions appear as “wormlike” movements of the bowel wall. In a recent publication, Richburg and Kim utilize US for the assessment of intestinal motility in preterm neonates [18]. Duodenojejunal manometry, another measure of small intestinal motility, has previously been found to be a sensitive predictor of feeding intolerance in preterm infants [19]. However, this method is more invasive than transabdominal ultrasonography, and requires more specialized equipment and operator training.
Near-Infrared Spectroscopy (NIRS) is a noninvasive technology available to obtain in vivo, real time, portable, and continuous measurement of tissue oxygen saturation of hemoglobin (StO2). NIRS exploits the different absorptive properties of deoxyhemoglobin, oxyhemoglobin, and underlying tissue to measure StO2. Physiologically, StO2 is a measure of a combination of oxygen saturation in venous, arterial, and capillary blood. Thus, StO2 is dependent on the regional delivery and consumption of oxygen in the tissues [20,21]. While US may be operator dependent and assesses function at the time of the intervention, it remains the current gold standard to directly visualize motility. The advantage of NIRS is that it can be used without an operator over relatively long time intervals. Our group recently studied continuous cerebral StO2 over 24 h to determine the hemodynamic effects of umbilical cord milking in preterm neonates [22].
Several studies have utilized transabdominal NIRS [23–33]. Although blood flow through the superior mesenteric artery (SMA), was found to correlate with StO2 measurements [26], no study to date has validated splanchnic NIRS measurements with a local imaging method that measures intestinal function.
Correlating a method of continuous NIRS monitoring with a validated functional imaging technique may open the door to routine monitoring of splanchnic StO2 in the NICU. The objective of our study is to explore the relationship between US derived assessment of motility and levels of StO2 derived from NIRS instrumentation.
Material and Methods
This pilot study was conducted in the level III neonatal intensive care unit (NICU) at Sharp Mary Birch Hospital for Women and Newborns in San Diego, California between December 2013 and May 2014. The data was collected as part of the validation study of the Fore-Sight II NIRS tissue oximeter monitor for splanchnic StO2. The study was approved by the Sharp Healthcare Institutional Review Board and registered on clinical trials.gov (NCT02017652). Informed written consent was obtained prior to study enrollment.
Inclusion criteria were as follows: (i) gestational age greater than 32 weeks; (ii) weight of less than 5000g; and (iii) umbilical venous catheterization to obtain a venous partial pressure of oxygen for device calibration as part of a multicenter trial (currently ongoing). A gestational age of 32 weeks was chosen to enroll those that would be large and well enough to have NIRS monitoring but also require a NICU admission. No infants who required any significant respiratory support (mechanical ventilation or nasal ventilation), or cardiac inotropes were included. A venous blood sample from the umbilical venous catheter was drawn for the validation study of the NIRS technology. Exclusion criteria were perinatal asphyxia events, intraventricular hemorrhage, vascular or coagulation disorders, congenital anomalies, or a history of NEC.
Upon enrollment, bowel ultrasound scans were performed using a Vivid E9 (GE Healthcare, Wauwatosa, Wisconsin) system and an 11 MHz linear probe. The scans were performed by a blinded ultrasonographer (DA). The scans were performed immediately prior to NIRS placement once consent was obtained. The transducer was placed on the left, middle and right abdomen (Figure 1A), and the peristaltic movements of the intestine were observed for 10 s at each location. Immediately after the ultrasound scan, a NIRS infrared probe (Casmed, Fore-Sight II, Branford, CT, USA) was placed on the left lower quadrant of the abdomen (Figure 1B). Splanchnic StO2 data was recorded into a laptop every 2 s for 24 h.
Figure 1.
