TO THE EDITOR
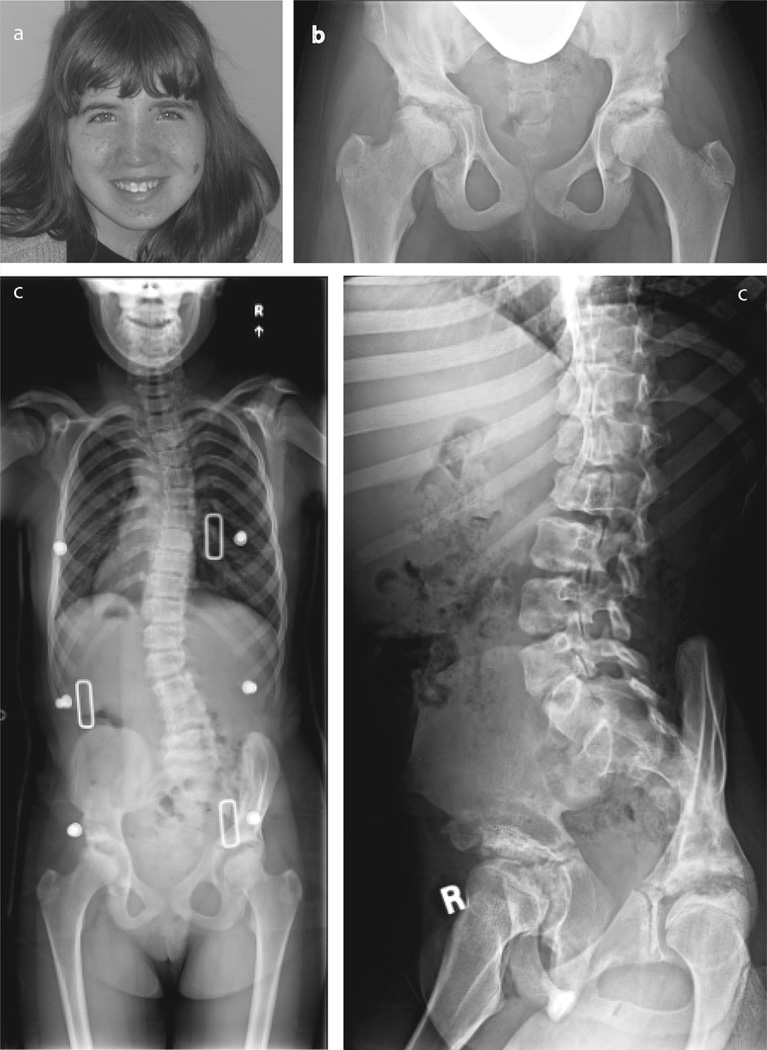
The mechanism whereby a chromosomal deletion uncovers a recessive mutation on the non-deleted chromosome was first proposed by Hatchwell [1996]. Such presentations combine two phenotypes: the consequences of the deletion, typically due to haplo-insufficiency of dosage-sensitive genes in the deleted segment; and the recessive syndrome itself [Coman and Gardner, 2007; Hochstenbach et al., 2012]. Herein,we describe a patientwith three separate Mendelian disorders with a chromosome 16p deletion: tuberous sclerosis, polycystic kidney disease, and mucolipidosis III gamma (MLIII γ). The patient is a Caucasian female born at 40 weeks gestation weighing 3.43 kg to a 28-year-old mother and a 33-year-old father, who were healthy and unrelated. Two older full siblings were normal. The pregnancy was complicated by decreased fetal movements. She had developmental dysplasia of the hips, a vascular malformation of the left cheek, and bilateral clubfeet that responded to serial casting. At 8 months of age, she was noted to have telecanthus, short palpebral fissures, and tapered fingers. At 9 months, she had a prolonged episode of unresponsiveness with an abnormal EEG and was diagnosed with a seizure disorder. She was treated with anticonvulsants for 1 year with no further seizures. Hip dysplasia was treated with bilateral pelvic osteotomies at 3 years of age. At age 4 years, facial angiofibromas in a malar distribution and hypopigmented macules led to a diagnosis of Tuberous Sclerosis. Formal neuropsychological testing at age 4 years showed moderate intellectual disability (FSIQ 48). Renal ultrasound at age 5.5 years was normal. She underwent pulsed dye laser treatment of angiofibromas at ages 8, 11, and 13 years (Fig. 1a). At age 10 years a renal ultrasound showed bilateral multicystic kidneys. A head MRI at 12 years of age showed cortical tubers and subependymal nodules. She subsequently developed genu valgum, stiffness of finger joints, decreased shoulder mobility, scoliosis, pain in back and upper legs, and decreased exercise tolerance. Multiple radiologic abnormalities were detected (Fig.1b,c). The wrists and distal forearms had multiple abnormalities including decreased carpal compartments bilaterally with small and malformed proximal carpal row bones, most evident in the scaphoid and lunate bones; beaking and thinning of the medial aspect of the distal radial epiphyses; and flattening of distal ulnar epiphyses. She developed thoracolumbar scoliosis, malformed and irregular appearing vertebral bodies, and spondylolisthesis of L5 on S1, as well as bilateral hip dysplasia with femoral head irregularities (Fig. 1b). In view of the progression of the symptoms the patient was re-evaluated by the genetics service.
