Abstract
Intravesical immunotherapy with bacille-Calmette-Guerin (BCG) is indicated in the treatment of high-risk and intermediate-risk non-muscle invasive bladder cancer (NMIBC). Our goal is to describe the various disease states following induction and maintenance BCG and to describe contemporary treatment options and the current and projected clinical trial landscape for patients who recur following BCG therapy.
Keywords: Bladder cancer, bacille-Calmette-Guerin (BCG), refractory, failure
Introduction
The definition of bacille-Calmette-Guerin (BCG) failure must be specified in the context of previous treatments and the time interval of recurrence. Valrubicin is the only FDA approved intravesical agent in this setting and the approval is restricted to patients with BCG refractory carcinoma in situ (CIS) and those who refuse or are not medically fit to undergo radical cystectomy. There are multiple other intravesical therapies available for these patients as well as others with Ta or T1 papillary urothelial cancer. Device assisted treatments designed to improved chemotherapy delivery are another option but await approval in the US. Ongoing clinical trials are testing strategies to augment the immune response including pre-treatment BCG vaccination, immune checkpoint inhibition, gene therapy and other novel delivery systems, targeted agents (e.g., FRFR3 inhibitors) and combination intravesical therapy (Table 1).
Table 1. Ongoing clinical trials for optional treatments of BCG refractory urothelial carcinoma.
| Trial | Phase | Sponsor | Trial status |
|---|---|---|---|
| Efficacy study of recombinant adenovirus for non-muscle invasive bladder cancer (BOND) | II/III | Cold Genesys | Open—recruiting |
| Study of BCG combined with PANVAC versus BCG alone course of BCG in adults with high grade non-muscle invasive bladder cancer who failed at least one course of BCG | II | National Cancer Institute | Open—recruiting |
| AD001 and intravesical gemcitabine in BCG-refractory primary or secondary carcinoma in situ of the bladder | I/II | Memorial Sloan Kettering Cancer Center | Open—recruiting |
| Sunitinib malate in treating patients with recurrent transitional cell bladder cancer | II | Case Cancer Center | Ongoing—not recruiting |
| Phase 2b, trial of intravesical DTA-H19/PEI in patients with intermediate-risk superficial bladder cancer | IIb | Case Cancer Center | Ongoing—not recruiting |
| Radiation therapy and chemotherapy in treating patients with stage I bladder cancer | II | Radiation Therapy Oncology Group | Open—recruiting |
| Dovitinib in BCG refractory urothelial carcinoma with FGFR3 mutations or over-expression | II | Hosier Cancer Research Network | Ongoing—not recruiting |
| Intravesical administration of Instiladrin (rAd-IFN with Syn3) in patients with bladder cancer | II | FKD Therapies | Ongoing—not recruiting |
BCG, bacille-Calmette-Guerin.
BCG is a live-attenuated mycobacterium originally developed as a vaccine against tuberculosis. Dr. Morales first demonstrated its therapeutic potential in bladder cancer in 1976 (1). While the initial application for NMIBC included the co-administration of transdermal and intravesical BCG, the current standard of care is full dose induction intravesical therapy weekly for 6 weeks followed by maintenance therapy (2).
BCG is approved by the US Food and Drug Administration for treatment of patients with CIS and high-grade papillary Ta and T1 urothelial bladder cancer. Meta analyses of EORTC trials suggest that BCG with maintenance is also an option for treatment of patients with multifocal and or recurrent or large solitary Ta and T1 low-grade disease (3,4).
In the current management of NMIBC at least eight different BCG strains are being used which all have been derived from the original BCG strain which was created from the attenuation experiments by Drs. Calmette and Guerin in 1921 at the Pasteur Institute in Lille, France (5). When the lyophilized form of BCG entered into mass production in 1961, the dispersion caused a drift in the genotype and with resulting substrains that were named after their site of origin and the manufacturer. The strains that are most commonly used are TICE (Chicago) and Connaught (Toronto). With a manufacturing shortage of the latter, the TICE strain is the most commonly available BCG strain at this time.
A Japanese study compared the efficacy of intravesical BCG with the Tokyo strain or the Connaught strain in a randomized study in 133 patients without prior intravesical treatment. The complete response (CR) rate was 90.3% and 85.0% respectively, which was not statistically significantly different. Despite randomization significantly more patients with CIS were allocated to the Tokyo BCG arm (6).
A recent single center randomized clinical trial reported a significant superiority in the treatment of NMIBC with BCG Connaught versus BCG TICE significantly improving 5-year recurrence-free survival (7). This has been attributed to a more effective TH-1 immune response as shown in in vitro experiments in mice. A genomic analysis reported in the same study, demonstrated significant differences in the mutation patterns between the strains. While the trial has many limitations in design and power, it raises a provocative question regarding the potential that BCG strain differences may affect relative efficacy in the absence of maintenance BCG. The drift in the genome of BCG has been comprehensively studied as a potential modulator of vaccine efficacy (8). Investigators hypothesize that early BCG strains may be more effective than BCG strains widely used today likely due to independent tandem duplications (DU1 and DU2).
