Abstract
This review discusses the most commonly employed techniques in the repair of proximal hypospadias, highlighting the advantages and disadvantages of single versus staged surgical techniques. Hypospadias can have a spectrum of severity with a urethral meatus ranging from the perineum to the glans. Associated abnormalities are commonly found with proximal hypospadias and encompass a large spectrum, including ventral curvature (VC) up to 50 degrees or more, ventral skin deficiency, a flattened glans, penile torsion and penoscrotal transposition. Our contemporary understanding of hypospadiology is comprised of a foundation built by experts who have described a number of techniques and their outcomes, combined with survey data detailing practice patterns. The two largest components of hypospadias repair include repair of VC and urethroplasty. VC greater than 20 degrees is considered clinically relevant to warrant surgical correction. To repair VC, the penis is first degloved—a procedure that may reduce or remove curvature by itself in some cases. Residual curvature is then repaired with dorsal plication techniques, transection of the urethral plate, and/or ventral lengthening techniques. Urethroplasty takes the form of 1- or 2-stage repairs. One-stage options include the tubularized incised urethroplasty (TIP) or various graft or flap-based techniques. Two-stage options also include grafts or flaps, including oral mucosal and preputial skin grafting. One stage repairs are an attractive option in that they may reduce cost, hospital stay, anesthetic risks, and time to the final result. The downside is that these repairs require mastery of multiple techniques may be more complex, and—depending on technique—have higher complication rates. Two-stage repairs are preferred by the majority of surveyed hypospadiologists. The 2-stage repair is versatile and has satisfactory outcomes, but necessitates a second procedure. Given the lack of clear high-quality evidence supporting the superiority of one approach over the others, hypospadiologists should develop their own algorithm, which gives them the best outcomes.
Keywords: Hypospadias, urethroplasty, children, grafts, ventral curvature (VC)
Introduction
Hypospadias can have a spectrum of severity with a urethral meatus ranging from the perineum to the glans. Hypospadias affects in 1 in 200 to 1 in 300 live births, with the majority of hypospadias occurring in the glans or subcoronally (1). Proximal hypospadias are estimated to comprise under 25% of all hypospadias repairs and include those defects located at the penoscrotal junction, scrotum or perineum (2). Associated abnormalities are commonly found with proximal hypospadias and encompass a large spectrum, including ventral curvature (VC) up to 50 degrees or more, ventral skin deficiency, a flattened glans, penile torsion and penoscrotal transposition (3) (Figure 1).
Figure 1.
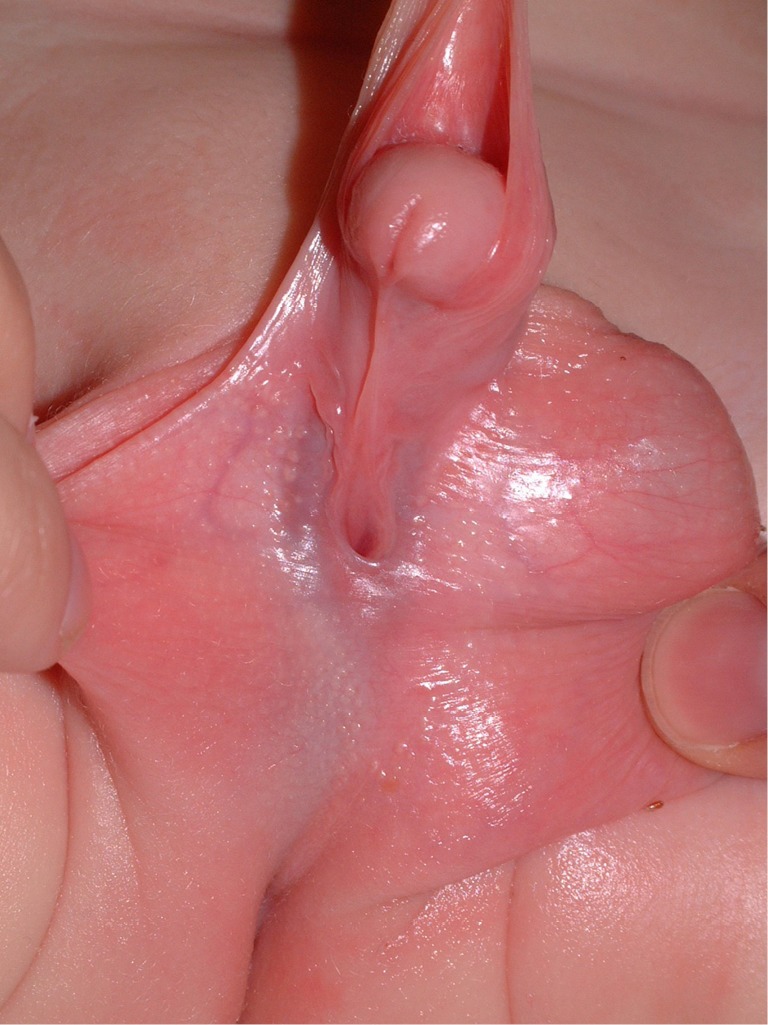
Appearance of a patient with proximal hypospadias.
Hypospadias surgery has been characterized historically by a multitude of techniques available for repair (2). A few key concepts have been described along the way, each being frequently modified (2). As with other fields in pediatric urology, the hypospadias literature is of low methodologic quality—generally based on small retrospective series (4). Our contemporary understanding of hypospadiology is comprised of a foundation built by experts who have described a number of techniques and their outcomes, combined with survey data detailing practice patterns. This review discusses the most commonly employed techniques in the repair of proximal hypospadias, highlighting the advantages and disadvantages of single versus staged surgical procedures.
Survey data
Our contemporary understanding of practice patterns in hypospadias repair comes from a number of recently published surveys. The geographic distribution, practitioner specialty distribution, time period, and topics of each survey differ significantly but several trends are noticed in their results, as discussed in relevant sections of this review.
In 1999, Bologna et al. reported the first of a number of informative surveys (5). This survey included 236 members of the American Academy of Pediatrics, Section on Urology, with a 52% response rate, who expressed their opinions on the VC aspect of the hypospadias complex (5). This survey defined the minimum degree of VC that most practitioners would repair as 20 degrees, and suggested that approaches to VC repair were quite heterogeneous.
In 2005, Cook et al. published a survey of hypospadias practice patterns of 121 pediatric urologists with an 83% response rate (6). The majority of respondents were full time academic pediatric urologists in North and South America and Europe who performed 10 or less hypospadias surgeries monthly. Respondents were reasonably distributed in terms of number of years in practice between 0 and more than 20. The survey concluded that practice patterns for proximal hypospadias were variable with no preference of one surgical technique over the others.
