Abstract
Background
Vascular endothelial growth factor (VEGF) is considered as a prime mediator of angiogenesis, and has been implicated in carcinogenesis and metastasis. Various studies examined the relationship between VEGF overexpression with the clinical outcome in patients with prostate cancer, but yielded conflicting results.
Methods
Electronic databases updated to July 2013 were searched to find relevant studies. A meta-analysis was conducted with eligible studies which quantitatively evaluated the relationship between VEGF status and survival of patients with prostate cancer. Survival data were aggregated and quantitatively analyzed.
Results
We performed a meta-analysis of 9 studies that evaluated the correlation between VEGF overexpression and survival in patients with prostate cancer. Combined hazard ratios suggested VEGF overexpression had an unfavorable impact on overall survival (OS) [hazard ratio (HR) =1.54, 95% CI (confidence interval): 1.25-1.83], but not disease free survival (DFS) (HR=1.23, 95% CI: 0.99-1.47) in patients with prostate cancer. No significant heterogeneity was observed among all studies.
Conclusions
VEGF overexpression indicates a poor prognosis for patients with prostate cancer.
Keywords: Vascular endothelial growth factor (VEGF), prostate cancer, prognosis, meta-analysis
Introduction
Prostate cancer is the most commonly diagnosed internal malignancy among males and is the second leading cause of cancer-related deaths in the US (1). Recently, the number of prostate cancer patients has increased significantly in China. Surgery and radiation therapy are effective for localized disease (2). However, no effective therapeutic strategy is yet available for recurrent or metastatic disease from failed surgery, radiation, chemotherapy, or hormonal therapy. With these limitations, more sensitive and more specific biomarkers are needed to provide valuable information for the diagnosis and identification of prostate cancer development and progression. The main prognostic factors are clinicopathological characteristics of the disease, including tumor size, stage, and grade. However, the prognostic factors do not fully predict individual clinical outcome. There is the need for better markers to identify patients with poor prognosis at the time of diagnosis. Researches have focused on the potential role of new biological factors involved in the carcinogenic process as prognostic markers in patients with prostate cancer.
Angiogenesis, the formation of new blood vessels from existing vasculature, is an important process in many malignancies including prostate cancer. It is the result of an intricate balance between pro-angiogenic and anti-angiogenic factors. Vascular endothelial growth factor (VEGF) (also referred to as VEGF-A, vascular permeability factor) is a critical pro-angiogenic factor in cancer. The role of VEGF in the regulation of angiogenesis is the object of intense investigation for more than a decade. The VEGF family is composed of several subtypes, including VEGF-A, -B, -C and -D which exist as numerous splice variant isoforms (3,4). Many anti-angiogenic compounds are being developed, most of which target VEGF and/or its receptors. It is necessary to establish whether VEGF expression is a prognostic marker in prostate cancer.
Many studies have evaluated whether VEGF overexpression may be a prognostic factor for survival in patients with prostate cancer. However, the results of the studies are inconclusive and no consensus has been reached. It is unknown whether differences in these investigations have been mostly due to their limited sample size or genuine heterogeneity. Thus, we conducted a meta-analysis of all available studies relating VEGF overexpression with the clinical outcome in patients with prostate cancer.
Materials and methods
Search strategy and study selection
The electronic databases PubMed and CNKI (China National Knowledge Infrastructure) were searched for studies to include in the present meta-analysis. An upper date limit of July 1, 2013 was applied; we used no lower date limit. Searches included the terms “prostate cancer or Pca”, “VEGF”, “vascular endothelial growth factor”, and “prognosis”. We also reviewed the Cochrane Library for relevant articles. The references reported in the identified studies were also used to complete the search.