The AUA, EAU, and NCCN guidelines recommend treatment with BCG for high-grade non-muscle invasive cancer including Ta high grade, CIS and/or T1 high-grade disease, which do not meet the criteria for a primary cystectomy (9-11). BCG has been shown to have a durable response rate of about 50% over a median follow-up of 4 years but this number drops to only 30% of patients who are free of tumor recurrence or progression at 10 years. Level I evidence supports the use of full dose BCG plus 3 years of maintenance treatment in patients with high risk disease (12) and meta-analyses suggest that BCG is superior to Mitomycin or Epirubicin for intermediate risk disease but only when administered with maintenance treatment (3,4).
SWOG 8507 randomized patients with high-risk disease to BCG induction alone vs. induction plus maintenance BCG for 3 years. Maintenance BCG was associated with both reduced recurrence and disease worsening defined as biopsy proven invasive cancer or a change in treatment strategy implying progression or worsening of the disease state. Five-year RFS was 60% vs. 41% with and without maintenance treatment (2). EORTC 30962 compared low dose vs. high dose and 1- vs. 3-year maintenance therapy in a non-inferiority trial design. While the trial failed to meet the primary endpoint, subset analyses in patients with high-risk disease confirmed the requirement for full dose and 3 years of maintenance and suggested that patients with intermediate-risk disease could be treated with 1 year of maintenance therapy with a similar efficacy as 3 years of maintenance (12).
The foremost challenge for the urologist who manages patients on BCG is to recognize and accurately define BCG failure and to determine the optimal treatment strategy. Failure to intervene with definitive radical cystectomy prior to progression to muscle invasive cancer is associated with a significant reduction in long-term survival probability (13). The definition of BCG non-responders has been modified over time. O’Donnell et al. have recommended the following categories (14): intolerance to BCG describes the inability of a patient to tolerate side effects from the treatment. Resistance to BCG refers to a recurrence or persistence of bladder cancer at 3 months after the first induction cycle but of lesser degree (stage or grade), which is absent at 6 months after either a re-induction cycle of 6 weeks or a first maintenance cycle of 3 weeks. Patients are refractory to BCG when there is persistent disease after a second course of BCG (either maintenance or second induction course). This also includes any progression or worsening of the tumor with regards to stage, grade and disease extent by 3 months after the first cycle of BCG. Formerly called BCG relapse refers to a recurrence of the disease after initial achievement of a disease free state within 6 months of initiation of treatment. A recurrence may be classified as early (within 12 months), intermediate (12-24 months) or late (>24 months). The 2013 FDA/AUA Workshop on clinical trial design in NMIBC expanded the patient population defined as BCG refractory to include those treated with two induction courses or induction plus 3 weeks of maintenance and fail to achieve a CR within 6 months of initiation of BCG (15). The FDA has also considered including patients who recur within 6 months after an initial CR. Single arm phase II trials may be considered for registration with this expanded patient population. Patients who recur with T1HG after an initial induction course may also be included in this group considered BCG unresponsive. BCG failure is a unique subset of patients with persistent TaHG or CIS after a single induction course of BCG or recurrent disease more than 1 year after an initial CR. Phase III trials comparing BCG to BCG plus an experimental drug should be considered in this patient population.
What to do when BCG fails the patient?
Patients who recur after an initial CR to BCG or have persistent disease not requiring a cystectomy after a single induction course can be re-induced with a second induction course or proceed to maintenance therapy with three weekly instillations. Patients who recur or have persistent disease after two induction courses or induction plus maintenance in general should not receive additional BCG. Cystectomy is the standard of care in these patients. For patients who refuse cystectomy or are at higher risk for morbidity or mortality due to co-morbidites can be re-induced with intravesical Valrubicin which is FDA approved for patients with CIS who meet these criteria. While patients with intermediate risk tumors i.e., multifocal and/or recurrent low-grade disease should be offered intravesical chemotherapy, any high-risk disease must be managed in a more aggressive fashion.
The initial CR rate for patient with CIS is 55% at 3 months following initiation of BCG therapy. Without any further BCG, an additional 10-15% of patients will have a CR by 6 months. With the addition of 3 weeks of maintenance, the 6-month CR rate for these patients improves to 84% (2) . The message from these data from SWOG 8507 is that one should wait until the 6-month evaluation time point in order to determine whether a patient is refractory to BCG. The exception is patients with persistent T1 disease at the first evaluation at 3 months who should strongly consider cystectomy. An algorithm is presented in Figure 1 describing the management of disease states following BCG therapy (16-18).
Figure 1.