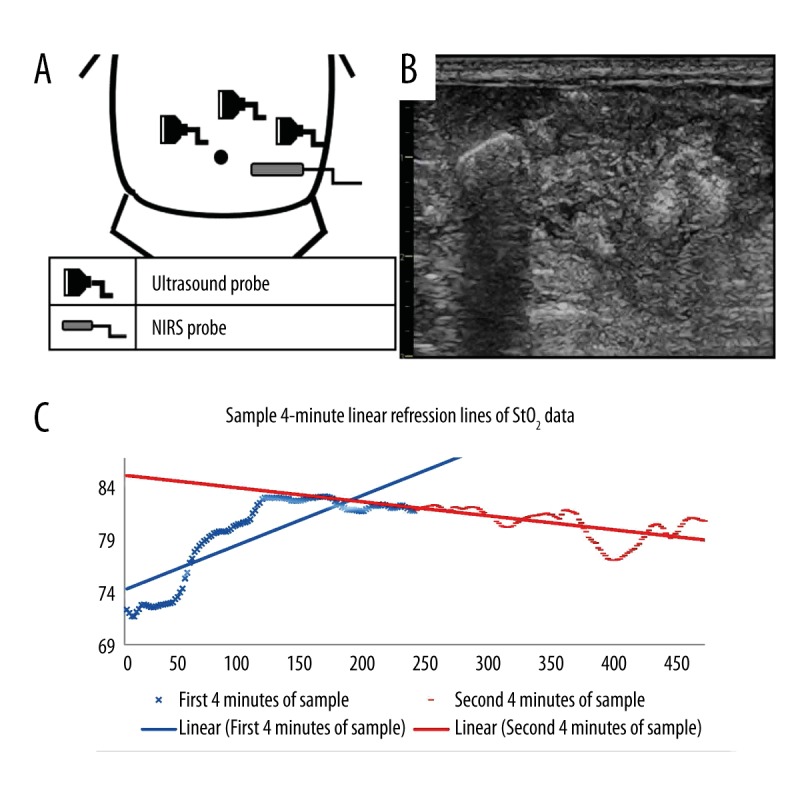
(A) Diagram that summarizes the approximate position of ultrasound transducer and NIRS probe. (B) Sample image of an abdominal ultrasound scan of the right side of the abdomen. Multiple short echogenic lines representing bowel lines can be seen. (C) StO2 vs. Time plot of an 8-min sample of tissue oximetry. The solid blue line is the line of best fit for the first 4 minutes of the 8-min sample. The solid red line is the line of best fit of the second 4 minutes of an 8-min sample.
The blinded ultrasonographer (DA) classified the babies into the respective motility levels, based on the observed peristaltic activity. Image features of a peristaltic movement of the bowel wall included rotational movement, displacement or sudden disappearance/reappearance of the echogenic bowel over 30 s (3 consecutive 10-s clips). To be considered a peristaltic activity, the movement must be localized to a small section of the image. The four categories used by Richburg et al. [18] (i.e. no, low, normal, hyperactive), were reduced to two distinct categories of visible levels of motility. The lower level categories (i.e. “no”, “low”) were merged into a single “no/low” level, while the upper two categories (i.e. “normal”, “hyperactive”) were merged into a “normal/hyperactive” category. The no/low category was assigned to subjects with minimal or no motility (0–2 peristaltic movements) observed in any 10-s image. An alternative normal/hyperactive category was assigned to a subject with multiple peristaltic movements (≥3 peristaltic movements) in any 10-s image. Movement of echogenic lines that are not localized to a small region of the image, are considered artifacts due to movement of the baby, movement of the US probe, or displacement of the abdominal contents (for example due to breathing).
Doppler ultrasonography of the superior mesenteric artery [26] was performed using an 8 MHz phased array transducer to measure the peak systolic velocity (vsystole) and end diastolic velocity (vdiastole). The arterial resistivity index was determined by using the following relation:
For analysis of the recorded NIRS measurements, the data was initially smoothed with a moving-window of 20 s. For each 24-h dataset, a representative distribution of measurements was generated from a uniform random sampling of multiple overlapping 4-min intervals, such that the average difference between the centers of the intervals was 1 min. Figure 1C illustrates an 8-min interval of StO2 samples with two 4-min linear fits highlighted in red.
A linear fit was performed for each of the randomly selected 4-min intervals. For visualizing the distributional properties of the representative measurements, the average StO2 (i.e. bias values of the linear fits) of all selected 4-min intervals over the 24 h were summarized into a single box plot. This was repeated for all cases, for a total of 19 Boxplots.