More recently, Springer et al. have electronically surveyed 413 pediatric urologists/surgeons from several professional associations on their hypospadias practice patterns (7). Only 6.4% of respondents were from the United States or Canada—with 31.8% from Asia/Australia and 31.3% from Europe. The majority of respondents performed between 11 and 50 cases a year (57%) with only 8.8% performing less than 10 cases a year. Like previous surveys, this survey suggested that techniques for urethroplasty and VC repair were quite variable amongst practitioners—although certain trends were evident. As urethral meatus location went from coronal to perineal, a preference for tubularized incised urethroplasty (TIP) repair decreased from 71% to 0.9% of respondents—with 2-stage repairs increasing from 0.5% to 76.6%.
Finally, Steven et al. (8) have published the results of an online survey of attendants at the 2011 World Congress of the International Society for Hypospadias and Disorders of Sex Development in London, England. The authors had 93 respondents with an estimated response rate of 78%. The majority of respondents were from Europe (57%). Respondents were pediatric urologists in 48% of cases and pediatric surgeons in 35% of cases. A total of 41% of respondents performed 51 or more procedures a year. This survey also reported that a variety of techniques were preferred by survey respondents for proximal hypospadias—although 2-stage repairs were preferred by the majority.
Components of proximal hypospadias repair
Repair of proximal hypospadias involves correction of several components of the hypospadias complex with the intent of optimizing long-term functional and cosmetic outcomes. These components include ventral penile curvature, proximal location of the urethral meatus, ventral skin deficiency, abnormal glans morphology, abnormal division of the corpus spongiosum, penile torsion and penoscrotal transposition (3).
Ventral curvature (VC)
VC of the penis exists to varying extents in proximal hypospadias. Some of the apparent VC may be simply due to ventral skin deficiency and skin tethering. This type of skin tethering is generally corrected by degloving the penis at the beginning of the hypospadias operation and, normally, does not need further repair. A series from The Hospital for Sick Children, Toronto, Canada has looked at the improvement of VC solely from penile degloving (9). This study included 137 patients, of which 9 had mild (<30 degrees) curvature, 44 had moderate (30-45 degrees) and 84 severe (>45 degrees) (Table 1). Seventy seven percent of patients with mild VC, 52% of those with moderate VC and 40.4% of those with severe VC had an improvement of their curvature solely after degloving. The percentage of patients who had complete resolution of curvature with degloving was 77%, 30%, and 2.4%, in the mild, moderate and severe curvature groups, respectively.
Table 1. Percentage (%) of patients with no ventral curvature after penile degloving in a series of 137 proximal hypospadias repairs from Sickkids, Toronto, Canada (9).
| Type of VC | Preop (before degloving) (n=137) | Residual VC after degloving (n) | % with no VC post degloving |
|---|---|---|---|
| Mild [<30] | 9 | No VC 7; mild 2 | 77.3 |
| Moderate [30-45] | 44 | No VC 13; mild 10; moderate 21 | 29.5 |
| Severe [>45] | 84 | No VC 2; mild 2; moderate 31; severe 49 | 2.4 |
VC, ventral curvature.
An artificial erection test, originally described by Gittes and McLaughlin (10), is used by most hypospadias surgeons to assess initial and residual VC in the corpora after penile degloving (8). Two decisions must be made in the operative management of VC that persists after degloving—whether the urethral plate is to be divided and whether a dorsal shortening or ventral lengthening approach should be used. These factors are highly dependent on the degree of VC and in some cases, VC is so mild as to not require any correction (5) (Figure 2).
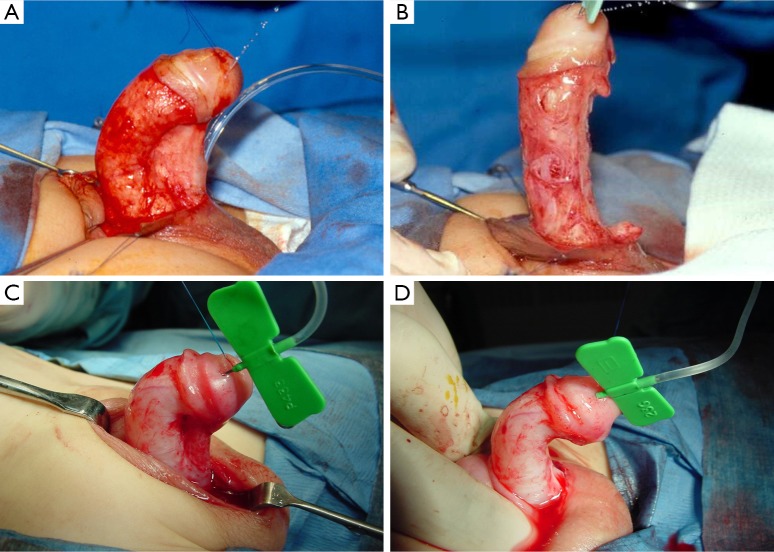
Figure 2.
In some cases of severe ventral curvature, tension arising from a tethered urethral plate is evident (A). Division of this tethering urethral plate (B) may then allow for penile straightening with preservation of penile length. In other cases (C) disproportion of the ventral corpora contributes to curvature that is not released by urethral plate transection (D). In these cases, additional ventral lengthening maneuvers are warranted.
Minor VC
Patients with less than 30-40 degrees of curvature can be considered to have minor VC. This group of patients with minor VC is fairly prevalent in proximal hypospadias, with Snodgrass and Prieto (11) reporting that approximately 50% of their proximal hypospadias repairs have 30 degrees of curvature or less after degloving. Most surveyed physicians would perform repair of VC beginning at around 20 degrees of curvature and the preference for a dorsal plication technique to repair curvature remains fairly homogeneous until around 40 degrees of curvature (5). An important consideration is that it is uncertain how these consensus-based paradigms surrounding curvature correction relate to patient-centered outcomes. Whether a 20 degree curvature is significant and should be corrected is unknown from a patient perspective.
Surgical repair of minor VC generally includes urethral plate preservation and dorsal shortening techniques. With minor VC, urethral plate transection is performed less than 25% of the time (5,7). Dorsal shortening techniques include different types of dorsal plications, such as the Nesbit and Baskin procedures (1). In the survey by Cook et al. (6) 83% of respondents reported using a dorsal plication technique for VC of up to 30-40 degrees. Springer et al. (7) and Bologna et al. (5) also reported that most would perform either a single dorsal plication suture or a Nesbit procedure in this curvature range.
