Abstract
Newborns commonly develop physiological hyperbilirubinemia (also known as jaundice). With increased bilirubin levels being observed in breast-fed infants, breast-feeding has been recognized as a contributing factor for the development of neonatal hyperbilirubinemia. Bilirubin undergoes selective metabolism by UDP-glucuronosyltransferase (UGT) 1A1 and becomes a water soluble glucuronide. Although several factors such as gestational age, dehydration and weight loss, and increased enterohepatic circulation have been associated with breast milk-induced jaundice (BMJ), deficiency in UGT1A1 expression is a known cause of BMJ. It is currently believed that unconjugated bilirubin is metabolized mainly in the liver. However, recent findings support the concept that extrahepatic tissues, such as small intestine and skin, contribute to bilirubin glucuronidation during the neonatal period. We will review the recent advances made towards understanding biological and molecular events impacting BMJ, especially regarding the role of extrahepatic UGT1A1 expression.
Keywords: Bilirubin, UDP-glucuronosyltransferase 1A1, humanized UGT1 mice, extra hepatic
Introduction
Bilirubin is the terminal metabolite of heme catabolism, is metabolized solely by UDP-glucuronosyltransferase (UGT) 1A1 through glucuronidation (Bosma et al., 1994), and accumulates in newborns because of a developmental delay in the expression of the UGTs (Burchell et al., 1989). Convincing evidence indicates that breast-fed infants have higher levels of total serum bilirubin (TSB) compared to bottle-fed infants (Arias et al., 1964; Schneider, 1986; Gourley, 2002; Stiehm and Ryan, 1965), suggesting that breast milk is a risk factor for the development of neonatal hyperbilirubinemia. While jaundice is usually benign, neonatal phototherapy treatment is often recommended in infants with severe jaundice. The complications of phototherapy include mother-infant separation that limits their interactions and has been linked to the development of postnatal depression (Crowell and Treboux, 1995; Onozawa et al., 2001; Coyl et al., 2002), but if not treated, infants with high levels of TSB become prone to unconjugated bilirubin-induced encephalopathy (Shapiro, 2003, 2005, 2010).
Arias et al. (1963) and Newman et al. (1963) first reported that breast milk was a contributing factor towards the development of neonatal jaundice. Substances in breast milk, including pregnane −3α,20β-diol, non-esterified fatty acid, β-glucuronidase, and other factors such as sub-optimal fluid intake, weight loss, increased enterohepatic circulation of bilirubin, and pre-mature birth were suspected of causing breast milk jaundice (BMJ) (Arias et al., 1964; Bevan and Holton, 1972; Gourley and Arend, 1986; American Academy of Pediatrics, 2004; Ip et al., 2004; Maisels et al., 2009). While these aforementioned factors may potentiate hyperbilirubinemia in jaundiced infants, key factors leading to BMJ have not yet been conclusively identified (Ramos et al., 1966; Constantopoulos et al., 1980; Gaffney et al., 1986). In 1979, Onishi et al. demonstrated that bilirubin UGT activity was repressed in human liver during neonatal development, suggesting the delayed development of hepatic UGTs leads to increased TSB levels. Since then, many lines of new evidence associated with neonatal jaundice and BMJ have been revealed; in particular, significant findings have been made in 2 areas, which are the main focus of this review: the important role of extrahepatic UGT1A1 expression and UGT1A1 polymorphism that is clinically relevant to hyperbilirubinemia.
UDP-Glucuronosyltransferase (UGT)
UGTs constitute a super family of endoplasmic reticulum (ER) bound proteins that form glucuronides by transferring glucuronic acid from UDP-glucuronic acid to the selective substrates (Dutton, 1980). The mammalian UGT gene superfamily, comprising four families, currently has more than 100 members (Mackenzie et al., 2005). In human, 19 UGTs had been identified, each being classified into the UGT1 (UGT1A1, -1A3, -1A4, -1A5, -1A6, -1A7, -1A8, -1A9 and -1A10) or UGT2 family (-A1, -A2, -A3, -B4, -B7, -B10, -B11, -B15, -B17, -B28) (Williams et al., 2004). The major UGTs involved in xenobiotic metabolism are UGT1A1, -1A3, -1A4, -1A6, -1A9 and -2B7 (Rowland et al., 2013), whereas a distinct yet overlapping set of UGTs is responsible for metabolism of various endogenous substances, such as bilirubin, estradiol, testosterone, 5-hydroxytryptamine, thyroid hormones (thyroxine and triiodothyronine), and bile acids (Tukey and Strassburg, 2000). It is now understood that the UGTs are expressed in a highly coordinated tissue specific fashion. The different complement of expressed UGTs in different tissues dictates the ability of each tissue to metabolize the large range of UGT substrates. With the number of UGTs well documented in different human tissues, many of them are expressed in the GI tract with great abundance and are subject to regulation by food derived nutrients and other substances that are consumed in the diet. With regard to newborns, these nutrients are being provided directly by breast milk or supplemented with formula.
While the UGTs exhibit broad substrate specificity, certain compounds are glucuronidated selectively. UGT1A1 is the sole UGT isoform responsible for the glucuronidation of bilirubin (Bosma et al., 1994). Since bilirubin is hydrophobic and exists in the blood mostly bound to serum proteins prior to its movement into cells and metabolism by UGT1A1 glucuronidation, certain physiological events can influence the relative saturation limits of serum proteins. In the event that bilirubin production exceeds the body’s ability to maintain a steady-state balance with serum proteins, the circulating unconjugated bilirubin is free to move across cell membranes and concentrates in various tissues that are getting a rich supply of blood, such as the tissues of the central nervous system. In adults, the dense network of capillaries that form the blood brain barrier is well developed and serves as a barrier to prevent access of miscellaneous agents that are found free in the blood. But in newborns, the blood brain barrier is poorly defined and substances, such as unconjugated bilirubin if not bound to serum proteins, can accumulate in brain tissue3. If bilirubin is not metabolized, its accumulation can lead to the initiation of cellular inflammation and eventually gliosis of glial cells (kernicterus) (Shapiro, 2005; Yueh et al., 2014). The central molecular events involved in the homeostatic balance between conjugated and unconjugated bilirubin during neonatal development is the control and expression of the UGT1A1 gene. Thus, developmental regulation of UGT1A1 is the rate limiting step in determining the severity of neonatal hyperbilirubinemia, and an appreciation of such regulation will play a significant role in preventing bilirubin induced neurological toxicities.