Studies eligible for inclusion in this meta-analysis met the following criteria: (I) measure VEGF expression in the primary prostate cancer with IHC (immunohistochemistry) or RT-PCR/ELISA (reverse transcription-polymerase chain reaction/enzyme linked immunosorbent assay); (II) provide information on survival [i.e., disease free survival (DFS) and/or overall survival (OS), studies investigating response rates only were excluded] and (III) When the same author reported results obtained from the same patient population in more than one publication, only the most recent report, or the most complete one, was included in the analysis. Two reviewers (P.Z. and Y.J.) independently determined study eligibility. Disagreements were resolved by consensus.
Data extraction and quality assessment
Data retrieved from the reports included author, publication year, patient source, test method, definition of positivity (cut-off value), VEGF positive ratio and survival data (Table 1). If data from any of the above categories were not reported in the primary study, items were treated as “not applicable”. We did no contact the author of the primary study to request the information. We did not use prespecified quality-related inclusion or exclusion criteria and did not weigh each study by a quality score, because the quality score has not received general agreement for use in a meta-analysis, especially observational studies (5). The data extraction and quality assessment were reported in previous meta-analysis (6-9).
Table 1. Main characteristics and results of the eligible studies.
| First author-year | Patients source | N pts | Method | Cut-off | Positive (%) | HR estimation | HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Weber-2012 | Switzerland | 103 | IHC | Immunoreactive score | 23.3 | HR | PFS 0.97 (0.39-2.42) |
| Wang-2011 | China | 148 | IHC | 50% | 22.9 | HR | OS 4.18 (2.17-8.05) |
| Peyromaure-2007 | France | 40 | IHC | Immunoreactive score | 47 | HR | PFS 1.38 (0.99-1.94) |
| Green-2007 | UK | 50 | IHC | Immunoreactive score | 56 | Surv curve | OS 2.58 (1.47-3.45) |
| Fukuda-2007 | Japan | 270 | IHC | Immunoreactive score | NA | HR | PFS 1.04 (0.44-2.48) |
| Shariat-2004 | USA | 423 | ELISA | 9.9 pg/mL | NA | HR | PFS 1.21 (1.00-1.61) |
| West-2001 | UK | 67 | IHC | 25% | 53 | Surv curve | OS 1.32 (1.05-1.72) |
| George-2001 | USA | 390 | ELISA | 260 pg/mL | 50 | HR | OS 2.42 (1.29-4.54) |
| Bok-2001 | USA | 100 | ELISA | 28 pg/mL | 50 | HR | OS 1.72 (1.09-2.71) |
Abbreviations: VEGF, vascular endothelial growth factor; IHC, immunohistochemistry; ELISA, enzyme linked immunosorbent assay; NA, not applicable; HR, hazard ratio; CI, confidence interval; OS, Overall survival; DFS, Disease-free survival.
Statistical methods
Included studies were divided into two groups for analysis: those with data regarding OS and those regarding DFS. For the quantitative aggregation of the survival results, we measured the impact of VEGF status on survival by HR between the two survival distributions. HRs and 95% confidence intervals (CIs) were used to combine as the effective value. If the HRs and their 95% CIs were given explicitly in the articles, we used crude ones. When these variables were not given explicitly, they were calculated from the available numerical data using methods reported by Parmar et al. (10).