Based on anatomic studies by Baskin et al. (12), the 12 o’clock position has been found to be free of nerves. For this reason, dorsal plication is recommended at the 12 o’clock position with one or more sutures. Baskin and Ebbers (1) recommend against the use of this approach when more than two rows of plication sutures or four permanent sutures are required, or when VC cannot be manually corrected with a finger. It is the practice of the senior author not to use dorsal plication when more than one suture is required. Dorsal plication is reported to have a 7% short-term recurrence (13). The Nesbitt procedure (14) is performed by excising corporal fascia dorsally and reapproximating fascial edges with a buried suture, although a number of modifications exist (2).
Major VC
With higher degrees of VC (40 degrees or more), urethral plate transection, ventral lengthening techniques, or both, are more frequently employed. In the experience of the senior author, a urethral plate that is evidently the site of tethering on an artificial erection test, demonstrating residual moderate or severe VC after extensive degloving down to the bulbar urethra will require urethral plate transection. The degree of tension arising from a tethered urethral plate is evident when the penis pivots around the urethral plate during attempts to manually straighten the penis. Urethral plate transection may then reveal the true residual curvature within the corpora requiring correction. In cases of a tethering urethral plate, this approach may also contribute to increased penile length.
The main downside of urethral plate division is that the urethral plate is no longer available for use during urethroplasty. The urethral plate was historically considered to be dysplastic and viewed as contracted scar tissue that had to be divided to in order to correct the VC. This approach has fallen out of favor with the understanding that the urethral plate may be comprised of healthy tissue (15) and preservation of the urethral plate can be performed in selected cases.
Prior to transecting the urethral plate in cases of severe curvature, some authors may attempt to mobilize the urethral plate from the corpora cavernosa all the way to the bulbar urethra. This results in some improvement in VC and may allow for urethral plate preservation. Concerns about use of the urethral plate in urethroplasty following extensive urethral mobilization include the risk of stricture. With the adoption of an algorithm that promoted more extensive mobilization of the urethral plate to reduce curvature as opposed to early division, Snodgrass and Prieto (11) increased rates of preservation of the urethral plate from 57% to 87% in two consecutive time periods. None of the patients in the later time period had recurrent curvature at a mean follow-up of 11 months. A follow-up study, however, demonstrated four cases of recurrent VC and a 17% stricture rate in the group that was extensively mobilized (16), leading these authors to be slightly less enthusiastic about extensive urethral plate mobilization. Bhat (17) has described that mobilization of the urethral plate may correct curvature in 88% of cases with only 6% requiring urethral plate division for VC correction—an approach that resulted in no recurrent VC or strictures with a mean follow-up of 23 months. Others have also reported reasonable outcomes with urethral plate mobilization (18,19).
Even with the option for urethral plate mobilization, VC may still persist and urethral plate transection will be required. At 50 degrees of curvature, approximately 35% of practitioners would divide the urethral plate (5,7). Similarly, most practitioners are performing urethroplasty techniques that do not preserve the urethral plate at this degree of curvature (6). VC recurrence rates after urethral plate transection are acceptably low (4,20).
The other way to address severe VC is by lengthening the ventral aspect of the penis instead of using dorsal plication techniques that risk shortening the phallus. Dorsal shortening techniques may lead to significant length loss with severe VC correction, which is especially concerning in patients with proximal hypospadias, who already have a smaller penis compared to those with more distal defects.
Braga et al. have published a study comparing dorsal plication versus ventral lengthening techniques in proximal hypospadias with severe curvature (greater than 45 degrees) (20). A total of 32 patients were in the ventral lengthening group and 68 were in the dorsal plication group. Patients were followed for a mean of approximately 5 years in each group and the primary outcome of recurrent curvature was assessed by physical examination with reflex erection, erection test under general anesthesia (usually during repeat surgery for complications), or parent report. The urethral plate was transected in 94% of patients with ventral lengthening vs. 23.5% of patients with dorsal plication. Recurrent curvature was seen in 9.4% of the ventral lengthening group compared to 28% of the dorsal plication group (P=0.03 for difference). Interestingly, none of the 16 patients who underwent dorsal plication with urethral plate transection had recurrent curvature. Although limited by its retrospective nature and pre-pubertal follow-up, this study provides some evidence that a ventral lengthening technique may be more durable than a dorsal shortening approach in cases of severe VC. The role of urethral plate transection in protecting against recurrent curvature is also suggested, but low numbers prevented further exploration of this hypothesis.
Ventral lengthening options include multiple ungrafted incisions (fairy cuts), grafting with dermis, tunica vaginalis, or 1-ply small intestinal submucosa (21), and tunica vaginalis flap techniques (22). Snodgrass and Bush (13) have identified a number of series supporting <10% VC recurrence rates with these techniques. It is the practice of the senior author to make multiple ventral corporotomies without grafting in cases of severe curvature (>70 degrees) that persists after extensive degloving and urethral plate transection. This avoids graft related complications including graft contracture and aneurysm formation. Additionally, use of this technique combined with a 2-stage repair avoids the need to place the urethroplasty graft on top of the corporal graft. This procedure is performed by making up to 3 transverse corporotomies at the point of maximum curvature from the 4 to 8 o’clock positions. The incision is through tunica albuginea until erectile tissues are visible (23).
Theoretical concerns with ventral lengthening procedures include aneurysmal dilation (Figure 3), penile instability, predisposition to penile fracture, and erectile dysfunction secondary to venous leak—of which at least one case has been identified (24). Similarly, in a minority, dorsal plication procedures may lead to dissatisfaction with penile shortening, erectile dysfunction, and reduced penile sensation (13).
Figure 3.
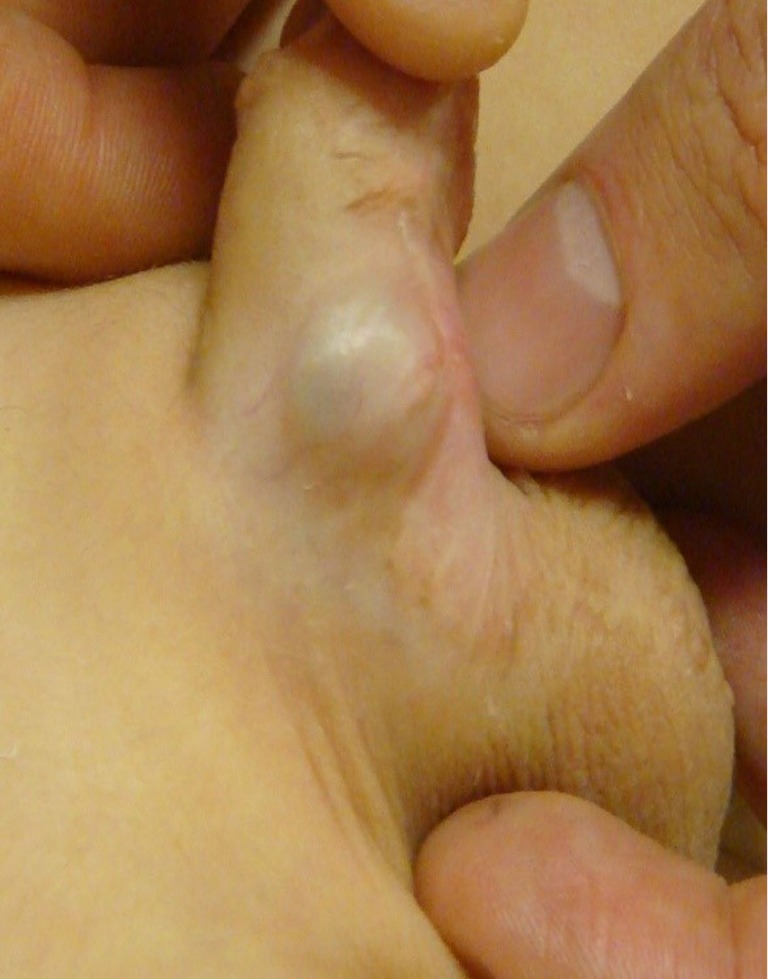
Aneurysmal dilation arising from a grafted ventral corporotomy.