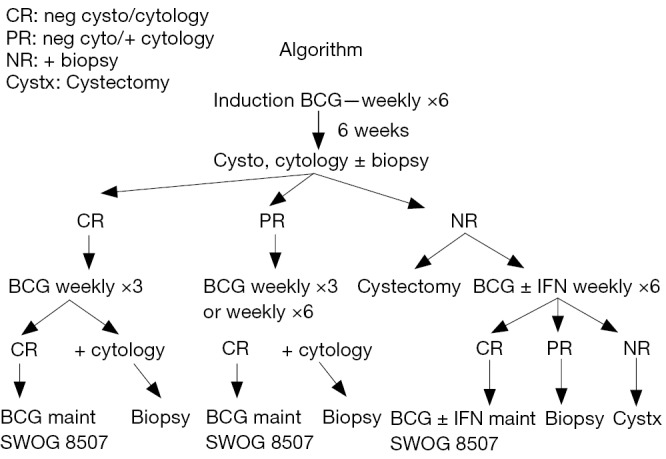
Algorithm for initial treatment with BCG and subsequent therapy depending on response (SWOG 8507 Phase III randomized trial comparing BCG induction and BCG induction plus maintenance to 36 months). BCG, bacille-Calmette-Guerin.
In a retrospective secondary analysis of the SWOG 8507 trial, we examined the association of a CR following induction BCG with overall survival (19). The 5-year survival probability was 77% for patients who had an initial CR vs. 62% who did not. Age and history of prior intravesical chemotherapy were also associated with worse survival.
In patients with a positive cytology but no visible disease in the bladder it is imperative to consider extravesical sites as the source of the malignant cells. This should include staging of the upper urinary tract (UUT) and the prostatic urethra as recommended by the EAU and NCCN guidelines. A recent study from Giannarini et al. reported that of 110 patients who were treated with a single or two courses of BCG, 52% had a second primary tumor either in the UUT or the prostatic urethra (20). Huguet et al. found that the involvement of the prostatic urethra was the strongest predictor for upstaging to muscle-invasive disease in patients treated with cystectomy for BCG failure (21). Moreover there is a clear association between the depth of invasion in prostatic urothelial carcinoma and survival as previously reported by our group and others (22). Ductal CIS or lamina propria invasion of the prostatic urethra and prostatic stromal invasion were associated with a 5-year survival of 44% and 32% respectively while the 5-year overall survival in the absence of any prostatic involvement was about 64%. Transurethral resection biopsy adjacent the verumontanum is imperative for the detection of CIS and invasive prostatic urothelial carcinoma arising from the prostatic urethra (23).
BCG failure—what next?
Radical cystectomy must be considered for patients who fail to achieve a CR or recur after treatment with BCG, but the best time point on when to abandon conservative treatment and consider radical cystectomy is unclear. There is a broad consensus among experts and this is also reflected by the guidelines that any patient not tolerating BCG or where BCG treatment is contraindicated should be offered a radical cystectomy. Contraindications include active tuberculosis or any form of congenital or acquired immunosuppression including (package insert for TICE http://www.fda.gov UCM163039):
Cancer therapy related immunosuppression (radiation or cytotoxic drug related);
Long-term treatment of corticosteroids;
Treatment with Remicade which is an inhibitor of tumor necrosis factor and blocks T cell immunity.
Moreover any patient with a BCG refractory tumor or any high-grade recurrence after BCG should be considered for radical cystectomy. This is especially important in patients with a high grade T1 tumor who have a high risk of progression. An analysis from Denzinger et al. in 2008 revealed significant differences in the survival rates for patients with initial T1 high-grade tumors who opted for an early versus a deferred cystectomy with a 10-year CSS of 78% and 51%, respectively (24). In patients who opted for BCG treatment and underwent deferred cystectomy the median time in treatment delay from the initial TURBT to cystectomy was 11.2 months. Notable predictors of worse survival were delayed cystectomy and concomitant CIS.
For medically fit patients, radical cystectomy must be performed prior to the development of muscle-invasive disease. In a series of 3,200 patients who underwent RC for CIS, 36% of patients were upstaged to invasive cancer (pT1-T4) and 22.6% of patients were upstaged to muscle-invasive disease (≥ pT2). Cancer specific survival at 3, 5 and 10 years was 91%, 85% and 73%, respectively (25).
There is no rationale for further BCG treatment after two courses unless the recurrence takes place ≥1 year after the last BCG treatment. However, there are several options for second line therapy that are available for patients who are medically unfit or unwilling to undergo cystectomy. Several of these must still be considered experimental.
Boosting the immune response
Transdermal vaccination prior to BCG
The mechanisms involved in the immune response generated by intravesical BCG immunotherapy for patients with non-muscle-invasive bladder cancer (NIMBC) are not completely understood, but evidence suggests a role played by both innate and adaptive arms (26). BCG induces a robust CD4 and CD8 T cell infiltrate and up-regulation of cytokine induction such as interferon-γ, IL-2 and IL-12, which are associated with a predominant T-helper cells (Th1) response. Previous trials have investigated the concomitant use of intradermal vaccination and intravesical therapy. The largest trial randomized 154 patients to intravesical therapy (BCG Pasteur strain) with or without combined intradermal inoculation (27). There was no benefit with regards to recurrence-free or progression-free survival similar to other trials (27-29).