Metabolic pathway of bilirubin
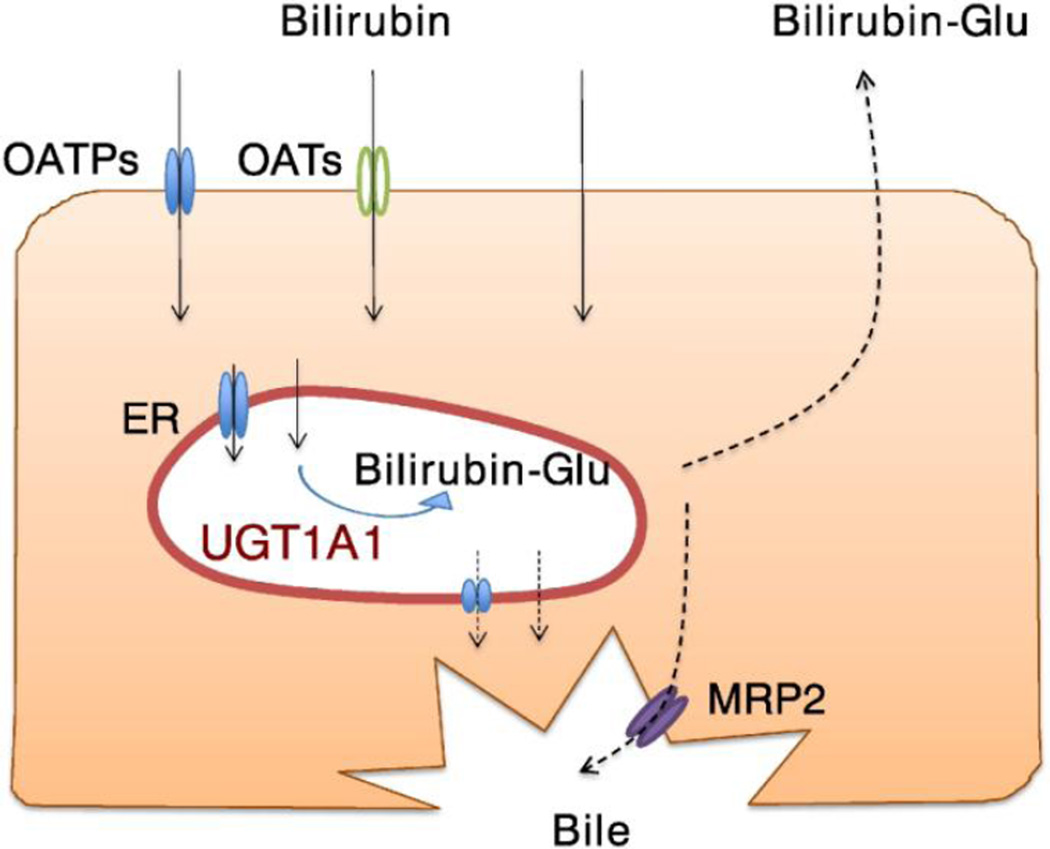
Liver tissue plays an essential role in the metabolism of drugs, xenobiotics, and many exogenous compounds (Kutsuno et al., 2013 and 2014). In the liver, sinusoidal membrane transporters, such as organic anion transporting polypeptides (OATPs) and organic anion transporters (OATs), contribute to the uptake of bilirubin from blood to hepatocytes (Kamisako et al., 2000; Zhang et al., 2007), although passive diffusion may be substantial in the transport of bilirubin into liver cells. Characterization of the membrane-bound UGTs demonstrates that the majority of the protein, including its active site, is located on the luminal surface of the ER (Rowland et a., 2013; Shepherd et al., 1989; Vanstapel et al., 1988), indicating that, UGT substrates, including bilirubin, need to be transported to the luminal side of the ER to be metabolized. Subsequently, it is necessary to transport the glucuronides back to the cytoplasm following glucuronidation (Fig. 1). A study by Csala et al. suggested that transporter(s) mediate the movement of hydrophobic compounds and their glucuronides in the ER membrane (Csala et al., 2007). Reported accumulation of bilirubin glucuronides within the lumen of the ER, which was found in liver microsomes from a jaundiced patient (Waddell et al., 1995), might have resulted from a genetic deficiency of such transporters in the ER membrane. Efforts have been made to identify ER membrane transporters that are responsible for the transport of bilirubin and its glucuronides across the ER membrane (Fujiwara and Itoh, 2014a and 2014b); however, such transporters have not been characterized to date. Protein interactions between UGT-UGT and -cytochrome P450 (CYP) might be involved in the transport of the substances across the ER membrane (Fujiwara et al., 2007a, 2007b and 2010; Nakajima et al., 2007; Finel and Kurkela, 2008; Ishii et al., 2007). Following bilirubin metabolism in liver cells, its glucuronides are transported out of these cells through the apical surface of the hepatocytes into the bile ducts by the multidrug resistance protein 2 (MRP2/ABCC2)(Fig. 1), leading to their movement through the GI tract where they eventually come into contact with microbes in the large intestine (Keppler et al., 1997; Kamisako et al., 2000). Bilirubin glucuronide is a substrate for microbial β-glucuronidase, which can cleave the glucuronide and liberate bilirubin for reabsorption through the basolateral surfaces of the intestines where it can undergo further metabolism or pass directly back into the circulation. This process, known as enterohepatic circulation, can extend the half-life of bilirubin while adding to the total serum bilirubin load. In addition, the presence of β-glucuronidase in breast milk has been linked to BMJ since it can directly increase the rate of enterohepatic circulation of bilirubin (Gourley, 2002).
Fig. 1. Metabolic pathway of bilirubin in hepatocytes.
OATPs and OATs uptake bilirubin into hepatocytes, while bilirubin is passively absorbed into the hepatocytes. Bilirubin is further transported into the luminal side of ER to be conjugated by UGT1A1. There might be a carrier that transport bilirubin and bilirubin-glucuronide across the ER membrane. Bilirubin-glucuronide is transported into the bile ducts by MRP2.