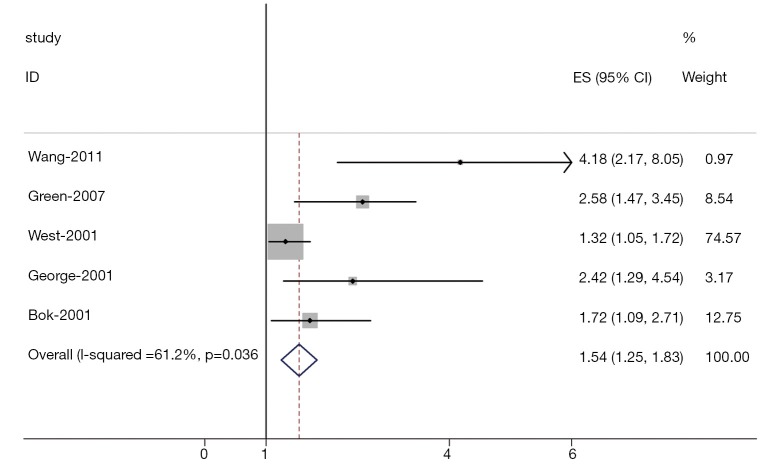
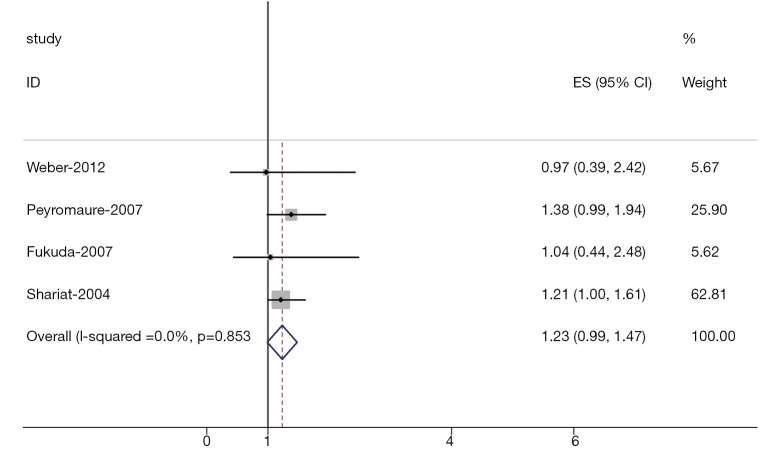
Heterogeneity of the individual HRs was calculated with χ2 tests according to Peto’s method (11). Heterogeneity test with inconsistency index (Ι2) statistic and Q statistic was performed. If HRs were found to have fine homogeneity, a fixed effect model was used for secondary analysis; if not, a random-effect model was used. DerSimonian-Laird random effects analysis (12) was used to estimate the effect of serum VEGF overexpression on survival. By convention, an observed HR >1 implies worse survival for the group with serum VEGF overexpression. The impact of VEGF on survival was considered to be statistically significant if the 95% confidence interval (CI) did not overlap with 1. Horizontal lines represent 95% CIs. Each box represents the HR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (HR=1.0).
Evidence of publication bias was sought using the methods of Egger et al. (13) and of Begg et al. (14). Intercept significance was determined by the t test suggested by Egger (P<0.05 was considered representative of statistically significant publication bias). All of the calculations were performed by STATA version 11.0 (Stata Corporation, College Station, TX).
Results
Study selection and characteristics
Nine studies (15-23) published between 2001 and 2012 were eligible for this meta-analysis. All reported the prognostic value of VEGF status for survival in prostate cancer patients. The total number of patients included was 1,591, ranging from 40 to 423 patients per study (median 177). The major characteristics of the 9 eligible publications are reported in Table 1. The studies were conducted in 6 countries (China, Japan, UK, France, Switzerland and USA).
All of the studies reported the prognostic value of VEGF status for survival in patients with prostate cancer. Of the 9 studies, 7 directly reported HRs (multivariate analysis), while the other 2 studies provided survival curves. Among them, the proportion of patients exhibiting serum high VEGF expression in individual studies ranged from 22.9% to 56%. Estimation using survival curves were segregated according to either OS or DFS. A HR on DFS and OS could be extracted for 4 publications and 5 publications of studies, respectively. Six of the 9 studies identified high VEGF expression as an indicator of poor prognosis, and the other 3 studies showed no statistically significant impact of high VEGF expression on survival.
Meta-analysis
The results of the meta-analysis were shown in Table 2 and Figures 1,2. Overall, the combined HR for all 5 eligible studies evaluating high VEGF status on OS was 1.54 (95% CI: 1.25-1.83), suggesting that high VEGF overexpression was an indicator of poor prognosis for prostate cancer. However, significant heterogeneity was observed among the studies (Q=9.39, I2=61.2%, P=0.036). In addition, for DFS analysis, no statistically significant effect of high VEGF level (HR=1.23, 95% CI: 0.99-1.47) in patients with prostate cancer was also observed. (Q=4.38, I2=0.0%, P=0.853).