It is uncertain how VC repair with either technique holds up with penile growth during puberty. Very few studies have reported post pubertal follow-up. Badawy and Morsi (24) described the case of one patient with erectile dysfunction and two insignificant recurrent VC in 16 patients that had postpubertal follow-up after dermal grafting for VC correction. Looking at things the other way, Vandersteen and Husmann (25) have reported on their experience of 22 adult patients with recurrent VC after prior proximal hypospadias repair that included VC correction with a Nesbit procedure (n=19) or tunica vaginalis graft (n=3). Patients generally noticed the development of VC as puberty progressed and they were all symptomatic, generally with sexual dysfunction. This study is limited by the fact that the study design did not allow us to determine the real incidence of recurrent VC (denominator was missing) and the major technique of urethroplasty in this series was a tube graft, no longer performed today. Nonetheless, all of these patients were apparently free of complications at discharge from their pediatric urologist, presenting with delayed VC only at the time of puberty. This observation raises the importance of post-pubertal follow-up studies and the fact that techniques which may provide for excellent VC correction in the short term still have uncertain long-term outcomes.
Urethroplasty
Correction of the location of the urethral meatus to a position in the glans with a cosmetically appealing appearance is the second component of proximal hypospadias repair. The decision on which urethroplasty technique to use depends on whether the urethral plate is preserved after or during correction of VC. In contemporary hypospadias surgery, there has been an increasing trend to preserve the urethral plate where possible (11). This stems from a recognition that the urethral plate is not always comprised of fibrotic tissue that leads to VC, as once thought (15).
Urethroplasty with preservation of urethral plate
In cases where the urethral plate is preserved and the surgeon feels it is healthy (i.e., elastic and supple) and usable, a TIP or Onlay urethroplasty may allow for a one-stage repair. In the experience of the senior author, proximal cases where the urethral plate may be preserved are fairly uncommon—in most peno-scrotal and perineal hypospadias, the plate tends to be inelastic, narrow, and associated with poorly developed spongiosum. Additionally, transection is required for VC correction with appropriate preservation of penile length.
Nonetheless, for the appropriate cases—Snodgrass has described a TIP repair for proximal hypospadias following similar principles to his TIP repair for distal defects (26). Glans wings are first separated from the urethral plate at the level of the glans. The corpus spongiosum is then mobilized from the corpora cavernosa along its length for later coverage of the urethroplasty. After artificial erection and repair of VC, the urethral plate is incised in the midline and tubularized in two-layer. Tubularization stops 3 millimeters shorter ventrally than the dorsal extent of the urethral plate to create a slit-like meatus. The spongiosum is then reapproximated over the urethra and covered with a tunica vaginalis barrier flap.
A systematic review has identified eight studies describing 260 patients undergoing proximal TIP repairs (4). The mean complication rate was 24.2% with a fistula/dehiscence rate of 20% and a stricture/stenosis rate of 2.7%. A number of hypothesis surround the etiology of the high fistula rate in long TIP urethroplasty for proximal hypospadias include high urethral pressure caused by a long suture line (27) and technical factors such as inadequate coverage of the repair (23). With adoption of a two-layer urethroplasty covered by a tunica vaginalis flap, Snodgrass noted no fistulas in his last 22 cases (26).
According to Snodgrass’ initial reports on proximal hypospadias, extensive urethral plate mobilization allowed him to perform TIP urethroplasty in 86% of cases (11), reducing 2-stage repair rates to only 14%. However, a follow-up study demonstrated that 17% of patients who had extensive urethral plate mobilization developed symptomatic strictures (16). He hypothesized that urethral strictures were related to devascularization of the urethra caused by extensive mobilization, and no longer advocates proximal TIP in combination with extensive urethral mobilization for penoscrotal hypospadias with severe VC. Other studies have not demonstrated significant stricture rates for TIP combined with extensive urethral plate mobilization for VC correction (17-19), nonetheless caution is warranted in combining these two techniques. Fortunately, approximately 50% of patients had VC <30 degrees after degloving and did not require this extensive urethral plate mobilization for VC correction, still allowing for ample use of the proximal TIP technique with acceptable outcomes (16). Cook et al. (6) have reported that a proximal TIP repair would be preferred by up to 43% of practitioners when the patient had no VC, but only by 3% when the patient had greater than 50 degrees of VC. Springer et al. (7) and Steven et al. (8) reported the preference of proximal TIP by only 10-15% of practitioners.
Another option for urethroplasty with preservation of the urethral plate is the Onlay transverse island flap (TVIF). This technique has the benefit of allowing for 1-stage repair in cases where the urethral plate does not need to be transected, but is not wide enough for tubularization (TIP repair). The Onlay TVIF was well described in a 1987 paper by Elder, Duckett, and Snyder (28), a modification of a prior tubularized TVIF technique by Duckett, which did not utilize the urethral plate (29). This technique begins similar to the proximal TIP in that the penis is degloved, urethral plate is preserved, and VC corrected if necessary. A rectangular flap is then harvested from the dorsal inner prepuce, ventrally rotated, and anastomosed to the urethral plate ventrally (27). The TVIF thus forms the ventral aspect of the neourethra with the urethral plate forming the dorsal aspect. Coverage for the suture line is provided by the vascular pedicle of the flap. A systematic review (4) identified 12 studies describing 367 patients that had undergone Onlay urethroplasty. The complication rate was similar to proximal TIP—means complication rate was 27.5%, mean fistula/dehiscence rate was 17.2%, and the mean stricture/stenosis rate was 4.4%. Neourethral diverticula and recurrent VC also rarely occur (26). Surveys suggested that Onlay island flaps were preferred by 10-30% of surgeons, with decreasing use in patients with >50 degrees of curvature and perineal hypospadias (6-8).