Hereditary unconjugated hyperbilirubinemia
Bilirubin undergoes glucuronidation selectively by UGT1A1 (Bosma et al., 1994). Genetic polymorphisms in the UGT1A1 gene can impair its enzymatic activity and cause hereditary unconjugated hyperbilirubinemia, specifically Crigler-Najjar syndrome (CN) type I (MIM #21880), type II (MIM #606785), and Gilbert’s syndrome (MIM #143500) (Bosma et al., 1992, 1994, and 1995; Aono et al., 1993; Monaghan et al., 1996 and 1999). The severity of hyperbilirubinemia is based upon the degree of deficiency of UGT1A1 activity as determined by the mutations in the UGT1A1 gene. Among over 40 mutations in the UGT1A1 gene that are associated with inheritable unconjugated hyperbilirubinemia (Tukey and Strassburg, 2000; Strassburg, 2008), the majority of these mutations leads to one of three major hyperbilirubinemia syndromes: CN type I and type II and Gilbert’s syndrome. Mutations in CN type I cause complete loss of UGT1A1 enzymatic bilirubin glucuronidation, resulting in a chronic, life-threatening condition of potential encephalopathy (Ritter et al., 1992 and 1993). Patients with CN type II have mutations that greatly reduce UGT1 activity and have moderate to high serum unconjugated bilirubin levels with rare occurrences of CNS toxicity (Crigler and Najjar, 1952; Arias et al., 1969; Robertson et al., 1991; Moghrabi et al., 1993). By comparison, Gilbert’s syndrome is a common, mild form of hyperbilirubinemia with hepatic UGT1A1 activity being about 30% of the normal level (Strassburg, 2008; Burchell and Hume, 1999). Typically, it is often first noticed as intermittent mild jaundice during adolescence (Gilbert and Lereboullet, 1901). The prevalence of Gilbert’s syndrome is approximately 3–9% of the population globally, with 1 in 3 affected patients being unaware that they have it (Owens and Evans, 1975; Sieg et al., 1987). The most common genotype of Gilbert’s syndrome is the homozygous polymorphism of a TA insertion in the (TA)6TAA region of the UGT1A1 promoter (the 7/7 allele, also known as UGT1A1*28), whereas a missense mutation in coding exon 1 (G71R, also known as UGT1A1*6) is more prevalent in Asian populations, especially in jaundiced Japanese newborns (Maruo et al., 1999 and 2004; Bosma et al., 1995; Beutler et al., 1998; Monaghan et al., 1999; Strassburg, 2008). Newborns that express the UGT1A1*6 allele are at increased risk of developing BMJ.
Neonatal jaundice and breast milk-induced jaundice
While hyperbilirubinemia can develop at any age, physiological jaundice is common in newborns. One of the molecular mechanisms underlying the development of neonatal jaundice involves the delayed and reduced expression of hepatic UGT1A1 and the simultaneous increase in TSB levels following destruction of fetal hemoglobin shortly after birth. UGT expression is subject to developmental regulation (Onishi et al., 1979; de Wildt et al., 1999; Burchell, 1973 and 1974). Adults express abundant levels of hepatic UGT1A1, as confirmed by experimental data with mRNA and protein expressions and enzymatic activity (Ritter et al., 1999; Nakamura et al., 2008; Izukawa et al., 2009; Ohno and Nakajin, 2009; Strassburg et al., 1997, 1998a, 1998b, 1999a, and 1999b), whereas fetuses and neonates – in contrast – have very limited UGT1A1 expression and bilirubin glucuronidation activity in liver (Onishi et al., 1979; Strassburg et al., 2002). Through analysis of mutations in the UGT1A1 gene it has been conclusively demonstrated that UGT1A1 is the sole enzyme responsible for bilirubin glucuronidation (Bosma et al., 1994), and its reduced expression (or absence) causes a correlated increase in levels of TSB during the neonatal period. With this statement, researchers logically assume that reduced UGT1A1 expression in the liver is the key factor determining the developmental onset of neonatal jaundice.
Accumulating evidence indicates that breast-fed infants have a higher risk for kernicterus development than formula-fed infants (Gourley, 1998 and 2002; Alonso et al., 1991; Bhutani et al., 2005), and the American Academy of Pediatrics (AAP) and the National Institute for Health and Clinical Excellence (NICE) of the U.K. have cited that breast-feeding is a risk factor for hyperbilirubinemia and potentially kernicterus formation. Components in breast milk, such as steroids, fats, cytokines, B-glucuronidase and the epidermal growth factor (EGF), have been tied to the accumulation of TSB through increased uptake of bilirubin by enterohepatic recirculation, a decrease in bilirubin excretion, or the inhibition of UGT1A1 activity (Arias et al., 1964; Bevan and Holton, 1972; Gourley and Arend, 1986; Shibuya et al., 2013). BMJ is defined as jaundice that occurs in neonates that consume adequate levels of breast milk. At the same time, inadequate breast milk-intake can lead to the onset of neonatal jaundice, which is called inadequate breastfeeding jaundice. In contrast to humans, experimental animals, such as mice or rats, do not naturally develop BMJ. Until recently, conclusive evidence explaining the mechanism of BMJ had not been obtained due to the lack of a suitable hyperbilirubinemia animal model.