To measure the variability of the 4-min linear trends, the residual errors of the linear-fits were determined. The residual errors are deviations from the line-of-best fit for each 4-min interval. The averages of the residual errors for each 4-min interval over the 24 h were summarized into box plots. Small residuals indicate highly-linear consecutive measurements over a given 4-min measurement interval.
For further analysis, the distributions of average StO2 and respective residual errors were normalized to zero mean and unit standard deviation. Pairwise products of the normalized means and residual errors were applied.
A multivariate linear regression model [34] was created using the following factors as training and test inputs: means, residual errors, and pairwise products. A nonlinear variant (3-node single hidden-layer neural network) [34] of the regression model was applied using the same means, residual errors, and pairwise products. Training and test outputs were class assignment (−1 to 1). Two sets of box plots were created for the global linear and non-linear regression models.
As this was a pilot study without prior data comparing NIRS to US, a convenience sample size of 19 subjects was obtained.
For initial exploratory analysis purposes, the differences between the StO2 distributions for the no/low versus normal/hyperactive motility assignments were assessed using multivariate analysis of variance (MANOVA) techniques. For the results reported herein, the lm() and anova() commands in R (Ver. 3.2.1; 2015-06-18), were utilized for generating the linear fits and comparison of the means and medians of the respective distributions. The nnet() command in R (nnet package) was utilized for generating the nonlinear variant of the linear regression model.
The nutritional intake was recorded at the bedside over the course of 24 h and additional data relevant to the study (maternal and infant characteristics) were collected from the patient’s electronic medical record and managed using REDCap database application (REDCap Software v5.7.1 2014 Vanderbilt University). Mann-Whitney U, chi-squared, or t tests were used for data analysis where appropriate.
Results
Of the 35 cases assessed for eligibility, 19 subjects were enrolled. Figure 2 is a flow diagram that quantifies progress through the trial. Twelve parents declined consent. Four patients were excluded from the study, 2 because of inability to obtain a venous blood gas from the UVC lines, and 2 due to insufficient data. After undergoing abdominal ultrasound imaging, 8 subjects had negligible peristaltic movements visible on bowel ultrasonography and were classified into the no/low group. Eleven were classified as having normal/hyperactive motility.
Figure 2.
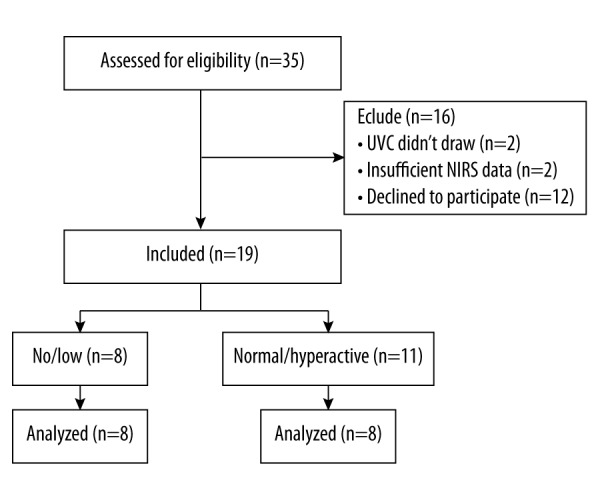
Consort flow diagram.
Table 1 summarizes the maternal characteristics of the participants assigned to each category. Table 2 summarizes the relevant patient characteristics collected from the electronic health records. Differences in gestational age and day of life at enrollment were not significant between the 2 categories. Birthweight was higher in the normal/hyperactive group and the mean magnesium levels were higher in the no/low group; however, these results were not statistically significant (p=0.11 and p=0.09, respectively). The no/low group had a higher incidence of abdominal distention (2 vs. 0; p=NA), gastric residuals (7 vs. 4; p=0.03), and gastric residual volume (2.03 mL vs. 0.87 mL; p=0.06). The no/low group had a lower volume of enteral nutrition during the duration of the study (13 mL vs. 98 mL; p=0.02).
Table 1.