Urethroplasty without preservation of the urethral plate
If the urethral plate is not used, a substitution urethroplasty is required. Despite the increasing popularity of urethral plate preservation, familiarity with a substitution urethroplasty technique is essential for any surgeon operating on proximal hypospadias, as these cases still constitute a significant proportion of repairs (16). Substitution urethroplasty may take the form of a 1- or 2-stage repair with graft or flap.
Although once a popular technique for hypospadias repair (25), 1-stage repairs with tubularized graft have fallen out of favor due to poor outcomes. A 46% mean complication rate was identified with 1-stage free graft techniques, including a 26.4% stricture or stenosis rate (4). More recently, there has been some resurgence in 1-stage graft procedures with the use of the Snodgraft procedure (30). The Snodgraft procedure is similar to the TIP procedure with the addition of an oral mucosal graft inlayed in the defect created by urethral plate incision. The Snodgraft procedure has been employed in reoperative cases (31,32), but comprehensive primary outcome data is unavailable.
Contemporary 1-stage substitution urethroplasty thus generally involves the use of flaps. The most popular flap for 1-stage repair is the tubularized preputial island flap, as popularized by Duckett (29). This technique involves an island flap of preputial skin which is tubularized and brought ventrally to serve as a neourethra. This is very similar to the Onlay TVIF previosuly described (28), but involves tubularization of the flap instead of using it in an Onlay fashion. In a total of 11 studies including 535 patients, the mean complication rate of this technique was 38%, with a 22.4% mean fistula/dehiscence rate and a 12.5% mean stricture/stenosis rate (4). Diverticuli, megalourethra, and recurrent VC have also been reported (26). Vallasciani et al. have described 5 cases of megalourethra associated with TVIF (33). They found that none of the cases were associated with distal obstruction, suspecting that they originated from a lack of support of the neourethra allowing for progressive dilation. These authors corrected these cases of megalourethra by tapering the urethra and did not observe any recurrences after a mean follow-up of 9.3 years. Cook et al. report that this technique was preferred by 40% of practitioners with greater than 50 degrees of curvature (6).
Variations of this technique include the Onlay-Tube-Onlay modification as described by Upadhyay and Khoury (34). This technique is a combination of both, the Onlay TVIF and the tubularized TVIF repairs. After the urethral plate is transected, the proximal and distal ends are incorporated into the urethroplasty in an Onlay fashion. The middle part of the urethroplasty is a tubularized TVIF. Using this approach in 16 patients, Khoury achieved a normal-looking penis in 70% of cases, with a 31% reoperation rate (5 patients) (34). Rigamonti and Castagnetti have described a modification of this technique named Onlay on Albuginea (35). In this procedure, the urethral plate is transected and a TVIF is sutured to the tunica albuginea in an Onlay fashion, with the proximal and distal ends Onlayed onto the remnants of the transected urethral plate. Over time, epithelialization of the tunica albuginea incorporated into the neourethra occurs. In their 14 reported cases with a mean follow-up of 7 months, these authors observed 1 fistula, 1 partial urethroplasty breakdown, and 1 patient with ballooning of the neourethra.
Another option for 1-stage repair is the Koyanagi procedure (36). This technique involves a flap of ventral skin extending to the dorsal prepuce and used to create a tubularized neourethra. In a total of 9 studies with 351 patients, the mean complication rate of this technique was 32% with a fistula/dehiscence rate of 28% and a stricture/stenosis rate of 7.4%. More recent studies have reported a lower complication rate with technical modifications (37,38), nonetheless in some series the majority of patients had a complication and required additional procedures (39). Surveys have generally not included this technique as a separate option, despite a number of published series supporting its use.
Two-stage repairs involve correction of curvature, excision of the urethral plate, and harvesting a graft or flap to create a neourethral plate in the first stage (Figure 4). As a result of the first stage, the meatus remains in its proximal location. The second stage involves tubularization of the graft or flap that was placed during the first stage to move the urethral meatus to a location in the glans. Surveys vary in their results regarding preferences for 2-stage repairs although, like tubularized TVIF flaps, they are more popular in cases of severe curvature and proximal hypospadias. Cook et al. (6) reported that 37% of practitioners would use a staged repair in cases of >50 degrees of curvature—29% with a buccal graft and 8% with a prepucial graft. These contrasts to only 5% who would prefer to use either when there was no curvature, representing a preference for techniques that make use of the urethral plate in this case by 86%. In the survey by Springer et al. (7), 43% of the respondents would use a 2-stage technique for penoscrotal hypospadias while 77% of practitioners preferred a 2-stage repair in patients with perineal hypospadias. The survey by Steven et al. (8) did not stratify proximal hypospadias but around 50% of respondents preferred two-stage repairs overall.
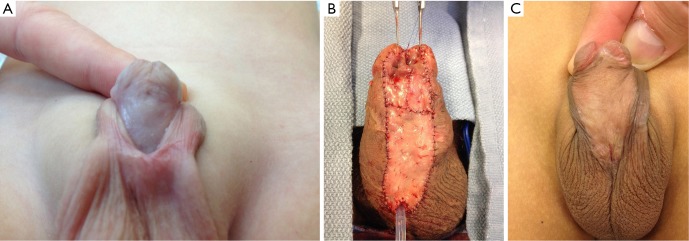
Figure 4.
Grafting in 2-stage urethroplasty. (A) A first stage preputial graft that has taken well; (B) a buccal mucosal graft; (C) another preputial graft that has taken well.
Another option for 2-stage repair is the use of Byars flaps (2). In the first stage, the penis is degloved, VC corrected, the urethral plate is excised, a glans cleft is created, and the Byars flaps are sutured to the ventral penile shaft. In the second stage, the Byars flaps are tubularized in 2-layers to bring the meatus to the glans. Although the reported mean complication rate of this technique is relatively low compared to other proximal hypospadias repair techniques (21%) (26) a number of unique problems may occur. These include neourethral diverticuli resulting from stretch of the distensible preputial skin, poor fixation of the Byars flaps to the corpora cavernosa due to interposed dartos pedicle, and difficulty making the redundant flap into a uniform smooth neourethra (26,30).
The two most commonly used graft options for a 2-stage urethroplasty include preputial skin and buccal mucosa. An important consideration in the use of grafts is that if a graft is used primarily to correct the VC by lengthening the ventral aspect of the penis, further graft to serve as a new urethral plate for the second stage urethroplasty should not be placed on top of the first one, as evidence suggests that graft take may be poor (26). Given the fact that urethral plate transection is often performed for severe VC that will then require correction with a ventral lengthening technique—this is an important point to remember.