Table 2. Meta-analysis: HR value of OS and DFS in prostate cancer.
| Nb | Random effects HR (95% CI) | χ2 heterogeneity test (P) | |
|---|---|---|---|
| Overall for OS | 5 | 1.54 (1.25-1.83) | 0.036 |
| Overall for DFS | 4 | 1.23 (0.99-1.47) | 0.853 |
Abbreviations: HR, hazard ratio; Nb, number of studies; OS, Overall survival; DFS, Disease-free survival.
Figure 1.
Meta-analysis (Forest plot) of the 5 evaluable studies assessing VEGF in prostate cancer for overall survival.
Figure 2.
Meta-analysis (Forest plot) of the 4 evaluable studies assessing VEGF in prostate cancer for disease-free survival.
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias in the literature. All 5 eligible studies investigating VEGF expression on OS yielded a Begg’s test score of P=0.142 and an Egger’s test score of P=0.054, meanwhile according to the funnel plot (Figure 3), the absence of publication bias was found. Moreover, the absence of publication biases were found for investigating VEGF expression on DFS (a Begg’s test score of P=0.174 and an Egger’s test score of P=0.452) (Figure 4).
Figure 3.
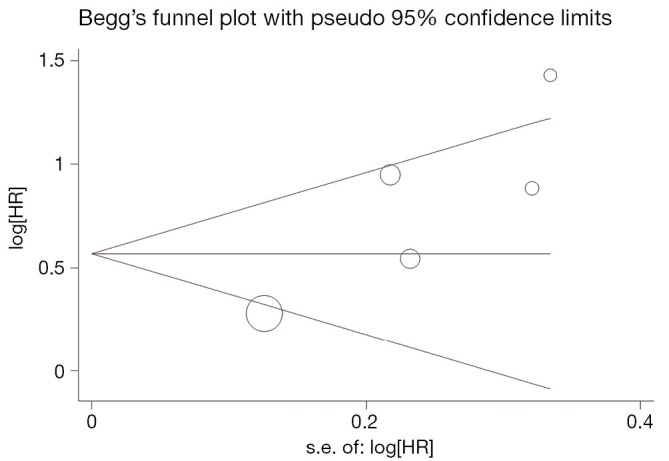
Funnel plot of the 5 evaluable studies assessing VEGF in prostate cancer for overall survival.
Figure 4.
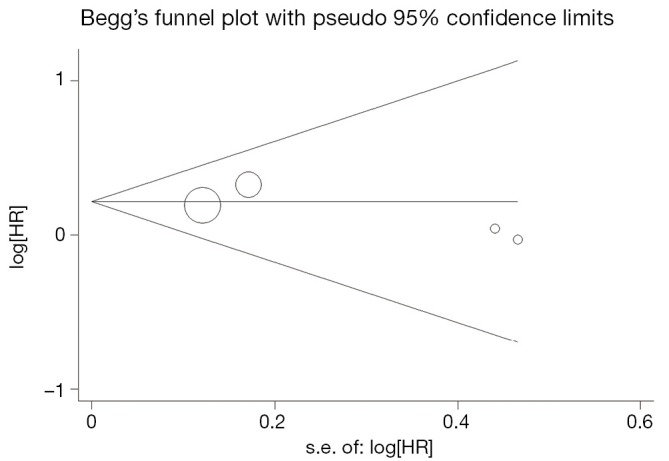
Funnel plot of the 4 evaluable studies assessing VEGF in prostate cancer for disease-free survival.