Maternal characteristics.
| Maternal characteristics | No/low (n=8) | Normal/hyperactive (n=11) | P-value |
|---|---|---|---|
| Maternal age (years) | 28±2 | 29±2 | 0.29 |
| Preterm labor | 5 (63%) | 7 (64%) | 0.96 |
| Premature rupture of membranes | 0 | 3 (27%) | 0.11 |
| Chorioamnionitis | 2 (25%) | 1 (9%) | 0.35 |
| Pre-eclampsia | 4 (50%) | 2 (18%) | 0.14 |
| Magnesium sulfate | 5 (63%) | 3 (27%) | 0.12 |
Table 2.
Neonatal characteristics and outcome.
| Maternal characteristics | No/low (n=8) | Normal/hyperactive (n=11) | P-value |
|---|---|---|---|
| Gestational age (weeks) | 33.8±0.7 | 34.4±0.7 | 0.28 |
| Postnatal age at enrollment (days) | 1.6±0.3 | 1.9±0.6 | 0.35 |
| Birthweight (grams) | 1907±177 | 2370±273 | 0.11 |
| Male (%) | 3 (38%) | 9 (81%) | 0.05 |
| Continuous positive airway pressure (CPAP) during study | 6 (75%) | 1 (9%) | 0.06 |
| Caffeine administration during study | 2 (25%) | 0 | 0.2 |
| Magnesium level at enrollment (miliequivalents per liter) | 2.20±0.10 | 2.03±0.08 | 0.09 |
| Distended abdomen during study | 2 (25%) | 0 | 0.2 |
| Stool passage during the study | 7 (88%) | 10 (91%) | 0.81 |
| Gastric residuals during study | 7 (88%) | 4 (36%) | 0.03 |
| Gastric residual volume (mililiters) | 2.03±0.59 | 0.87±0.43 | 0.06 |
| Volume of enteral nutrition (mililiters per 24 hours) | 13±11 | 98±30 | 0.02 |
Table 3 summarizes the data obtained from the ultrasonography measurements. On all three 10-s ultrasound images, the no/low category had an average of 1.9±1.3 peristaltic movements and the normal/hyperactive group had an average of 9.2±2.2 peristaltic movements. The peak systolic velocity through the superior mesenteric artery was higher in the normal/hyperactive (77.2±6.3 cm/s vs. 58.5±8.1 cm/s; p=0.04). The resistivity index was not significantly different in both categories.
Table 3.
Ultrasound findings.
| Ultrasound findings | No/low (n=8) | Normal/hyperactive (n=11) | P-value |
|---|---|---|---|
| Peristaltic movements on all US | 1.9±1.3 | 9.2 ±2.2 | – |
| Peak systolic velocity (centimeters/second) | 58.5±8.1 | 77.2±6.3 | 0.04 |
| Resistivity index | 0.71±0.06 | 0.76±0.04 | 0.21 |
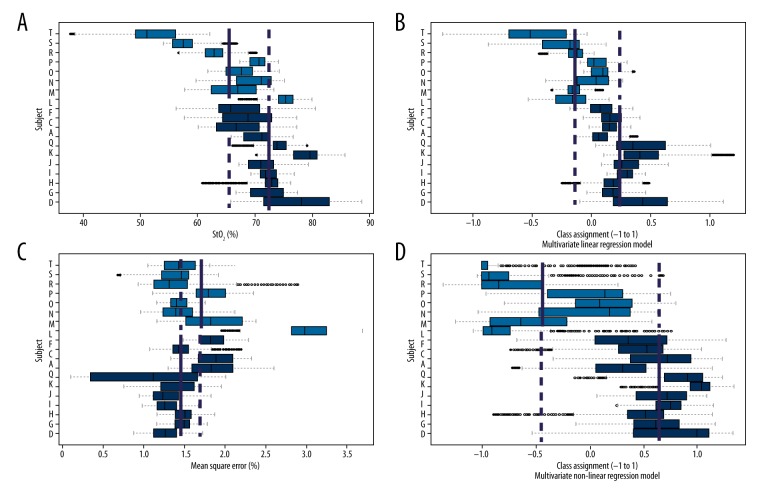
Neonates who were classified as having normal/hyperactive motility had on average higher StO2 over 24 h (Figure 3A) than those classified as having no/low motility (72.3±4.4 vs. 65.5±7.9, p=0.03, F=5.65). On average, no/low motility neonates also had higher variability, or deviations (i.e., residual errors) from the 4-min linear fits as shown in Figure 3B. As a univariate factor, the difference in the residual error (goodness-of-fit) was not considered statistically significant (1.48±0.26 vs. 1.73±0.58, p=0.213, F=1.67).