Two clinical trials are in developments that revisit the concept of intradermal application of BCG. The European study BOOST and the US SWOG study PRIME will independently evaluate the role of transdermal vaccination with BCG and subsequent BCG intravesical treatment versus BCG alone. In a pre-clinical experiment mice were treated with subcutaneous vaccination with BCG 3 weeks before the initiation of the intravesical induction BCG, which resulted in 100% survival compared to controls and a modest delay in tumor growth and mortality (30). The investigators found that an accelerated immune response was associated with rapid recruitment of T-cells. A retrospective analysis of a BCG treated patient cohort revealed that a previous exposure to BCG and positive Mantoux (PPD) test resulted in a better response to BCG. While simultaneous intradermal and intravesical therapy have been evaluated these upcoming trials might shine a light on whether intradermal BCG followed in 3 weeks by induction intravesical BCG treatment might provide better treatment responses (27-29).
BCG plus interferon-α (INF-α)
The treatment of high-grade bladder cancer with INF-α has been reported mostly as an intravesical therapy. Interferon monotherapy does not appear to have an effect on recurrence-free survival but has been shown to reduce progression to a higher grade or stage (31).
With the goal of reducing toxicity of BCG the immuno-modulator interferon-alpha has been studied in combination with dose-reduced BCG and as an enhancer of the immune response. A multicenter phase II trial led by the National BCG/Interferon Investigator group and published in 2006 studied a combination of BCG and INF-α, which was administered to patients who were BCG naïve or previously treated with one or two courses of BCG. All patients received 50 million units of IFN α-2b and BCG dosage was reduced in those previously treated with BCG or in those patients deemed BCG intolerant. Inclusion criteria allowed for patients with primary or recurrent bladder cancer, previous intravesical chemotherapy and patients who recurred after treatment with BCG. Of all 1,007 patients at 24 months, 59% and 45% were free of disease in the BCG naïve and the BCG failure group, respectively. Patients who received three or more cycles of BCG had an inferior outcome suggesting they had resistant disease. Based on these results a third cycle of BCG is not warranted as a general rule of practice. Importantly the study did not prove an advantage of the combination treatment to BCG monotherapy. A recent phase III trial however, showed no benefit to the addition of interferon alpha in a BCG naïve population (32). Rosevear et al. reported in a subset analysis data on patients with CIS from the same phase II trial. While initial response rates were similar, 24-month disease-free rates were 60% in BCG naïve patients and 57% or 23% after one or two cycles of previous BCG (33). The treatment response to the drug combination was also associated with a significantly shortened disease-free survival in patients who recurred <12 months following BCG treatment compared to patients who recurred after >12 months. In practice many clinicians may consider adding INF-α in the setting of a relapse after 12 months or at the time of a second BCG induction course if there is persistent disease after the first 6-week induction course.
Checkpoint inhibitors
The class of immunotherapy drugs under the common term checkpoint inhibitors refers to monoclonal antibodies, which block the pathways of CTLA-4 (cytotoxic T lymphocyte antigen-4) or programmed cell death protein 1 (PD-1/PD-L1) (34). PD1 and CTLA-4 are negative regulators of the T cell activity and their expression may be up-regulated in the context of bladder cancer. Checkpoint blockade can reverse this suppression and enhance anti-tumor T cell activity. A single-arm phase II trial is planned to assess the efficacy of first-line gemcitabine—cisplatin with ipilimumab for metastatic urothelial cancer of the bladder (NCT01524991).
Nivolumab, an anti PD-1 antibody, is currently being evaluated in a phase II clinical trial to evaluate the efficacy of a combined treatment with nivolumab plus/minus ipilimumab in advanced or metastatic bladder cancer (NCT01928394).
While these drugs essentially enhance the T-cell response of cytotoxic CD8+ T-cells they may offer an opportunity to modulate T-cell response with BCG immunotherapy as well. Trials are planned to evaluate checkpoint inhibition in combination with BCG and as monotherapy for patients for whom BCG is no longer a treatment option (BCG unresponsive).
Mycobacterium cell wall-DNA complex
The treatment with an intravesical mycobacterium cell wall-DNA complex (MCNA) was first reported by Morales et al. in 2009 after treatment of 55 consecutive mostly BCG refractory patients as an alternative to BCG. The study was the first to report treatment efficacy as well as safety of the drug, which is formulated as an emulsion and diluted in saline for an intravesical administration. With a dose of 8 mg 46.4% of patients had a CR at 12 and 26 weeks (35). Adverse events were mild to moderate in 90% of patients.