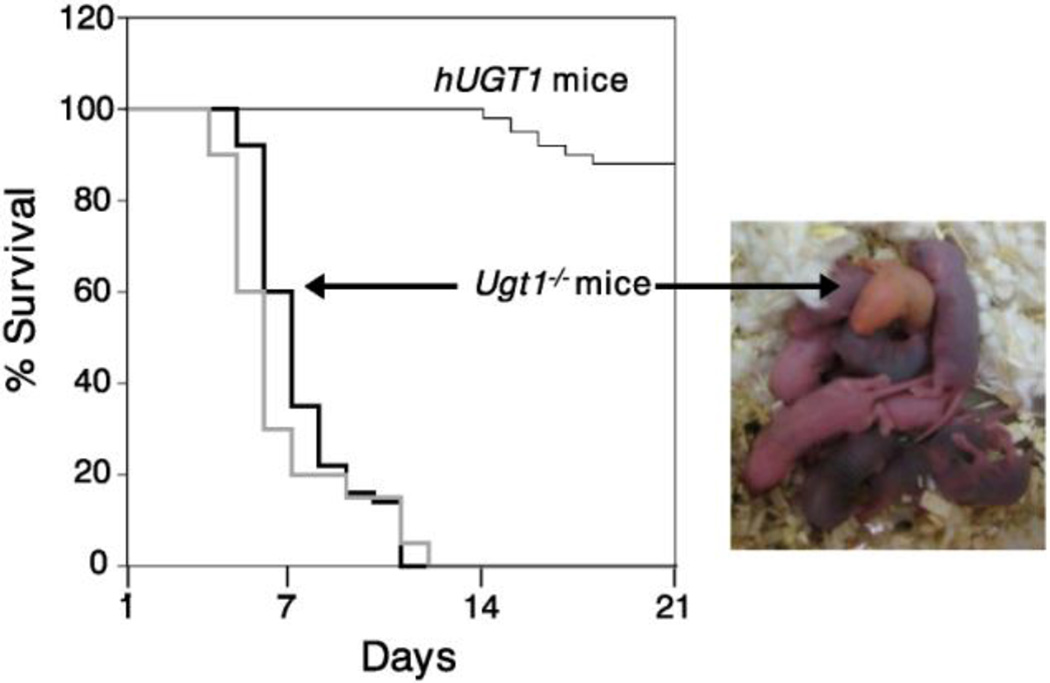
The recent development of humanized UGT1 (hUGT1) mice – expressing the entire human UGT1 locus in a Ugt1-null background – has provided the first appropriate animal model to study the mechanisms associated with neonatal hyperbilirubinemia. The generation of humanized UGT1 mice was accomplished in a few steps. First, a transgenic mouse line (TgUGT1 mice), harboring the entire human UGT1 locus and expressing all nine of the UGT1A genes, was generated (Chen et al., 2005). Second, the entire murine Ugt1 locus was inactivated (Ugt1−/− mice) through the introduction of a genetic mutation into exon 4, eliminating the nine mouse UGT1A proteins. Finally, crossing heterozygous Ugt1+/− mice with TgUGT1 mice led to the generation of a hybrid mouse line (TgUGT1/Ugt1+/−) and eventual Tg(UGT1)Ugt1−/− mice, which represent fully humanized UGT1 (hUGT1) mice. The use of the heterozygous Ugt1+/− line was necessary because the Ugt1−/− mice exhibit the accumulation of lethal levels of TSB (Nguyen et al., 2008; Bortolussi et al., 2012), manifesting as an orange skin color early after birth, and most of them die within 7 days after birth (Fig. 2). Humanization with the UGT1 locus, including the UGT1A1 gene, rescues neonatal lethality pertaining to the Ugt1-null locus and the absence of the Ugt1a1 gene. The expression pattern of the human UGT1A genes was nearly identical to that documented in human tissues (Tukey and Strassburg, 2000). Thus, hUGT1 mice seem to be a relevant animal model for studying the molecular events regulating UGT1A1 gene expression during development.
Fig. 2. Survival curves of hUGT1 and Ugt1−/− mice.
Due to the accumulation of lethal levels of TSB, most of Ugt1−/− mice die within 7 days after birth (thicker lines). Humanization with the UGT1 locus rescues neonatal lethality, although 5–10% of newborn hUGT1 mice are still lethal (thin line). Black thick line indicates the survival curve of Ugt1−/− mice reported by Nguyen et al., (2008). Gray thick line indicates the survival curve of Ugt1−/− mice reported by Bortolussi et al., (2012).
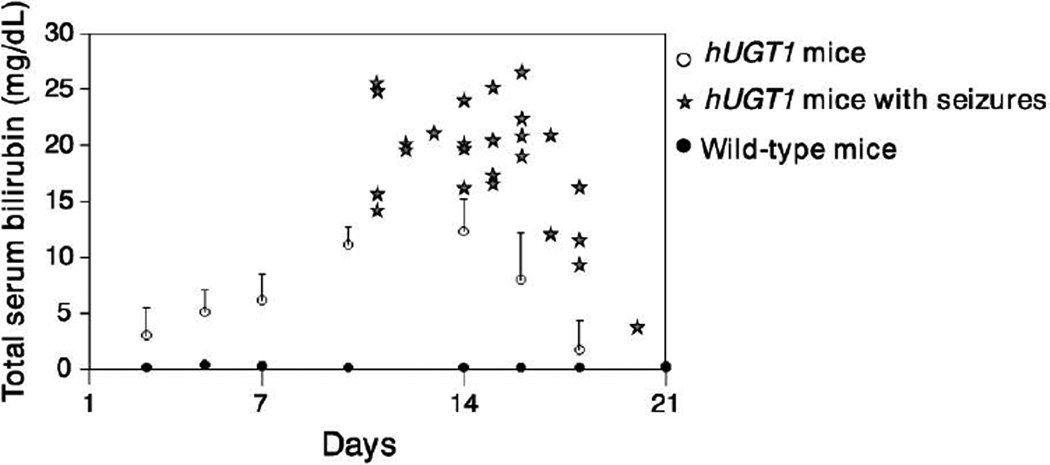
Shortly after birth, hUGT1 mice develop severe hyperbilirubinemia, with peak levels of TSB being achieved approximately 14 days after birth and ranging from 12 to 15 mg/dL, compared with < 0.2 mg/dL in wild-type mic (Fig. 3). Overly extreme hyperbilirubinemia occurs in 5–10% of newborn hUGT1 mice, with the TSB levels often reaching > 20 mg/dL, leading to the development of seizures and eventual death due to bilirubin accumulation in brain tissue. Mimicking the hUGT1 model, children with CN type I also develop seizures as a result of severe hyperbilirubinemia. Studies examining the UGT1A1 gene expression pattern and the regulatory role of xenobiotic receptors in hUGT1 mice have revealed that the steady accumulation of TSB levels in the first two weeks after birth is attributed to significantly repressed expression of liver UGT1A.
Fig. 3. Total serum bilirubin levels in neonatal hUGT1 and wild-type mice.
Mean TSB levels in neonatal hUGT1 and wild-type mice were shown. Stars indicate the TSB levels of hUGT1 mice that developed seizures.
We discovered that reduction in liver UGT1A1 gene expression during development is a programmed event, with the pregnenalone X receptor (PXR) serving as a transcriptional co-repressor, as evidenced by the fact that humanized UGT1 mice that are deficient in PXR do not develop extreme hyperbilirubinemia (Chen et al., 2012). While the reduction in hepatic UGT1A1 continues 14 days after birth, we observed that TSB levels start to gradually decline and eventually reach normal levels as intestinal UGT1A1 expression and activity coincidently progressively increase, implying the role of intestinal UGT1A1 in bilirubin glucuronidation (Fujiwara et al., 2010).