Preputial skin grafting involves use of skin from the undersurface of the prepuce to create a neourethral plate. Bracka is credited with popularizing this grafting technique for hypospadias repair (40). The strengths of this graft are that it is thin and flexible, takes reliably, is designed to be moist, has no potential for hair growth and has an expendable donor site (30). After the graft is harvested and the dartos layer removed, it is secured to the corpora, ventrally to form the neourethral plate. Tubularization is then performed 6 months later.
Oral mucosa serves as another important source of graft for hypospadias repair. Again, there is little donor site morbidity and no potential for hair growth. Donor site complications from this graft are rare and minor (41). Graft can be harvested from the cheek or lip, and is particularly useful in reoperative cases when the foreskin has been used or lichen sclerosus is present. Important principles in graft harvesting include limiting buccal graft harvests to not cross the angle of the mouth into the lower lip, preventing cosmetic facial deformities during wound healing. Buccal mucosal graft is relatively stiff which prevents diverticula and does not prolapse (2). In a prospective study of 350 adults undergoing buccal mucosa graft harvesting, 98% would have the technique performed again—85% experienced no pain, 4% had oral numbness at 3 months, 2% reported changes in oral sensitivity, and 100% encountered no difficulties in smiling (42).
1- vs. 2-stage repairs
One stage repairs are an attractive option in that they may reduce cost, hospital stay, anesthetic risks, and time to the final result. As described above, favorable long-term results with acceptable complication rates have been described for 1-stage repairs using the urethral plate such as proximal TIP and Onlay TVIF repairs (4).
Preference for one-stage repairs requires the surgeon to be more versatile and master a number of different techniques that involve preservation and transection of the urethral plate depending on the circumstances, as well as familiarity with a 1 or more 2-stage repairs for complex and reoperative cases. The mastery of one or more additional techniques is thus a challenge inherent to this approach. Additionally, if the urethral plate is not used for the urethroplasty, 1-stage repairs may have higher complication rates (4). Although limited by the lack of controlled studies—a recent systematic review suggested that the mean complication rate with 2-stage repairs (22.2%) was lower than that of 1-stage repairs that did not utilize the urethral plate (32-46%) (4), but similar to reported mean complication rates for proximal TIP (24.2%) and Onlay TVIF (27.5%) (4).
Two-stage repairs permit compartmentalization of the repair, providing the opportunity to reevaluate the situation along the way for complex hypospadias cases. The time interval between the first and second stage allows for growth of the penile structures and reveals complications that relate to recurrent VC or graft contracture. Dealing with these complications earlier can be simpler than after urethral tubularization, leading to a more successful final outcome. Problems after the second stage are then generally isolated to the urethroplasty.
Complications after 1-stage repairs can be quite complex. Although rare, complete flap necrosis or dehiscence of the entire repair may occur, leading to multiple reoperations. With this in mind, approximately 70% of patients undergoing 1-stage repairs do avoid a secondary procedure (4). The current preference for 2-stage repairs in severe hypospadias cases among pediatric urologists presumably reflects the versatility of staged procedures in contrast to the increased complexity of single stage repairs and their higher complication rate. Accordingly, survey data has shown that staged repairs were favored in cases of scrotal and perineal hypospadias with greater than 50 degrees of VC (6) and (7). Although not included in those surveys, we hypothesize that other factors that increase the complexity of a case and the chance for complications would also serve as predictors for a preference for a 2-stage repair—including reduced width and lack of elasticity of the urethral plate, small glans size, reoperative cases and medical comorbidities.
In the experience of the senior author, proximal hypospadias cases amenable to 1-stage urethral plate preserving techniques are rare. This is due to the predominance of unfavorable characteristics for this repair in this patient population—a narrow and inelastic urethral plate, poorly developed spongiosum, scrotal or perineal meatal location, and significant urethral plate tethering resulting in severe VC. The use of a single stage repair preserving the urethral plate in these sorts of cases may result in penile shortening, residual or recurrent VC and urethral strictures due to excessive plate mobilization and inadequate correction of curvature. In addition, the use of a 1-stage repair that discards the urethral plate increases the complexity of the single stage repair and may contribute to even higher complication rates. As a result, a 2-stage preputial graft technique is most commonly used by the senior author.
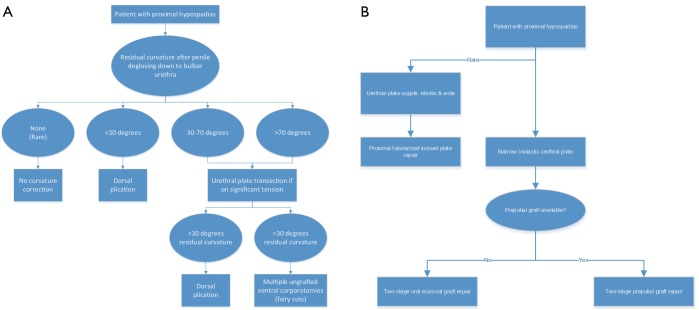
Given the lack of clear high-quality evidence supporting the superiority of one approach over the others, hypospadiologists should adopt an algorithm that gives them the best outcomes in their hands. For many, this may include a 1-stage approach in cases of proximal hypospadias with “healthy” urethral plates and VC less than 45 degrees and a 2-stage approach for the more severe cases (Figure 5).
Figure 5.
An algorithm for approaching ventral curvature (A) and urethroplasty (B) is presented. In the experience of the senior author, a finding of a wide and elastic urethral plate that does not contribute to ventral curvature is rare.
Barrier layer and skin closure
The hypospadias complex includes a redundant dorsal foreskin and ventral skin deficiency. This disproportion can sometimes make skin closure very challenging. Skin closure after VC correction and urethroplasty should be symmetric and without excessive tension to prevent recurrent skin tethering and curvature. In the experience of the senior author, midline closure can almost always be accomplished even in cases of severe ventral skin deficiency. If primary closure is not possible, a Byars skin flap may be utilized. Cosmetic outcomes of this approach may be less than ideal. In cases where a buried penis is anticipated, there may be value in suturing shaft skin at the base of the penis to Buck’s fascia.
In addition to skin closure, creation of protective intermediate layers is an important concept in urethroplasty. The intent of this step is to provide mechanical support for the neourethra, provide healthy tissue to aid in vascularization of the neourethra, and provide barrier layers to prevent fistulae. Snodgrass and Yucel describe the use of spongioplasty as part of their closure (43). The corpus spongiosum is mobilized and reapproximated over the neourethra in a Y-to-I fashion (44). Additional options for this barrier layer include tunica vaginalis flap, dorsal subcutaneous flap, dartos flap, or an external spermatic fascia flap (2). Snodgrass has found tunica vaginalis to be a more substantial flap than dartos when used as an interpositional layer (23). These interpositional layers may be used in addition to spongioplasty to provide added support (43). Speaking to the value of these techniques, Snodgrass has reduced his rate of urethrocutaneous fistulas significantly with the adoption of a tunica vaginalis barrier flap, spongioplasty, and a two-layer subepithelial closure technique (23).