MCNA is believed to activate cytokine induction similar to BCG. More recently Morales et al. published data from an open label study with 129 patients from 25 study sites after treatment failure with BCG. The dose regimen was 8 mg of MCNA and treatments were given on a 2-year maintenance schedule similar to BCG. The overall disease-free survival rate was 25% after 1 year and 19% after 2 years but the best outcome was observed in patients with papillary disease only (36).
Valrubicin
Valrubicin is the only FDA approved therapy for patients with BCG refractory CIS or for patients who are intolerant. The drug was approved in 1998 in patients in whom “cystectomy would be associated with unacceptable morbidity and mortality”.
In a phase II/III open-label study valrubicin was administered in 6 or 9 weekly instillation of 800 mg to patients with CIS (37). All patients had received at least one course of BCG and were either intolerant or BCG refractory. Thirty-five percent had no evidence of disease at the first control at 3 months, which included cystoscopy, biopsy and cytology. A positive cytology was allowed. A complete remission at 6 months was achieved in 18% of patients but the 2-year disease free probability was only 4% and 25% of patients underwent radical cystectomy as definitive treatment.
Gemcitabine
Several Phase I and Phase II studies indicate both the safety and potential efficacy of intravesical Gemcitabine. Dalbagni et al. found that the majority of high-risk patients who initially responded at 3 months had recurred by 12 months with a 1-year RFS of 21% (38). This suggests a potential role for maintenance gemcitabine therapy. The cost of this regimen is very high at about $1,000 per dose though gemcitabine is now off patent and the cost is likely to decrease. A prospective randomized phase II trial compared gemcitabine versus BCG in 80 patients with persistent disease after one BCG induction course. 2-year RFS were 19% and 3% respectively (39). The SWOG S0353 phase II trial investigated the role of gemcitabine in patients with NMIBC (intermediate and high-risk) after two prior courses of BCG (40). The treatment schedule included a 6 weeks induction treatment and monthly maintenance treatments up to 12 months. Forty-seven percent of patients responded at 3 months and 28% were free of recurrence at 1 year and 21% after 2 years. The response rates are similar to Valrubicin suggesting that this is an additional treatment option for patients unfit for major surgery.
Mitomycin
Intravesical mitomycin has been shown to have a higher efficacy when administered under optimized conditions as shown in a randomized phase III clinical trial. In the optimized arm, the dose and concentration were doubled (40 mg/20 cc), bicarbonate was given the evening and morning of treatment as MMC uptake in tissue is optimized under alkaline conditions. Patients were kept NPO in order to limit urine production and dilution of mitomycin. The bladder was scanned after catheter placement in order to minimize residual urine. Patients treated in the optimized were compared to patients treated with 20 mg/20 cc and no pharmacologic optimization demonstrated a longer median time to recurrence with 29 months versus 12 months for patients in the standard treatment arm (41).
Another promising approach has been device-assisted therapy for intravesical mitomycin using induced hyperthermia. Alfred Witjes et al. reported a response rate of 92% in patients with CIS (42). The series included BCG naive patients as well as patients with persistent or recurrent disease after previous BCG therapy but resulted in durable response rates which were around 50% after 2 years regardless of any previous BCG treatment. Side effects were transient and most commonly reported as pain and bladder spasms in about 13% of patients. Another report from Colombo et al. prospectively evaluated the response rate in patients with intermediate to high-risk bladder cancer in patients receiving thermo-chemotherapy versus chemotherapy alone (43). This study also included both BCG naïve as well as BCG pretreated patients. All patients received 8 weekly followed by 4 monthly treatments. The 10-year disease free survival rates were 53% and 15% respectively. Interestingly a history of multifocal tumor occurrence was associated with a reduction of disease-free survival only in patients treated with mitomycin alone while it had no bearing on outcome in patients treated with thermochemotherapy. The technology is not approved yet in the United States.
The benefit of intravesical electromotive drug administration (EMDA) of mitomycin has been recently reported from a prospective randomized clinical trial in the setting of a preoperative instillation prior to TURBT. The trial randomized 374 patients to mitomycin by passive instillation, EMDA or no mitomycin. EMDA mitomycin was administered preoperatively before the TURBT. Ninety-seven patients of 374 patients had high-grade tumors. The majority of patients had low or intermediate risk tumors based on the EORTC calculator. The disease-free interval for was significantly longer in patients treated with EMDA with 52 months versus 16 and 12 months after postoperative mitomycin or TURBT alone respectively. These effects were sustained when the disease-free interval was stratified by risk category with a significant benefit for multifocal high-risk disease (44).