Figure 3.
Relationship between StO2 measurements and US categories. Four sets of 19 horizontal box plots. Each box plot summarizes the mean and interquartile ranges for each StO2 parameter, obtained from the 4-min linear fits over a 24-h period. Green box plots correspond to neonates with normal/hyperactive motility. Yellow box plots correspond to neonates with no/low motility. Solid blue vertical lines represent the mean of each category. Dashed blue vertical lines were added as continuations of the solid lines for comparison. (A) Neonates assigned to normal/hyperactive group had higher average StO2 than those assigned to no/low motility (72.25±4.36 vs. 65.53±7.93; p<0.05, F value=5.65). (B) The variability (average of residual error of 4 min linear fits) was not significantly different between the 2 categories (1.48±0.26 vs. 1.73±0.58, p=0.213, F=0.213). (C) The multivariate linear regression model utilizing a combination of means, residuals, and pairwise products of both described higher separation (0.47±0.26 vs. −0.24±0.33, p<0.01, F=27.4). (D) The highest separation was found utilizing a multivariate nonlinear regression model (0.47±0.26 vs. −0.24±0.33 vs., p<0.01, F=27.4).
The global linear regression models showed a higher level of separation between the groups (0.47±0.26 vs. −0.24±0.33, p=0.0001, F=27.4), as illustrated in Figure 3C. The non-linear variant of the regression model showed the highest level of separation between the 2 groups (0.68±0.24 vs. −0.49±0.53, p=0.0001, F=41.9), as illustrated in Figure 3D.
Discussion
Emerging noninvasive continuous monitoring techniques, such as cerebral NIRS (cerebral StO2) and electrocardiography (cardiac output, stroke volume), have been utilized by our group to augment continuous recording of more common vital signs, such as heart rate, blood pressure, mean arterial pressure, and arterial oxygen saturation (SpO2) [22,35]. Previous studies by other groups developed NIRS-based methods for describing splanchnic StO2 characteristics in relation to: cerebral StO2 [24,36], superior mesenteric artery flow [26], blood transfusions, hyperemia after feeds [23,24,37], apneic episodes [29,31], feeding intolerance, patent ductus arteriosus [27,30] and necrotizing enterocolitis [25,28,32]. The methods of NIRS data recording, statistical analysis, and instrumentation vary widely among these studies. To date, no other study has validated splanchnic NIRS with a functional medical imaging technique which measures intestinal function, such as the one described by Richburg et al. [18]. In this pilot study, we were successful in describing a relationship between this method of medical imaging and NIRS.
This study found that higher motility levels correspond to higher average splanchnic StO2 over a 24-h period. We attribute this finding to increased delivery of oxygenated arterial blood in the normal/hyperactive group, as observed by Doppler US of the SMA (insert results). Although peristaltic contractions of the smooth muscle cells result in increased oxygen consumption (which would decrease StO2), some metabolic products of peristaltic activity (such as adenosine) are powerful vasodilators that increase arterial blood flow by as much as a factor of 4. However, motility and blood flow are dependent on many other factors: gestational age, postnatal age, time after administration of feeds (fed/unfed state), and overall maturation. The gestational age and postnatal age did not differ significantly between the 2 comparison groups. The total volume of feeds over the 24 h of monitoring was significantly higher in the normal/hyperactive group (results), which suggests that infants in the normal/hyperactive group had better overall intestinal function. To identify normal and abnormal intestinal function and development, larger stratified studies are needed to further describe the gestational postnatal age and postprandial evolution of motility and circulation.