It has been recognized for more than 50 years that newborns who are breastfed have a 3- to 6-fold greater probability of developing elevated TSB levels than formula-fed newborns (Newman and Gross, 1963; Arias et al., 1963; Schneider, 1986). Our descriptive observations that the timing of intestinal UGT1A1 gene expression in hUGT1 mice and the concordant reduction in TSB levels led us to examine the possibility that breast milk controlled expression of intestinal UGT1A1. When neonatal hUGT1 mice that were 10 days of age were removed from nursing and placed on a formula diet, TSB levels dropped dramatically within 24 hours (Fujiwara et al., 2012). The reduction in TSB levels was a direct result of the formula, because when we fed hUGT1 mice human breast milk (HBM), it had no impact on reducing TSB levels. This study suggested that breast milk, including HBM, directly suppresses expression of the UGT1A1 gene. When gene expression patterns were examined, formula feeding led to dramatic induction of intestinal UGT1A1 gene expression. This induction did not occur when hUGT1 mice were fed HBM. Importantly, formula treatment only induced intestinal UGT1A1 expression without affecting the expression patterns in liver. Two important conclusions were drawn from these studies. First, specific components in breast milk played an important role in repressing expression of intestinal UGT1A1. Second, regulation of intestinal UGT1A1 during neonatal development when liver gene expression was compromised appeared to play an important role in bilirubin metabolism and elimination.
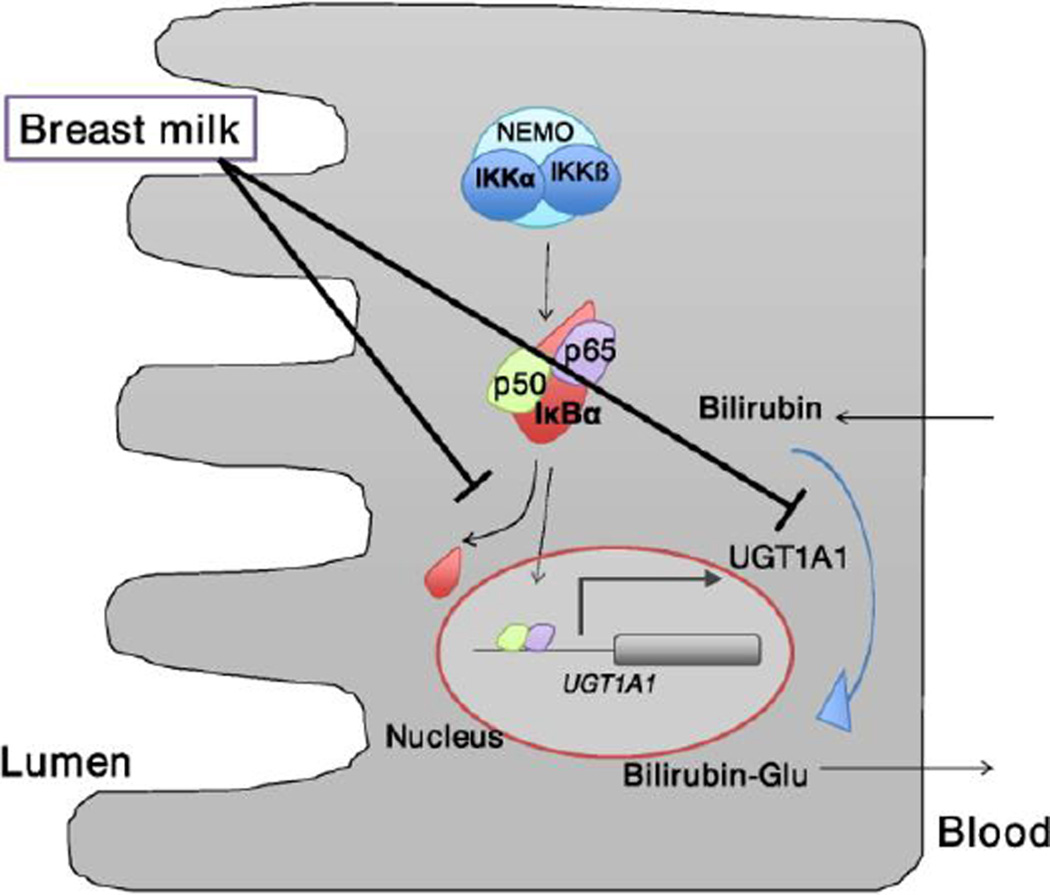
During the course of these studies, selective gene profiling demonstrated that formula treatment led to induction of the mouse macrophage inflammatory protein-2 (Mip-2) gene, the homologue of human IL-8, as well as the Cox-2 gene, both being classical NF-κB target genes. These findings were relevant since it had been reported previously that breast milk can inhibit nuclear factor κB (NF-κB)-dependent target gene expression (Minekawa et al., 2004). When neonatal hUGT1 mice were treated orally with agents known to activate the IκB kinase (Ikk)/NF-κB pathway, intestinal UGT1A1 gene expression was induced and TSB levels dropped. The correlation between Ikk/NF-κB activation by formula and induction of intestinal UGT1A1 gene by other selective agents known to activate NF-κB led us to speculate that the Ikk/NF-κB pathway plays an important role in regulation of intestinal UGT1A1 gene expression and the onset of BMJ. It is unclear how NF-κB is tied to regulation of intestinal UGT1A1, but we have speculated that NF-κB is important for intestinal UGT1A1 gene expression and that inhibition of the Ikk/NF-κB pathway by possible agents present in breast milk contributes to the onset of BMJ (Fujiwara et al., 2012 and Fig. 4).
Fig. 4. Schematic representation of pathways in intestinal cells.
In neonatal hUGT1 mice, UGT1A1 metabolizes bilirubin in the gastrointestinal tract to prevent the accumulation of bilirubin. Intestinal UGT1A1 is under control by IKK/NF-κB (p50/p65) signaling. In the breast-fed neonates, breast milk can inhibit the NF-κB-mediated transcription of UGT1A1. Meanwhile, breast milk can also inhibit the bilirubin glucuronidation. NEMO, NF-κB essential modulator.
Possible factors in breast milk that cause neonatal hyperbilirubinemia
Identifying the components of breast milk that lead to the onset of BMJ has been elusive. Pregnane-3α, 20β-diol, a steroid in breast milk, was first suspected of being an inhibitor of bilirubin-conjugating activity (Arias et al., 1963). Indeed, this steroid had the potency to inhibit bilirubin glucuronide formation in vitro (Arias et al., 1964). However, other findings over 20 years later demonstrated that the levels of pregnane-3α, 20β-diol were barely detectable in breast milk (Murphy et al., 1981).