The other issue with respect to skin closure is foreskin preservation. Within certain cultures, the goal of creating a normal penis includes that of an uncircumcised penis. Foreskin preservation can be employed when the dorsal prepuce was not used for urethroplasty. Isolated reports of its use in cases of proximal hypospadias have been described (23,45). At present, compared to distal hypospadias, foreskin preservation in proximal hypospadias has been infrequently reported and the complication rate of this approach is not yet well established.
Penoscrotal transposition
Penoscrotal transposition is also commonly observed in proximal hypospadias cases and a number of techniques are available for its correction (2). It can be corrected as part of a single stage repair or at the time of the first or second stage, during staged procedures, according to surgeon preference.
Reoperative hypospadias
Reoperative proximal hypospadias operations are more complex due to scar tissue and variability of penile and foreskin anatomy. Additionally, commonly used donor sites for grafts or flaps may be unavailable due to their prior use. Reoperative cases have to be approached with the goal of correcting the primary problem in mind (scarred urethral plate, residual VC due to inadequate correction of curvature, breakdown or dehiscence of the entire repair, graft contraction, etc.), and may require the use of a variety of surgical techniques (46).
As with primary repairs, reoperative hypospadias can be divided into those with a useable residual urethral plate and those without. After degloving and repair of VC, a decision is made whether to use the urethral plate or not. The urethral plate in this case may not be the congenital urethral plate but one constructed from previous graft or flap. If it appears healthy and usable, a TIP repair may be employed or a dorsal inlay graft can be used according to the “Snodgraft” principle (30).
In cases where the urethral plate is not useable, a 2-stage repair with buccal mucosal graft is favored because prior hypospadias repair will often have used or distorted the vascular supply of local grafts and flaps. Balanitis xerotica obliterans (BXO) may also be present in some of these multi-operated cases and it best treated by excising all scarred tissue and replacing it with oral mucosa. A buccal mucosal graft has the advantages of a versatile non-hair bearing graft that develops little redundancy and has good graft take (30). Acceptable results using oral mucosal grafts have been published for reoperative hypospadias cases (47).
Conclusions
Surgery for proximal hypospadias has been markedly advanced in the last 20 years with the popularization of a number of viable 1- and 2-stage repair techniques. At this time, short-term functional and cosmetic outcomes are satisfactory—repair generally allows for a straight normal appearing penis with adequate urinary function. Moving forward, understanding the true utility of these techniques will only be possible if we promote the publication of better quality, prospective studies with long-term follow up data.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg 2006;41:463-72. [DOI] [PubMed] [Google Scholar]
- 2.Hadidi AT, Azmy AF. eds. Hypospadias surgery: an illustrated guide. Heidelberg: Springer Verlag, 2004. [Google Scholar]
- 3.van der Toorn F, de Jong TP, de Gier RP, et al. Introducing the HOPE (Hypospadias Objective Penile Evaluation)-score: a validation study of an objective scoring system for evaluating cosmetic appearance in hypospadias patients. J Pediatr Urol 2013;9:1006-16. [DOI] [PubMed] [Google Scholar]
- 4.Castagnetti M, El-Ghoneimi A. Surgical management of primary severe hypospadias in children: systematic 20-year review. J Urol 2010;184:1469-74. [DOI] [PubMed] [Google Scholar]
- 5.Bologna RA, Noah TA, Nasrallah PF, et al. Chordee: varied opinions and treatments as documented in a survey of the American Academy of Pediatrics, Section of Urology. Urology 1999;53:608-12. [DOI] [PubMed] [Google Scholar]
- 6.Cook A, Khoury AE, Neville C, et al. A multicenter evaluation of technical preferences for primary hypospadias repair. J Urol 2005;174:2354-7. [DOI] [PubMed] [Google Scholar]
- 7.Springer A, Krois W, Horcher E. Trends in hypospadias surgery: results of a worldwide survey. Eur Urol 2011;60:1184-9. [DOI] [PubMed] [Google Scholar]
- 8.Steven L, Cherian A, Yankovic F, et al. Current practice in paediatric hypospadias surgery; a specialist survey. J Pediatr Urol 2013;9:1126-30. [DOI] [PubMed] [Google Scholar]
- 9.Braga LH, Pippi Salle JL, Dave S, et al. Outcome analysis of severe chordee correction using tunica vaginalis as a flap in boys with proximal hypospadias. J Urol 2007;178:1693-7; discussion 1697. [DOI] [PubMed]
- 10.Gittes RF, McLaughlin AP, 3rd. Injection technique to induce penile erection. Urology 1974;4:473-4. [DOI] [PubMed] [Google Scholar]
- 11.Snodgrass W, Prieto J. Straightening ventral curvature while preserving the urethral plate in proximal hypospadias repair. J Urol 2009;182:1720-5. [DOI] [PubMed] [Google Scholar]
- 12.Baskin LS, Erol A, Li YW, et al. Anatomy of the neurovascular bundle: is safe mobilization possible? J Urol 2000;164:977-80. [DOI] [PubMed] [Google Scholar]
- 13.Snodgrass WT. eds. Pediatric Urology: Evidence for Optimal Patient Management. New York: Springer, 2013. [Google Scholar]
- 14.Nesbit RM. Operation for correction of distal penile ventral curvature with or without hypospadias. Trans Am Assoc Genitourin Surg 1966;58:12-4. [PubMed] [Google Scholar]
- 15.Snodgrass W, Patterson K, Plaire JC, et al. Histology of the urethral plate: implications for hypospadias repair. J Urol 2000;164:988-9. [DOI] [PubMed] [Google Scholar]
- 16.Snodgrass WT, Granberg C, Bush NC. Urethral strictures following urethral plate and proximal urethral elevation during proximal TIP hypospadias repair. J Pediatr Urol 2013;9:990-4. [DOI] [PubMed] [Google Scholar]