Doublet intravesical chemotherapy
Lightfoot et al. reported the combination of gemcitabine and mitomycin as a sequential treatment retrospective case review (45). Forty-seven patients who were unfit or unwilling to undergo cystectomy were included in the analysis. Thirty-six patients had undergone previous BCG treatment (one or two cycles). The sequential regimen consisted of the instillation of one gram of gemcitabine with subsequent bladder drainage followed by instillation of 40 mg of mitomycin. All patients received a 6-week induction course and 1 year of monthly maintenance treatments. Response rates were directly correlated with the number of prior BCG treatments. Patients who were BCG refractory had a CR rate of 69%. The recurrence-free survival was 50% and 32% after 1 and 2 years of treatment. The retrospective nature and heterogeneous patient population do not allow for management recommendations but the concept of combining treatment modalities with different targets is an excellent opportunity for future drug trials. A new study from the University of Iowa using the doublet gemcitabine and docetaxel was recently reported in Bladder Cancer and found treatment success of 32% at 2 years.
Docetaxel
Docetaxel is a taxane and used as a chemotherapy agent in metastatic prostate and breast cancer. McKiernan et al. have previously demonstrated that it can be safely used in intravesical therapy and showed a 56% response rate in patients with BCG refractory disease (46). More recently Barlow et al. presented results from a single center analysis of 54 patients who had failed at least one course of prior BCG with or without interferon (47). After the first induction cycle 59% of patients had a CR to docetaxel. One- and three-year recurrence-free survival rates were 40% and 25% respectively. The analysis did not reveal a benefit for a monthly maintenance schedule for up to 9 months after the first three months control.
Abraxane
A recently published phase II trial evaluated treatment response to nanoparticle albumin-bound paclitaxel (Abraxane®). The 28 patients who were enrolled had recurrent high-grade disease after at least on cycle of prior intravesical treatment BCG + intravesical chemotherapy and refused or were unfit for cystectomy. Nab-paclitaxel was administered weekly for 6 weeks and after CR continued on a monthly maintenance schedule for a total of 6 months. Initial response rate was 35.7% at 6 weeks defined as a negative biopsy and cytology. Recurrence-free survival was 30.6% at 2 years of follow-up (48).
Gene therapy
The basis of gene therapy relies on viral or non-viral delivery systems that can safely transduce the urothelium at high efficiency. Vectors can deliver genes of various sizes depending on the packaging systems. One immunotherapy approach is based on induction of high levels of interferon-alpha endogenously secreted from transduced urothelial and bladder tumor cells. The technique relies on utilization of an adenoviral vector adenovirus (Ad)-IFN-α encoding for a secreted gene product of interferon-alpha and therefore bypassing the requirement to transduce every tumor cell (49). Syn3 is used as an excipient to overcome the lack of the coxsackie and adenovirus receptor (CAR), which is frequently absent in urothelial cancer. An ongoing phase II clinical trial is evaluating this treatment with the primary endpoint of prevention of high-grade recurrence in a BCG refractory patient population (NCT01687244). This trial has completed accrual and if positive will be followed with a single arm Phase II registration trial.
The adenovirus vector CG0070 is a replication competent adenovirus, which stimulates production of GM-CSF, is currently studied in a phase II clinical trial (NCT01438112). The treatment concept is based on enhancing the immune response by stimulating dendritic and effector cells.
Targeted therapies
The Cancer Genome Atlas Project has reported a comprehensive integrated analysis of genomic data from muscle-invasive bladder tumors (50). Twelve percent of bladder tumors had FGFR3 mutations, which affected kinase-activating sites. Activating point mutations of FGFR3 occur in up to 80% of patients with noninvasive tumors suggesting this as a rationale target. A current phase II trial of the receptor tyrosine kinase inhibitor dovitinib is directed at patients with BCG-refractory NMIBC with FGFR3 mutations or overexpression (NCT01732107).
Over 40% of urothelial cancers of the bladder have been shown to have alterations in the PI3K/AKT/mTOR pathway (51). The mTOR pathway acts downstream of the PI3K pathway and regulates metabolism of the cell promoting growth and cell proliferation. The mTOR inhibitor everolimus is being evaluated in a phase I/II trial in combination with intravesical gemcitabine for the management of patients with BCG refractory bladder cancer (NCT01259063).
Trial design for registration trials
In 2013 the AUA and the FDA held a workshop on trial design for patients with non-muscle-invasive bladder. There was consensus that clinical trials should include high-grade papillary disease and CIS. The meeting also addressed the challenges of defining standardized endpoints in the setting of NMIBC. Uniformly this should include:
Failure to achieve a CR in patients with CIS;
Recurrence with CIS or papillary high-grade disease.
The panel recommended a definition of a successful treatment as a 40-50% response rate at 6 months and durable response rate of 30% at 18-24 months. Moreover there was consensus that any clinical trial with BCG refractory patients should not be placebo controlled as the panel felt this was unethical due to the aggressive nature of the disease. On the contrary placebo controlled trials may be feasible for low-grade disease or perioperative intravesical chemotherapy instillations (15).