Breast milk contains a high amount of fatty acids, including oleic acid, linoleic acid, and docosahexaenoic acid, along with other C18 and C20 unsaturated fatty acids and has been shown to inhibit UGT activities in vitro (Bevan and Holton, 1972; Hargreaves, 1973; Shibuya et al., 2013), indicating that unsaturated fatty acids may contribute to the development of BMJ. Although these unsaturated fatty acids strongly inhibited UGT1A1 activity in vitro by using the recombinant UGT1A1 system, they induced both hepatic and intestinal UGT1A1 expression and reduced TSB levels when they were treated to hUGT1 neonates (Shibuya et al., 2013). These findings suggest that the inhibitory potential of unsaturated fatty acids when being added directly to microsomes cannot mimic the actions seen in vivo, and the reason may be that UGT1A1 induction is secondary to the induction of cellular signaling by these fatty acids. One can speculate that the architecture of the intestinal villi allows sufficient contact of the fatty acids with the rich layers of the epithelial cells where nuclear receptor activation and subsequent UGT1A1 induction may occur.
Various cytokines are present in human breast milk, including interleukin (IL)-1β, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α. In particular, the concentration of IL-1β in colostrum was statistically higher in mothers of infants with jaundice, compared with mothers nursing infants who did not develop jaundice (Zanardo et al., 2007). A similar observation was reported in 2012 showing that IL-1β concentrations were significantly higher in the breast milk of mothers whose infants had BMJ (Apaydin et al., 2012). Epidermal growth factor (EGF) has also been reported to be elevated in human breast milk of mothers whose infants developed BMJ (Kumral et al., 2009). While the molecular mechanism involving IL-1β- and EGF-induced jaundice is unknown, evidence has shown that through facilitating gastrointestinal development and promoting systematic responses, EGF and IL-1β might interrupt intestinal absorption and enterohepatic circulation of bilirubin. Moreover, it has been reported that IL-1β can inhibit the constitutive androstane receptor (CAR)-induced expression of hepatic UGT1A1 gene expression (Assenat et al., 2004). However, research in our laboratory has demonstrated that knocking out CAR in hUGT1 (hUGT1/Car−/−) mice has no impact on the TSB levels in newborn mice (Fujiwara et al., 2012). It is interesting to note, however, that deletion of CAR seems to sensitize brain tissue to the elevated levels of unconjugated bilirubin, since over 50% of the hUGT1/Car−/− mice die shortly after birth. Thus, since breast milk contains higher concentrations of cytokines, including IL-1, IL-6, IL-10 and TNFα, inflammatory signaling may take place, leading to suppression of intestinal UGT1A1 gene expression. This hypothesis, which is aligned with the previous findings associating the Ikk/NF-κB pathway and intestinal UGT1A1 gene expression, needs further exploration (Fig. 4).
It was previously demonstrated that breast-fed infants had a higher prevalence of malnutrition compared to bottle-fed infants (Victora et al., 1984). Inadequate caloric intake or starvation has been implied as a cause of neonatal jaundice (Sato et al., 2013). While breast-fed newborns showed relatively severe hyperbilirubinemia, those newborns who received supplemental glucose along with breast milk showed significantly lowered TSB levels. Recently, the underlying mechanism of glucose-induced reduction in TSB levels was elucidated with a series of in vitro and in vivo studies. In hUGT1 mice, supplemental oral glucose treatments specifically induced UGT1A1 in the small intestine but not in the liver (Aoshima et al., 2014). Glucose-mediated UGT1A1 induction was also observed in human intestinal Caco-2 cells. We further identified that Specificity Protein 1 (SP1) was the key transcriptional factor controlling the induction of intestinal UGT1A1 by glucose (Aoshima et al., 2014). These findings indicate that additional caloric intake through glucose supplementation induces expression of UGT1A1 in the small intestine, alleviating hyperbilirubinemia in breast-fed infants. Further research is required to understand the underlying mechanism through which glucose enhances UGT1A1 gene expression. Inadequate caloric intake is common in low birth weight preterm infants (Rodriguez and Rice, 2014), in whom a higher incident of jaundice has been reported (Watchko and Oski, 1992); therefore, inadequate caloric intake could be a causal factor of inadequate breastfeeding jaundice. The fact that large variability exists in both nutrients and caloric content in human breast milk within mothers indicates that there may be other factors causing suppression of UGT1A1 expression.
Role of extrahepatic UGT1A1 in bilirubin metabolism
While liver plays a major role in bilirubin metabolism, several key experiments have demonstrated that extrahepatic tissues contribute to bilirubin metabolism and clearance. Over 50 years ago, broken cell preparations of guinea pig gastrointestinal mucosa was used to demonstrate its enzymatic potential to form bilirubin glucuronides (Stevenson and Dutton, 1962), while Hartmann and Bissell (1982) demonstrated that bilirubin-UGT activity was qualitatively similar to that present in liver tissue by using cell-free extracts of small intestinal mucosa from rats or humans. Two independent studies, in which Gunn rats - deficient in the Ugt1 locus and the Ugt1al gene - underwent small intestinal tissue transplantation from Wistar rats confirmed that intestinal tissue was capable of metabolizing bilirubin and reducing TSB levels (Takahashi et al. 1994; Medley et al, 1995). Since Gunn rats do not have the ability to conjugate bilirubin, it is assumed that the reduction of TSB levels in the small intestine-transplanted Gunn rat is associated with accelerated bilirubin metabolism and clearance in the tissue transplant. As described above, hUGT1 neonates develop hyperbilirubinemia, and their TSB levels are inversely correlated with the expression level of UGT1A1 in the small intestine in the second half of the developmental period (Fujiwara et al., 2010). Furthermore, small intestine-specific induction of the UGT1A1 gene resulted in a dramatic decrease in TSB levels in neonatal hUGT1 mice (Fujiwara et al., 2012). Combined, these studies provide indirect evidence that intestinal tissue is capable of serving as the conduit for bilirubin glucuronidation and clearance.
Employing mouse genetics and the Cre-lox system, Chen et al. (2013) demonstrated that liver-specific deletion of mouse Ugt1a1 resulted in only a slight increase in TSB levels during development. This is in stark contrast to whole animal deletion of the Ugt1 locus (Ugt1−/−), which has been shown to be a lethal mutation that results in dramatic increases in TSB levels shortly after birth (Nguyen et al., 2008). The dramatic increase in TSB levels in Ugtl−/− mice within the first week after birth leads to pronounced microglia neuroinflammation and severe brain impairment (Yueh et al., 2014). The targeted deletion of the Ugt1a1 gene in liver tissue and the resulting mild increase in TSB levels is direct confirmation that bilirubin is conjugated and excreted by extra-hepatic tissues, such as the intestines, during neonatal development.