Multivariate regression models using a combination of StO2 and goodness-of-fit, displayed the highest degree of separation between the 2 groups. In the normal/hyperactive group, 2-s measurements more consistently increased (positive slope), decreased (negative slope), or stayed the same value (zero slope) throughout 4-min sample intervals. Overall, the regression models indicate that at higher StO2 levels, the 4-min trends tend to be more linear and less random. We attribute the less random nature to more functional auto-regulatory mechanisms in the intestinal microcirculation.
One of the future applications of this study lies in the decision to advance or delay enteral feeds in preterm neonates. Feeding protocols are generally based on the gestational age (GA) and birthweight of neonates and subsequent trial and error of tolerance [3–7]. One method available to estimate tolerance of feeds is the collection of gastric residual (GR) aspirates. In the present study, the incidence of GRs was significantly higher in patients with no/low motility. However, even the presence of GRs is often not a sufficient reason to decrease or interrupt enteral feeds, as maintaining feeding may improve gastrointestinal maturation and feeding tolerance [38,39]. Clinicians consider gastric residuals suspect if they are >30–50% of the previous feed or bilious-stained [4]. Our findings that multivariate linear regression models of continuous StO2 measurements correlate closely with US motility measurements suggest that NIRS may be also used for gauging feeding readiness. NIRS is even less operator-dependent than bowel US.
Another future application of our results is in the early detection of neonates at high risk of NEC, which often occurs unpredictably, with subsequent high morbidity and mortality. StO2 has been found to be lower in infants who develop NEC. Patel et al. found that infants with NEC have a higher variability of StO2 [28]. The variability of StO2 measurements suggests an impairment of auto-regulation of blood flow. This is consistent with our finding that infants with higher intestinal function tend to adhere to a more linear trend of 4-min measurement intervals. This is also consistent with the study by Downard et al., which described a discontinuous “stop-and-go pattern” of flow in venules of rats with induced NEC [40]. In this context, our study suggests an alternative analysis of StO2 data that is potentially useful for future NEC studies.
One of the limitations of this study is the small sample size. The sample included relatively mature preterm babies of gestational age >32 weeks, and the timing of the scans were not predefined. This was an exploratory study to describe the general correspondence between 2 techniques which are not currently used in our NICU. More work is needed to apply our results to neonates who may benefit the most from this type of noninvasive monitoring. The presence of artifacts in the US images is another limitation. Movement of the baby, movement of abdominal contents due to contraction of the diaphragm during breathing, movement of the ultrasound probe, and presence of small amounts of air can create artifacts that affect the quality of the images. In future studies, we may refine our technique to account for this limitation. NIRS instrumentation is also subject to motion artifacts and light interference which limit the accuracy of NIRS measurements.
Conclusions
This is the first study to demonstrate an association between NIRS-based StO2 measurements and peristaltic activity visualized by ultrasound imaging. In addition to continuously monitoring splanchnic StO2 level, NIRS may offer a continuous, noninvasive method to indirectly assess intestinal motility and function. Future research will focus on the applicability of the methods and results demonstrated in this study for predicting tolerance to feeds, advancing or withholding enteral feeding, and early detection of necrotizing enterocolitis.