Conclusions
BCG failure is one of the most complex and challenging scenarios in urologic oncology. It is imperative for the clinician to have a clear understanding of the indications for BCG treatment and the definition of BCG failure. The utilization of drug combinations and device-assisted treatments has the potential to improve treatment response. Inhibition of immune checkpoints and targeted therapy addressing central pathways of cell regulation are the subject of multiple clinical trials and could help to define new treatment algorithms. Patients who have failed BCG treatment are at a high risk for disease progression and a definitive treatment with cystectomy should be offered. The hopefully transient shortage of BCG may become a recurring problem in the future. It is imperative for any urologist to be aware of the potential alternatives and their limitations.
Acknowledgements
Funding: This study has been supported in part by NIH/NCI Career Development Award Grant to Guilherme Godoy (K23CA160664). Seth Lerner is a Co-Investigator on this Grant.
Footnotes
Conflicts of Interest: Seth P. Lerner has affiliations (grants) with Imalux, Photocure, Tengion, and FKD, and serves as an advisory board member/consultant for Genentech, Biocancell, Vaxxion, Theracoat, Dendreon, Sitka, and Nucleix.
References
- 1.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976;116:180-3. [DOI] [PubMed] [Google Scholar]
- 2.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 2000;163:1124-9. [PubMed] [Google Scholar]
- 3.Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247-56. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester RJ, van der Meijden AP, Witjes JA, et al. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 2005;174:86-91; discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. J Urol 2008;179:53-6. [DOI] [PubMed] [Google Scholar]
- 6.Sengiku A, Ito M, Miyazaki Y, et al. A prospective comparative study of intravesical bacillus Calmette-Guérin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol 2013;190:50-4. [DOI] [PubMed] [Google Scholar]
- 7.Rentsch CA, Birkhäuser FD, Biot C, et al. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol 2014;66:677-88. [DOI] [PubMed] [Google Scholar]
- 8.Brosch R, Gordon SV, Garnier T, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci U S A 2007;104:5596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw 2013;11:446-75. [DOI] [PubMed] [Google Scholar]
- 10.Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64:639-53. [DOI] [PubMed] [Google Scholar]
- 11.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314-30. [DOI] [PubMed] [Google Scholar]
- 12.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 2013;63:462-72. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Lotan Y, Bastian PJ, et al. Outcomes of patients with clinical T1 grade 3 urothelial cell bladder carcinoma treated with radical cystectomy. Urology 2008;71:302-7. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol 2006;24:481-7. [DOI] [PubMed] [Google Scholar]
- 15.Jarow JP, Lerner SP, Kluetz PG, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology 2014;83:262-4. [DOI] [PubMed] [Google Scholar]
- 16.Catalona WJ, Hudson MA, Gillen DP, et al. Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol 1987;137:220-4. [DOI] [PubMed] [Google Scholar]
- 17.Bui TT, Schellhammer PF. Additional bacillus Calmette-Guérin therapy for recurrent transitional cell carcinoma after an initial complete response. Urology 1997;49:687-90; discussion 690-1. [DOI] [PubMed] [Google Scholar]
- 18.de Reijke TM, Kurth KH, Sylvester RJ, et al. Bacillus Calmette-Guerin versus epirubicin for primary, secondary or concurrent carcinoma in situ of the bladder: results of a European Organization for the Research and Treatment of Cancer--Genito-Urinary Group Phase III Trial (30906). J Urol 2005;173:405-9. [DOI] [PubMed] [Google Scholar]
- 19.Lerner SP, Tangen CM, Sucharew H, et al. Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol 2009;27:155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannarini G, Birkhäuser FD, Recker F, et al. Bacillus Calmette-Guérin failure in patients with non-muscle-invasive urothelial carcinoma of the bladder may be due to the urologist's failure to detect urothelial carcinoma of the upper urinary tract and urethra. Eur Urol 2014;65:825-31. [DOI] [PubMed] [Google Scholar]
- 21.Huguet J, Crego M, Sabaté S, et al. Cystectomy in patients with high risk superficial bladder tumors who fail intravesical BCG therapy: pre-cystectomy prostate involvement as a prognostic factor. Eur Urol 2005;48:53-9; discussion 59. [DOI] [PubMed] [Google Scholar]
- 22.Shen SS, Lerner SP, Muezzinoglu B, et al. Prostatic involvement by transitional cell carcinoma in patients with bladder cancer and its prognostic significance. Hum Pathol 2006;37:726-34. [DOI] [PubMed] [Google Scholar]
- 23.von Rundstedt FC, Lerner SP, Godoy G, et al. Usefulness of transurethral biopsy for staging the prostatic urethra before radical cystectomy. J Urol 2015;193:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol 2008;53:146-52. [DOI] [PubMed] [Google Scholar]