Exposing jaundiced newborns to sunlight has been associated with lowering TSB levels (Salih, 2001). Ultraviolet (UV)-B exposure will isomerize bilirubin leading to its excretion from the body (Salih, 2001). Therefore, it was originally hypothesized that the UVB-induced isomerization of bilirubin was the underlying mechanism for decreasing TSB levels in sunlight-exposed human infants. Another line of evidence indicates that UVB exposure photo-oxidizes L-tryptophan in the skin and generates 6-formylindolo[3,2-b]carbazole (FICZ), which is a known ligand for the aryl hydrocarbon receptor (AhR) (Wincent et al., 2009). Indeed, the cytochrome P450 1A1 gene, a well-defined AhR target gene, is induced by FICZ in cultured cells (Sumida et al., 2013). Since UGT1A1 is also a target gene of the AhR (Yueh et al., 2003), we hypothesized that induction of UGT1A1 in the skin can contribute to bilirubin metabolism in sunlight-exposed neonates. Previous evidence has shown that selective UGT1A proteins, including UGT1A1, are expressed in human skin as well as in human skin derived keratinocytes (Sumida et al., 2013; Peters et al., 1987). Treatment of HaCaT keratinocytes with FICZ or UVB-exposed L-tryptophan resulted in an induction of UGT1A1 (Sumida et al., 2013). When hUGT1 neonates were exposed to UVB, we further demonstrated that UVB exposure induced UGT1A1 in the skin, accompanied by a reduction of TSB levels (Sumida et al., 2013). Since the skin receives a large supply of blood, it is reasonable to speculate that UGT1A1 expressed in the skin plays an important role in metabolism and clearance of circulating bilirubin. Gene expression of UGT1A1 has been detected in many extrahepatic tissues, including most of the GI tract (except esophagus), kidney, lung and brain (Nakamura et al., 2008; Shelby et al., 2003, Tukey and Strassburg, 2000). While the role of these tissues in the metabolic clearance of bilirubin is starting to be understood, we can speculate that UGT1A1 expression in the brain might exhibit protective effects against the accumulation of unconjugated bilirubin from bilirubin-induced brain damage.
Genetic polymorphisms in the UGT1A1 gene and their association with neonatal jaundice
Several lines of evidence indicate that polymorphisms in the UGT1A1 gene are linked to BMJ. For example, the UGT1A1*28 genotype in Gilbert’s syndrome is linked to the onset of BMJ. In a secluded hospital-based nested case-control study in Northern India, Agrawal et al. concluded that the UGT1A1*28 allele was a risk factor in newborns and was associated with TSB levels ≥ 18mg/dl, a condition requiring phototherapy. Other studies have documented that newborns homozygous for the Gilbert’s syndrome A(TA)7TAA polymorphism serves as one of the factors contributing to neonatal jaundice (Laforgia et al., 2002). These findings, however, appear to be controversial since in other studies the probability of developing neonatal jaundice was not statistically different between neonates who carry the UGT1A1*28 allele and those who carry the more common UGT1A1*1 allele (Maruo et al., 1999; Babaoglu et al., 2006; Azlin et al., 2011; Huang et al., 2004; Travan et al., 2014). Data obtained with hUGT1 mice also support the conclusion that the UGT1A1*28 allele does not impact TSB levels in the neonatal period. Humanized UGT1 mice that carry homozygous UGT1A1*1 or the UGT1A1*28 allele developed neonatal hyperbilirubinemia, and their TSB levels did not show statistical differences (Fujiwara et al., 2010).
In contrast, the UGT1A1*6 allele associated with Gilbert’s syndrome has been consistently linked to elevated TSB levels in newborns (Maruo et al., 1999 and 2000; Azlin et al., 2011; Huang et al., 2004; Chang et al., 2013; Sato et al., 2013) (Table 1). In a study with a large number of Japanese children, Maruo et al. (2014) reported that the allelic frequency of UGT1A1*6 in the hyperbilirubinemia group (0.694) was significantly higher than that in the non-hyperbilirubinemia group (0.182). It was further demonstrated that the impact of the UGT1A1*6 allele on TSB levels was significant in breast-fed infants (Maruo et al., 2014). The complete mechanism documenting the contribution of UGT1A1*6 towards BMJ has not been fully elucidated; however, it has been suggested that delayed bilirubin glucuronidation in newborns that express the UGT1A1*6 allele results from inhibition of the p.G71R-UGT1A1 protein by the breast milk component 5α-pregnane-3α, 20β-diol (Ota et al., 2011).
Table 1.
Genetic polymorphisms in UGT1A1 and their impact on hyperbilirubinemia and neonatal jaundice
| Polymorphism | Ethnic group | Frequency | Note | Reference | |
|---|---|---|---|---|---|
| Control* | Patient | ||||
| UGT1A1*6 | Japanese | N.D. | 0.68 | Among 17 BMJ neonates, 8 neonates were homozygous and 7 were heterozygous. | Maruo et al., 2000 |
| Japanese | 0.16 | 0.34 | Allelic frequency was statistically higher in neonates with hyperbilirubinemia. | Maruo et al., 1999 | |
| Malay | 0.04 | 0.13 | Allelic frequency was statistically higher in neonates with hyperbilirubinemia. | Azlin et al., 2011 | |
| 0.11 | 0.31 | Allelic frequency was statistically higher in neonates with hyperbilirubinemia. | Huang et al., 2004 | ||
| 0.13 | 0.23 | Allelic frequency was statistically higher in neonates with hyperbilirubinemia | Chang et al., 2013 | ||
| Japanese | 0.18 | 0.66 | Allelic frequency was statistically higher in neonates with hyperbilirubinemia. | Maruo et al., 2014 | |
| UGT1A1*28 | Japanese | N.D. | 0.03 | Among 17 BMJ neonates, just one neonate was heterozygous. | Maruo et al., 2000 |
| Japanese | 0.15 | 0.04 | Allelic frequency was lower in neonates with hyperbilirubinemia. | Maruo et al., 1999 | |
| North Indian | 0.29 | 0.487 | Allelic frequency was higher in neonates with hyperbilirubinemia. | Agrawal et al., 2009 | |
| Turkish | 0.27 | 0.25 | Allelic frequency was similar in neonates with hyperbilirubinemia. | Babaoglu et al., 2006 | |
| Malay | 0.001 | 0.035 | Allelic frequency was not statically higher in neonates with hyperbilirubinemia. | Azlin et al., 2011 | |
| 0.065 | 0.02 | Allelic frequency was lower in neonates with hyperbilirubinemia. | Huang et al., 2004 | ||
| Caucasia | 0.41 | 0.41 | Allelic frequency was similar in neonates with hyperbilirubinemia. | Travan et al., 2014 | |
| Asian | 0.16 | N.D. | Beutler et al., 1998 | ||
| European | 0.387 | N.D. | Beutler et al., 1998 | ||
| Africa | 0.426 | N.D. | Beutler et al., 1998 | ||
Allele frequency in neonates without hyperbilirubinemia.