Footnotes
Source of support: This study was supported by the Zakar Family, who paid for publication costs and Casmed (CT, USA), which supplied probes
References
- 1.Nowicki PT. Neonatal intestinal circulation. Comprehensive Physiology. 2010 [Google Scholar]
- 2.Chaaban H, Stonestreet BS. Intestinal hemodynamics and oxygenation in the perinatal period. Semin Perinatol. 2012;36:260–68. doi: 10.1053/j.semperi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Jadcherla SR, Kliegman RM. Studies of feeding intolerance in very low birth weight infants: Definition and significance. Pediatrics. 2002;109:516–17. doi: 10.1542/peds.109.3.516. [DOI] [PubMed] [Google Scholar]
- 4.Senterre T. Practice of enteral nutrition in very low birth weight and extremely low birth weight infants. World Rev Nutr Diet. 2014;110:201–14. doi: 10.1159/000358468. [DOI] [PubMed] [Google Scholar]
- 5.Li YF, Lin HC, Torrazza RM, et al. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatr Neonatol. 2014;55:335–40. doi: 10.1016/j.pedneo.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Torrazza RM, Parker LA, Li Y, et al. The value of routine evaluation of gastric residuals in very low birth weight infants. J Perinatol. 2015;35:57–60. doi: 10.1038/jp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S, Kim E-K, Neu J. Technologies for the evaluation of enteral feeding readiness in premature infants. Gastroenterology and Nutrition: Neonatology Questions and Controversies Series. 2012:339. [Google Scholar]
- 8.Cassia GS, Faingold R, Bernard C, Sant’Anna GM. Neonatal hypoxic-ischemic injury: sonography and dynamic color Doppler sonography perfusion of the brain and abdomen with pathologic correlation. Am J Roentgenol. 2012;199:W743–52. doi: 10.2214/AJR.11.8072. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Kim IO, Kim WS, Kang GH. Bowel sonography in sepsis with pathological correlation: an experimental study. Pediatr Radiol. 2011;41:237–43. doi: 10.1007/s00247-010-1806-4. [DOI] [PubMed] [Google Scholar]
- 10.Choi Y-H, Kim I-O, Cheon J-E, et al. Doppler sonographic findings in an experimental rabbit model of necrotizing enterocolitis. J Ultrasound Med. 2010;29:379–86. doi: 10.7863/jum.2010.29.3.379. [DOI] [PubMed] [Google Scholar]
- 11.Epelman M, Daneman A, Navarro OM, et al. Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics. 2007;27:285–305. doi: 10.1148/rg.272055098. [DOI] [PubMed] [Google Scholar]
- 12.Hata J, Kamada T, Haruma K, Kusunoki H. Evaluation of bowel ischemia with contrast-enhanced US: initial experience. Radiology. 2005;236:712–15. doi: 10.1148/radiol.2362040299. [DOI] [PubMed] [Google Scholar]
- 13.Faingold R, Daneman A, Tomlinson G, et al. Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology. 2005;235:587–94. doi: 10.1148/radiol.2352031718. [DOI] [PubMed] [Google Scholar]
- 14.Di Napoli A, Di Lallo D, Perucci CA, et al. Inter observer reliability of radiological signs of necrotising enterocolitis in a population of high risk newborns. Paediatric Perinatal Epidemiol. 2004;18:80–87. doi: 10.1111/j.1365-3016.2003.00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Buonomo C. The radiology of necrotizing enterocolitis. Radiol Clin North Am. 1999;37:1187–98. doi: 10.1016/s0033-8389(05)70256-6. [DOI] [PubMed] [Google Scholar]
- 16.Miller S, Seibert J, Kinder D, Wilson A. Use of ultrasound in the detection of occult bowel perforation in neonates. J Ultrasound Med. 1993;12:531–35. doi: 10.7863/jum.1993.12.9.531. [DOI] [PubMed] [Google Scholar]
- 17.Kodroff M, Hartenberg M, Goldschmidt R. Ultrasonographic diagnosis of gangrenous bowel in neonatal necrotizing enterocolitis. Pediatr Radiol. 1984;14:168–70. doi: 10.1007/BF01002304. [DOI] [PubMed] [Google Scholar]
- 18.Richburg DA, Kim JH. Real-time bowel ultrasound to characterize intestinal motility in the preterm neonate. J Perinatol. 2013;33:605–8. doi: 10.1038/jp.2013.17. [DOI] [PubMed] [Google Scholar]
- 19.Berseth CL, Nordyke CK. Manometry can predict feeding readiness in preterm infants. Gastroenterology. 1992;103:1523–28. doi: 10.1016/0016-5085(92)91173-2. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman G, Ghanayem N, Tweddell J. Non-invasive monitoring of oxygen delivery. In: Da Cruz EM, Ivy D, Jaggers J, editors. Pediatric and Congenital Cardiology, Cardiac Surgery and Intensive Care. Springer; London: 2014. pp. 835–55. [Google Scholar]