- 25.Tilki D, Reich O, Svatek RS, et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: an international study of 243 patients. J Urol 2010;183:1757-63. [DOI] [PubMed] [Google Scholar]
- 26.Breban R, Bisiaux A, Biot C, et al. Mathematical model of tumor immunotherapy for bladder carcinoma identifies the limitations of the innate immune response. Oncoimmunology 2012;1:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lüftenegger W, Ackermann DK, Futterlieb A, et al. Intravesical versus intravesical plus intradermal bacillus Calmette-Guerin: a prospective randomized study in patients with recurrent superficial bladder tumors. J Urol 1996;155:483-7. [DOI] [PubMed] [Google Scholar]
- 28.Lamm DL, DeHaven JI, Shriver J, et al. Prospective randomized comparison of intravesical with percutaneous bacillus Calmette-Guerin versus intravesical bacillus Calmette-Guerin in superficial bladder cancer. J Urol 1991;145:738-40. [DOI] [PubMed] [Google Scholar]
- 29.Witjes JA, Fransen MP, van der Meijden AP, et al. Use of maintenance intravesical bacillus Calmette-Guérin (BCG), with or without intradermal BCG, in patients with recurrent superficial bladder cancer. Long-term follow-up of a randomized phase 2 study. Urol Int 1993;51:67-72. [DOI] [PubMed] [Google Scholar]
- 30.Biot C, Rentsch CA, Gsponer JR, et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 2012;4:137ra72. [DOI] [PubMed]
- 31.Portillo Martín JA, Martín García B, Hernández Rodríguez R, et al. Clinical trial with intravesical alfa-2b interferon for the prevention of T1 transitional carcinoma of the bladder: preliminary results. Review of the bibliography. Arch Esp Urol 1995;48:479-88. [PubMed] [Google Scholar]
- 32.Nepple KG, Lightfoot AJ, Rosevear HM, et al. Bacillus Calmette-Guérin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol 2010;184:1915-9. [DOI] [PubMed] [Google Scholar]
- 33.Rosevear HM, Lightfoot AJ, Birusingh KK, et al. Factors affecting response to bacillus Calmette-Guérin plus interferon for urothelial carcinoma in situ. J Urol 2011;186:817-23. [DOI] [PubMed] [Google Scholar]
- 34.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015;33:1974-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales A, Phadke K, Steinhoff G. Intravesical mycobacterial cell wall-DNA complex in the treatment of carcinoma in situ of the bladder after standard intravesical therapy has failed. J Urol 2009;181:1040-5. [DOI] [PubMed] [Google Scholar]
- 36.Morales A, Herr H, Steinberg G, et al. Efficacy and Safety of MCNA in Patients with Nonmuscle Invasive Bladder Cancer at High Risk for Recurrence and Progression after Failed Treatment with bacillus Calmette-Guérin. J Urol 2015;193:1135-43. [DOI] [PubMed] [Google Scholar]
- 37.Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 2013;31:1635-42. [DOI] [PubMed] [Google Scholar]
- 38.Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin-refractory transitional cell carcinoma of the bladder. J Clin Oncol 2006;24:2729-34. [DOI] [PubMed] [Google Scholar]
- 39.Di Lorenzo G, Perdonà S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 2010;116:1893-900. [DOI] [PubMed] [Google Scholar]
- 40.Skinner EC, Goldman B, Sakr WA, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guérin. J Urol 2013;190:1200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au JL, Badalament RA, Wientjes MG, et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst 2001;93:597-604. [DOI] [PubMed] [Google Scholar]
- 42.Alfred Witjes J, Hendricksen K, Gofrit O, et al. Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: experience of the European Synergo working party. World J Urol 2009;27:319-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colombo R, Salonia A, Leib Z, et al. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int 2011;107:912-8. [DOI] [PubMed] [Google Scholar]
- 44.Di Stasi SM, Valenti M, Verri C, et al. Electromotive instillation of mitomycin immediately before transurethral resection for patients with primary urothelial non-muscle invasive bladder cancer: a randomised controlled trial. Lancet Oncol 2011;12:871-9. [DOI] [PubMed] [Google Scholar]
- 45.Lightfoot AJ, Breyer BN, Rosevear HM, et al. Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urol Oncol 2014;32:35.e15-9. [DOI] [PMC free article] [PubMed]
- 46.McKiernan JM, Masson P, Murphy AM, et al. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol 2006;24:3075-80. [DOI] [PubMed] [Google Scholar]
- 47.Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guérin therapy. J Urol 2013;189:834-9. [DOI] [PubMed] [Google Scholar]
- 48.McKiernan JM, Holder DD, Ghandour RA, et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guérin treatment failure. J Urol 2014;192:1633-8. [DOI] [PubMed] [Google Scholar]
- 49.Tao Z, Connor RJ, Ashoori F, et al. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: implications for clinical investigation. Cancer Gene Ther 2006;13:125-30. [DOI] [PubMed] [Google Scholar]
- 50.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Houédé N, Pourquier P. Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: its potential use in the treatment of bladder cancers. Pharmacol Ther 2015;145:1-18. [DOI] [PubMed] [Google Scholar]