FIG. 1.
a: Patient at 14 years of age with facial angiofibromas. b: Pelvis radiograph demonstrating sclerotic, irregular acetabulae, and central lucencies of femoral heads. c: Spine radiographs showing scoliosis and vertebral endplate irregularities at 14 years of age.
Prior investigation included a normal 46, XX karyotype (resolution greater than 550 bands). Fluorescence in situ hybridization for the 22q11.2 DiGeorge critical region was normal. However, at age 13 an oligonucleotide microarray analysis detected a 1.9 Mb interstitial deletion on chromosome 16p13.3 that spanned from 214,846 to 2,127,395 bp (UCSC version NCBI36/hg18. released in 2006). This deletion contains at least 96 genes, 54 of which were annotated in OMIM, including TSC2 and PKD1. Other genes in the region were examined for possible relationship to the musculoskeletal features exhibited by the patient. The GNPTG gene, which encodes the γ subunit of UDP-N-acetylglucosamine: lysosomal enzyme N-acetylglucosamine 1-phosphotransferase, was in the deleted interval. Biallelic mutations in GNPTG causemucolipidosis III γ (MLIII γ, OMIM #252605). Subsequently, a lysosomal enzyme panel showed normal leukocyte activity, but 10- to 20-fold elevation of the plasma activities of the seven lysosomal hydrolases tested, consistent with diagnosis of ML II/III. Confirmatory testing via GNPTG gene sequencing showed a c.376G>A which predicts p.Gly126Ser; this variant is conserved, had not been previously reported, and was predicted to be probably damaging. Analysis of the subcellular localization of p.Gly126Ser γ expressed in HeLa cells showed a marked difference compared to the wild-type protein (Supplemental Online Fig. 1). While the wild-type enzyme co-localized with the cis-Golgi marker GM130, the mutant protein showed a predominant endoplasmic reticulum (ER) localization, co-localizing with the ER marker calnexin. These results show that the p. Gly126Ser substitution has a harmful effect, preventing the mutant protein from being transported from the ER to the Golgi where it functions.
Mucolipidosis III γ is characterized by childhood onset of mild to moderate dysostosis multiplex; joint stiffness, and pain initially in the shoulders, hips, and fingers; and gradual mild coarsening of facial features [Raas-Rothchild and Spiegel, 2010]. Rarely, affected individuals have mild cognitive impairment. Mucolipidosis III γ is not readily apparent in early childhood given its slowly progressive course with absent or minimal face coarsening, and with most disease manifestations becoming apparent in late childhood or adolescence. In this patient, because of bone findings atypical for TSC or PKD, the diagnosis was made after a thorough review of the genes in the patient’s 1.9Mbinterstitial deletion. The bone findings in the patient are typical for ML III γ. The etiology of the developmental hip dysplasia and clubfeet is unclear as it is not characteristic of any of the three previously mentioned Mendelian disorders, however, it could be the consequence of haploinsufficiency of other dosage-sensitive genes within the microdeletion.
The CLCN7 gene is also in the 1.9 Mb chromosome 16p interstitial deletion. Heterozygous mutations in CLCN7 cause autosomal dominant osteopetrosis Type II (ADO II, OMIM #166600). This disorder is characterized by an increased bone density due to impaired bone resorption leading to sclerosis predominantly involving the spine, pelvis, and skull base [Waguespack et al., 2003; Del et al., 2006]. However, due to a lack of clinical and radiological findings, we concluded that the patient did notmanifest the ADOII phenotype. Mutations in CLCN7 impair the function of the CLCN7 protein by affecting its ability to form dimers, and mutations in the gene therefore have a dominant negative effect [Waguespack et al., 2003]. Since this proband was haploinsufficient, this may explain the lack of findings.