Discussion
Members of the VEGF family promote two very important processes in vivo, angiogenesis and lymphangiogenesis, which involve growth of new blood and lymphatic vessels from pre-existing vasculature, respectively. VEGF-A exists as a homodimer or can heterodimerize with either VEGF-B or non-VEGF factors such as PIGF (placenta growth factor) (24-26). VEGF-A and VEGF-B promote vascular angiogenesis primarily through activation of vascular endothelial cell associated VEGFR-1 (Flt1) and VEGFR-2 (Flk1/KDR). On the other hand, VEGF-C and VEGF-D which are ligands for VEGFR-2 and VEGFR-3, promote angiogenesis and lymphangiogenesis (27,28).
The present meta-analysis has combined 9 publications including 1,591 patients to yield statistics, indicating a statistically significant role of VEGF on overall survival, but not on disease-free survival in prostate cancer. Our data were consistent with the results of a previous meta-analysis (29) published in 2012 that showed an association between VEGF overexpression and poor survival of patients with prostate cancer. We have improved upon that previous meta-analysis by including more recent related studies and by generally using a more comprehensive search strategy. Screening, study selection and quality assessment were performed independently and reproducibly by two reviewers. We also explored heterogeneity and potential publication bias in accordance with published guidelines.
There were several meta-analyses studying the prognostic value of VEGF in other cancer types, such as head and neck squamous cancer (30), lung cancer (31), colon cancer (32), gastric cancer (33), and hepatocellular carcinoma (34). Association of VEGF overexpression with poor outcomes provides a rationale for anti-angiogenics use in the treatment of cancer. VEGF has become a leading therapeutic target for the treatment of cancer. Potentially therapeutic strategies to inhibit VEGF pathway include monoclonal antibodies directed against VEGF, tyrosine kinase inhibi-tors (TKIs), and antisense strategies (35). Bevacizumab (Avastin) is a humanized monoclonal antibody directed against VEGF (36). It binds to all isoforms of VEGF-A, thus blocking its binding to VEGFR, but it does not bind to other VEGF molecules, such as VEGF-B or VEGF-C.
The heterogeneity issue was complicated in the systematic review and meta-analysis was. We found no significant heterogeneity among all studies included and subgroup analysis. Another potential source of bias is related to the method of HR and 95% CI extrapolation. If these statistics were not reported by the authors, we calculated them from the data available in the article. If this was not possible, we extrapolated them from the survival curves, necessarily making assumptions about the censoring process. Data for multivariate survival analysis reported in the article were included in the present systematic review and meta-analysis; if these data were not available, data calculated from survival curves by univariate analysis were included. These results should be confirmed by an adequately designed prospective study. Furthermore, the exact value of VEGF overexpression status needs to be determined by appropriate multivariate analysis.
Publication bias (37) is a major concern for all forms of meta-analysis; positive results tend to be accepted by journals, while negative results are often rejected or not even submitted. The present analysis does not support publication bias; the obtained summary statistics likely approximate the actual average. However, it should be noted that our meta-analysis could not completely exclude biases. For example, the study was restricted to papers published in English and Chinese, which probably introduced bias.