- 21.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:i3–i13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 22.Katheria AC, Leone TA, Woelkers D, et al. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. J Pediatr. 2014;164:1045–50.e1. doi: 10.1016/j.jpeds.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Corvaglia L, Martini S, Battistini B, et al. Bolus vs. continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr Res. 2014;76(1):81–85. doi: 10.1038/pr.2014.52. [DOI] [PubMed] [Google Scholar]
- 24.Dave V, Brion L, Campbell D, et al. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J Perinatol. 2008;29:213–18. doi: 10.1038/jp.2008.189. [DOI] [PubMed] [Google Scholar]
- 25.Gay AN, Lazar DA, Stoll B, et al. Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J Pediatr Surg. 2011;46:1034–40. doi: 10.1016/j.jpedsurg.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillam-Krakauer M, Cochran CM, Slaughter JC, et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J Perinatol. 2013;33:609–12. doi: 10.1038/jp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier S, Eble B, Stapleton G, et al. Mesenteric oxyhemoglobin desaturation improves with patent ductus arteriosus ligation. J Perinatol. 2006;26:562–64. doi: 10.1038/sj.jp.7211559. [DOI] [PubMed] [Google Scholar]
- 28.Patel AK, Lazar DA, Burrin DG, et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med. 2014;15:735–41. doi: 10.1097/PCC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 29.Petros A, Heys R, Tasker R, et al. Near infrared spectroscopy can detect changes in splanchnic oxygen delivery in neonates during apnoeic episodes. Eur J Pediatr. 1999;158:173–74. doi: 10.1007/s004310051046. [DOI] [PubMed] [Google Scholar]
- 30.Petrova A, Bhatt M, Mehta R. Regional tissue oxygenation in preterm born infants in association with echocardiographically significant patent ductus arteriosus. J Perinatol. 2011;31:460–64. doi: 10.1038/jp.2010.200. [DOI] [PubMed] [Google Scholar]
- 31.Petrova A, Mehta R. Regional tissue oxygenation in association with duration of hypoxaemia and haemodynamic variability in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2010;95(3):F213–19. doi: 10.1136/adc.2009.161604. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton GE, Eble BK, Dickerson HA, et al. Mesenteric oxygen desaturation in an infant with congenital heart disease and necrotizing enterocolitis. Texas Heart Inst J. 2007;34:442–44. [PMC free article] [PubMed] [Google Scholar]
- 33.Teller J, Schwendener K, Wolf M, et al. Continuous monitoring of liver oxygenation with near infrared spectroscopy during naso-gastric tube feeding in neonates. Schweiz Med Wochenschr. 2000;130(18):652–56. [PubMed] [Google Scholar]
- 34.Haykin SS. Neural Networks: A Comprehensive Foundation. Macmillan; 1994. [Google Scholar]
- 35.Katheria AC, Sauberan JB, Akotia D, et al. A pilot randomized controlled trial of early versus routine caffeine in extremely premature infants. Am J Perinatol. 2015;32:879–86. doi: 10.1055/s-0034-1543981. [DOI] [PubMed] [Google Scholar]
- 36.Montaldo P, De Leonibus C, Giordano L, et al. Cerebral, renal and mesenteric regional oxygen saturation of term infants during transition. J Pediatr Surg. 2015;50(8):1273–77. doi: 10.1016/j.jpedsurg.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Teller J, Wolf M, Keel M, et al. Can near infrared spectroscopy of the liver monitor tissue oxygenation? Eur J Pediatr. 2000;159:549. doi: 10.1007/s004310051334. [DOI] [PubMed] [Google Scholar]
- 38.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85:629S–34S. doi: 10.1093/ajcn/85.2.629S. [DOI] [PubMed] [Google Scholar]
- 39.Burrin DG, Stoll B, Jiang R, et al. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr. 2000;71:1603–10. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 40.Downard CD, Grant SN, Matheson PJ, et al. Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg. 2011;46:1023–28. doi: 10.1016/j.jpedsurg.2011.03.023. [DOI] [PubMed] [Google Scholar]