In summary, we report on a patient with three separate Mendelian disorders as a result of a chromosome 16p deletion: tuberous sclerosis due to TSC2 deletion; adult polycystic kidney disease due to PKD1 deletion; and MLIII γ due to deletion of one GNPTG allele in trans to a GNPTG point mutation. Patients who have tuberous sclerosis complex and autosomal dominant polycystic kidney disease can have a contiguous gene syndrome involving TSC2 and PKD1 (OMIM #600273) [Laass et al., 2004; Smulders et al., 2003]. However, MLIII γ is not a part of this syndrome. There are less than 20 reports of chromosomal deletions unmasking recessive disorders. They may be under-recognized due to a tendency to believe that atypical findings represent the pleiotropic effect of the deletion. However, it is becoming evident that atypical, complex symptoms often reflect multiple diagnoses. This report highlights the importance of considering autosomal recessive conditions when evaluating a chromosomal microdeletion, for the unlikely co-occurrence of a mutation in the remaining allele and unmasking a recessive disorder.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patient and her family for their kind collaboration. We also thank Dr. Marilyn Jones for her valuable insights.
Grant sponsor: National Institutes of Health; Grant number: CA08759;
Grant sponsor: The Yash Gandhi Foundation Stuart Kornfeld.
Footnotes
Conflict of interest: none
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site
REFERENCES
- Coman DJ, Gardner RJ. Deletions that reveal recessive genes. Eur J Hum Genet. 2007;15:1103–1104. doi: 10.1038/sj.ejhg.5201919. [DOI] [PubMed] [Google Scholar]
- Del Fattore A, Peruzzi B, Rucci N, Recchia I, Cappariello A, Longo M, Fortunati D, Ballanti P, Iacobini M, Luciani M, Devito R, Pinto R, Caniglia M, Lanino E, Messina C, Cesaro S, Letizia C, Bianchini G, Fryssira H, Grabowski P, Shaw N, Bishop N, Hughes D, Kapur RP, Datta HK, Taranta A, Fornari R, Migliaccio S, Teti A. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet. 2006;43:315–325. doi: 10.1136/jmg.2005.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchwell E. Monozygotic twins with chromosome 22q11 deletion and discordant phenotype. J Med Genet. 1996;33:261. doi: 10.1136/jmg.33.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach R, Poot M, Nijman IJ, Renkens I, Duran KJ, Van’t Slot R, van Binsbergen E, van der Zwaag B, Vogel MJ, Terhal PA, Ploos van Amstel HK, Kloosterman WP, Cuppen E. Discovery of variants unmasked by hemizygous deletions. Eur J Hum Genet. 2012;20:748–753. doi: 10.1038/ejhg.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laass MW, Spiegel M, Jauch A, Hahn G, Rupprecht E, Vogelberg C, Bartsch O, Huebner A. Tuberous sclerosis and polycystic kidney disease in a 3-month-old infant. Pediatr Nephrol. 2004;19:602–608. doi: 10.1007/s00467-004-1442-z. [DOI] [PubMed] [Google Scholar]
- Qian Y, van Meel E, Flanagan-Steet H, Yox A, Steet R, Kornfeld S. Analysis of mucolipidosis II/III GNPTAB missense mutations identifies domains of UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase involved in catalytic function and lysosomal enzyme recognition. J Biol Chem. 2015;290:3045–3056. doi: 10.1074/jbc.M114.612507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raas-Rothschild A, Spiegel R. Mucolipidosis III Gamma. 2010 Jan 28 [updated 2012 Jul 05]. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 2003. 1993–2014. Available from http://www.ncbi.nlm.nih.gov/books/NBK24701/ [PubMed] [Google Scholar]
- Smulders YM, Eussen BH, Verhoef S, Wouters CH. Large deletion causing the TSC2-PKD1 contiguous gene syndrome without infantile polycystic disease. J Med Genet. 2003;40:E17. doi: 10.1136/jmg.40.2.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meel E, Qian Y, Kornfeld S. Mislocalization of phosphotransferase as a cause of mucolipidosis III αβ. Proc Natl Acad Sci USA. 2014;111:3532–3537. doi: 10.1073/pnas.1401417111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguespack SG, Koller DL, White KE, Fishburn T, Carn G, Buckwalter KA, Johnson M, Kocisko M, Evans WE, Foroud T, Econs MJ. Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res. 2003;18:1513–1518. doi: 10.1359/jbmr.2003.18.8.1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.