In conclusion, our meta-analysis estimated the association between prognostic significance of VEGF overexpression and patients with prostate cancer. As determined in our meta-analysis, we concluded that VEGF overexpression was associated with poor overall survival, but not disease-free survival, and there is no significant heterogeneity among all studies. To strengthen our findings, well-designed prospective studies with better standardized assessment of prognostic markers should help to explore the relation between VEGF overexpression and the outcome of patients with prostate cancer.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 2000;31:578-83. [DOI] [PubMed] [Google Scholar]
- 2.Klein EA, Kupelian PA. Localized prostate cancer: radiation or surgery? Urol Clin North Am 2003;30:315-30. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2002;2:795-803. [DOI] [PubMed] [Google Scholar]
- 4.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27. [DOI] [PubMed] [Google Scholar]
- 5.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan P, Qian Q, Yu LK. Prognostic value of COX-2 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2013;5:40-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan P, Qian Q, Wan B, et al. Prognostic value of TTF-1 expression in patients with non-small cell lung cancer: a meta-analysis. Transl Cancer Res 2013;2:25-32. [Google Scholar]
- 8.Zhan P, Wang Q, Qian Q, et al. Megestrol acetate in cancer patients with anorexia-cachexia syndrome: a meta-analysis. Transl Cancer Res 2013;2:74-9. [Google Scholar]
- 9.Zhan P, Wang Q, Qian Q, et al. Risk of venous thromboembolism with the erythropoiesis-stimulating agents (ESAs) for the treatment of cancer-associated anemia: a meta-analysis of randomized control trials. Chin Clin Oncol 2012;1:19. [DOI] [PubMed] [Google Scholar]
- 10.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed] [Google Scholar]
- 15.Weber DC, Tille JC, Combescure C, et al. The prognostic value of expression of HIF1α, EGFR and VEGF-A, in localized prostate cancer for intermediate- and high-risk patients treated with radiation therapy with or without androgen deprivation therapy. Radiat Oncol 2012;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Diao X, Sun J, et al. Stromal cell-derived factor-1 and vascular endothelial growth factor as biomarkers for lymph node metastasis and poor cancer-specific survival in prostate cancer patients after radical prostatectomy. Urol Oncol 2013;31:312-7. [DOI] [PubMed] [Google Scholar]
- 17.Peyromaure M, Camparo P, Badoual C, et al. The expression of vascular endothelial growth factor is associated with the risk of cancer progression after radical prostatectomy. BJU Int 2007;99:1150-3. [DOI] [PubMed] [Google Scholar]
- 18.Green MM, Hiley CT, Shanks JH, et al. Expression of vascular endothelial growth factor (VEGF) in locally invasive prostate cancer is prognostic for radiotherapy outcome. Int J Radiat Oncol Biol Phys 2007;67:84-90. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda H, Tsuchiya N, Narita S, et al. Clinical implication of vascular endothelial growth factor T-460C polymorphism in the risk and progression of prostate cancer. Oncol Rep 2007;18:1155-63. [PubMed] [Google Scholar]
- 20.Shariat SF, Karam JA, Walz J, et al. Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood-based biomarkers. Clin Cancer Res 2008;14:3785-91. [DOI] [PubMed] [Google Scholar]
- 21.West AF, O’Donnell M, Charlton RG, et al. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer 2001;85:576-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George DJ, Halabi S, Shepard TF, et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 2001;7:1932-6. [PubMed] [Google Scholar]
- 23.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res 2001;61:2533-6. [PubMed] [Google Scholar]
- 24.Parikh AA, Ellis LM. The vascular endothelial growth factor family and its receptors. Hematol Oncol Clin North Am 2004;18:951-71, vii. [DOI] [PubMed] [Google Scholar]
- 25.Xie K, Wei D, Shi Q, et al. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev 2004;15:297-324. [DOI] [PubMed] [Google Scholar]
- 26.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Gray A, Yuan J, et al. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci U S A 1996;93:1988-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997;276:1423-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, Peng HL, Li LK. Prognostic value of vascular endothelial growth factor expression in patients with prostate cancer: a systematic review with meta-analysis. Asian Pac J Cancer Prev 2012;13:5665-9. [DOI] [PubMed] [Google Scholar]
- 30.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res 2005;11:1434-40. [DOI] [PubMed] [Google Scholar]
- 31.Zhan P, Wang J, Lv XJ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol 2009;4:1094-103. [DOI] [PubMed] [Google Scholar]
- 32.Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006;94:1823-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng L, Zhan P, Zhou Y, et al. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol Biol Rep 2012;39:9473-84. [DOI] [PubMed] [Google Scholar]
- 34.Schoenleber SJ, Kurtz DM, Talwalkar JA, et al. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer 2009;100:1385-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 2006;12:5018-22. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004;3:391-400. [DOI] [PubMed] [Google Scholar]
- 37.Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J R Stat Soc A 1988;151:419-63. [Google Scholar]