- 17.Bhat A. Extended urethral mobilization in incised plate urethroplasty for severe hypospadias: a variation in technique to improve chordee correction. J Urol 2007;178:1031-5. [DOI] [PubMed] [Google Scholar]
- 18.Kajbafzadeh AM, Arshadi H, Payabvash S, et al. Proximal hypospadias with severe chordee: single stage repair using corporeal tunica vaginalis free graft. J Urol 2007;178:1036-42. [DOI] [PubMed] [Google Scholar]
- 19.Mollard P, Castagnola C. Hypospadias: the release of chordee without dividing the urethral plate and onlay island flap (92 cases). J Urol 1994;152:1238-40. [DOI] [PubMed] [Google Scholar]
- 20.Braga LH, Lorenzo AJ, Bägli DJ, et al. Ventral penile lengthening versus dorsal plication for severe ventral curvature in children with proximal hypospadias. J Urol 2008;180:1743-7. [DOI] [PubMed] [Google Scholar]
- 21.Castellan M, Gosalbez R, Devendra J, et al. Ventral corporal body grafting for correcting severe penile curvature associated with single or two-stage hypospadias repair. J Pediatr Urol 2011;7:289-93. [DOI] [PubMed] [Google Scholar]
- 22.Braga LH, Pippi Salle JL, Dave S, et al. Outcome analysis of severe chordee correction using tunica vaginalis as a flap in boys with proximal hypospadias. J Urol 2007;178:1693-7. [DOI] [PubMed] [Google Scholar]
- 23.Snodgrass W, Bush N.Tubularized incised plate proximal hypospadias repair: Continued evolution and extended applications. J Pediatr Urol. 2011;7:2-9. [DOI] [PubMed] [Google Scholar]
- 24.Badawy H, Morsi H. Long-term followup of dermal grafts for repair of severe penile curvature. J Urol 2008;180:1842-5. [DOI] [PubMed] [Google Scholar]
- 25.Vandersteen DR, Husmann DA. Late onset recurrent penile chordee after successful correction at hypospadias repair. J Urol 1998;160:1131-3. [DOI] [PubMed] [Google Scholar]
- 26.Wein AJ, Kavoussi LR, Campbell MF. eds.Campbell-Walsh urology. 10th ed. Philadelphia, PA: Elsevier Saunders, 2012. [Google Scholar]
- 27.Braga LH, Pippi Salle JL, Lorenzo AJ, et al. Comparative analysis of tubularized incised plate versus onlay island flap urethroplasty for penoscrotal hypospadias. J Urol 2007;178:1451-6. [DOI] [PubMed] [Google Scholar]
- 28.Elder JS, Duckett JW, Snyder HM. Onlay island flap in the repair of mid and distal penile hypospadias without chordee. J Urol 1987;138:376-9. [DOI] [PubMed] [Google Scholar]
- 29.Duckett JW, Jr. Transverse preputial island flap technique for repair of severe hypospadias. Urol Clin North Am 1980;7:423-30. [PubMed] [Google Scholar]
- 30.Bracka A. The role of two-stage repair in modern hypospadiology. Indian J Urol 2008;24:210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes MC, Malone PS. The use of a dorsal buccal mucosal graft with urethral plate incision (Snodgrass) for hypospadias salvage. BJU Int 1999;83:508-9. [DOI] [PubMed] [Google Scholar]
- 32.Ye WJ, Ping P, Liu YD, et al. Single stage dorsal inlay buccal mucosal graft with tubularized incised urethral plate technique for hypospadias reoperations. Asian J Androl 2008;10:682-6. [DOI] [PubMed] [Google Scholar]
- 33.Vallasciani S, Berrettini A, Nanni L, et al. Observational retrospective study on acquired megalourethra after primary proximal hypospadias repair and its recurrence after tapering. J Pediatr Urol 2013;9:364-7. [DOI] [PubMed] [Google Scholar]
- 34.Upadhyay J, Khoury A. Single-stage procedure for severe hypospadias: Onlay-Tube-Onlay modification of the transverse island preputial flap. In: Hadidi AH, Azmy OF. eds. Hypospadias Surgery an Illustrated Guide. 1st ed. Heidelberg: Springer-Verlag, 2004:173-85. [Google Scholar]
- 35.Rigamonti W, Castagnetti M. Onlay on albuginea: modified onlay preputial island flap urethroplasty for single-stage repair of primary severe hypospadias requiring urethral plate division. Urology 2011;77:1498-502. [DOI] [PubMed] [Google Scholar]
- 36.Koyanagi T, Matsuno T, Nonomura K, et al. Complete repair of severe penoscrotal hypospadias in 1 stage: experience with urethral mobilization, wing flap-flipping urethroplasty and “glanulomeatoplasty”. J Urol 1983;130:1150-4. [DOI] [PubMed] [Google Scholar]
- 37.Emir H, Jayanthi VR, Nitahara K, et al. Modification of the Koyanagi technique for the single stage repair of proximal hypospadias. J Urol 2000;164:973-5. [DOI] [PubMed] [Google Scholar]
- 38.Sugita Y, Tanikaze S, Yoshino K, et al. Severe hypospadias repair with meatal based paracoronal skin flap: the modified Koyanagi repair. J Urol 2001;166:1051-3. [PubMed] [Google Scholar]
- 39.Catti M, Lottmann H, Babloyan S, et al. Original Koyanagi urethroplasty versus modified Hayashi technique: outcome in 57 patients. J Pediatr Urol 2009;5:300-6. [DOI] [PubMed] [Google Scholar]
- 40.Bracka A.Hypospadias repair: the two-stage alternative. Br J Urol 1995;76:31-41. [DOI] [PubMed] [Google Scholar]
- 41.Markiewicz MR, Lukose MA, Margarone JE, 3rd, et al. The oral mucosa graft: a systematic review. J Urol 2007;178:387-94. [DOI] [PubMed] [Google Scholar]
- 42.Barbagli G, Vallasciani S, Romano G, et al. Morbidity of oral mucosa graft harvesting from a single cheek. Eur Urol 2010;58:33-41. [DOI] [PubMed] [Google Scholar]
- 43.Snodgrass W, Yucel S. Tubularized incised plate for mid shaft and proximal hypospadias repair. J Urol 2007;177:698-702. [DOI] [PubMed] [Google Scholar]
- 44.Yerkes EB, Adams MC, Miller DA, et al. Y-to-I wrap: use of the distal spongiosum for hypospadias repair. J Urol 2000;163:1536-8. [PubMed] [Google Scholar]
- 45.Snodgrass WT, Koyle MA, Baskin LS, et al. Foreskin preservation in penile surgery. J Urol 2006;176:711-4. [DOI] [PubMed] [Google Scholar]
- 46.Barbagli G, Perovic S, Djinovic R, et al. Retrospective descriptive analysis of 1,176 patients with failed hypospadias repair. J Urol 2010;183:207-11. [DOI] [PubMed] [Google Scholar]
- 47.Leslie B, Lorenzo AJ, Figueroa V, et al. Critical outcome analysis of staged buccal mucosa graft urethroplasty for prior failed hypospadias repair in children. J Urol 2011;185:1077-82. [DOI] [PubMed] [Google Scholar]