In 1990, Newman et al. reported that there were statistically significant differences in the frequency of severe neonatal hyperbilirubinemia among various ethnic groups (Newman et al., 1990). For example, the incidence of hyperbilirubinemia was 31% in Asian infants and 9% in black infants. The frequencies of the UGT1A1 polymorphisms that are associated with unconjugated hyperbilirubinemia were also very different amongst different racial groups worldwide (Table 1). Homozygous UGT1A1*28 is the most prevalent genotype causing Gilbert’s syndrome in Caucasians and Africans with frequencies of 0.36–0.40 and 0.42–0.48, respectively, which is significantly higher than that in East Asians (0.15, Japanese, Koreans, and Chinese) (Bosma et al., 1995; Monaghan et al., 1996; Maruo et al., 1999; Beutler et al., 1998). In contrast, the allelic frequency of UGT1A1*6 was 0.16 in East Asians, whereas this polymorphism was not detected in Caucasians and Africans (Maruo et al., 1999). These findings indicate that the varied frequency of neonatal hyperbilirubinemia and BMJ among different races can be attributed in part to the different frequencies of genetic polymorphisms of the UGT1A1 gene.
Conclusions
Along with epidemiological evidence that breast milk is a key factor in the development of neonatal hyperbilirubinemia, convincing new findings employing humanized and mouse genetics have demonstrated an important role for extrahepatic tissues in the metabolism of bilirubin during neonatal development (Table 2). Importantly, intestinal UGT1A1 plays a key role in bilirubin metabolism in BMJ and inadequate breastfeeding jaundice. These new experimental tools can be exploited to develop intervention therapies as needed in those extreme cases of severe neonatal hyperbilirubinemia.
Table 2.
Impact of extrahepatic tissues on bilirubin metabolism
| Tissue | Observations | Reference |
|---|---|---|
| Small intestine | UGT1A1 was highly expressed in small intestine |
Nakamura et al., 2008 Tukey and Strassburg, 2000 |
| Transplantation of normal small intestine to Gunn rat resulted in decreased serum bilirubin levels |
Takahashi et al., 1994 Medley et al., 1995 |
|
| Developmental expression of intestinal UGT1A1 correlated to serum bilirubin levels in neonatal hUGT1 mice | Fujiwara et al., 2010 | |
| Specific induction of UGT1A1 in small intestine resulted in a decrease in serum bilirubin levels in hUGT1 mice | Fujiwara et al., 2012 | |
| Breast milk suppressed UGT1A1 expression in small intestine of BMJ mice | Fujiwara et al., 2012 | |
| Knockout of liver Ugt1 locus and the Ugt1a1 gene resulted in only a slight increase in TSB levels | Chen et al., 2013 | |
| Skin | UGT1A1 was expressed in human skin and human keratinocyte HaCaT cells |
Sumida et al., 2013 Peters et al., 1987 |
| Expression of UGT1A1 was higher in the skin than that in the liver in neonatal hUGT1 mice | Sumida et al., 2013 | |
| UVB induced skin UGT1A1 and decreased serum bilirubin levels in hUGT1 mice | Sumida et al., 2013 | |
Acknowledgments
This work was supported by a Grant-in-Aid for Encouragement of Young Scientists B to R.F. [Grant 26870562 and 24890224]. This work was also supported in part by the National Institute of Environmental Health Sciences [Grant P42-ES010337] and the National Institute of General Medical Sciences [Grant R01-GM100481 and GM086713].
Abbreviations
- BMJ
breast milk jaundice
- UGT
UDP-glucuronosyltransferase
- hUGT1
humanized UGT1
- Tg
transgenic
- OATP
organic anion transporting polypeptide
- OAT
organic anion transporter
- ER
endoplasmic reticulum
- MRP2
multidrug resistance protein 2
- AAP
American Academy of Pediatrics
- NICE
National Institute for Health and Clinical Excellence
- CAR
constitutive androstane receptor
- FICZ
6-formylindolo[3,2-b]carbazole
- AhR
aryl hydrocarbon receptor
- UV
Ultraviolet
- SP1
Specificity Protein 1
- TSB
total serum bilirubin
- EGF
epidermal growth factor
- CN
Crigler-Najjar
- PXR
pregnenolone X receptor
- Mip-2
macrophage inflammatory protein-2
- TNF
tumor necrosis factor
- IL
interleukin
- IKK
IκB kinase
- NF-κB
nuclear factor κB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agrawal SK, Kumar P, Rathi R, Sharma N, Das R, Prasad R, Narang A. UGT1A1 gene polymorphisms in North Indian neonates presenting with unconjugated hyperbilirubinemia. Pediatr. Res. 2009;65:675–680. doi: 10.1203/PDR.0b013e31819ed5de. [DOI] [PubMed] [Google Scholar]
- Alonso EM, Whitington PF, Whitington SH, Rivard WA, Given G. Enterohepatic circulation of nonconjugated bilirubin in rats fed with human milk. J. Pediatr. 1991;118:425–430. doi: 10.1016/s0022-3476(05)82162-6. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- Aono S, Yamada Y, Keino H, Hanada N, Nakagawa T, Sasaoka Y, Yazawa T, Sato H, Koiwai O. Identification of defect in the genes for bilirubin UDP-glucuronosyl-transferase in a patient with Crigler-Najjar syndrome type II. Biochem. Biophys. Res. Commun. 1993;197:1239–1244. doi: 10.1006/bbrc.1993.2610. [DOI] [PubMed] [Google Scholar]
- Aoshima N, Fujie Y, Itoh T, Tukey RH, Fujiwara R. Glucose induces intestinal human UDP-glucuronosyltransferase (UGT) 1A1 to prevent neonatal hyperbilirubinemia. Sci. Rep. 2014;4:6343. doi: 10.1038/srep06343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaydin K, Ermis B, Arasli M, Tekin I, Ankarali H. Cytokines in human milk and late-onset breast milk jaundice. Pediatr. Int. 2012;54:801–805. doi: 10.1111/j.1442-200X.2012.03680.x. [DOI] [PubMed] [Google Scholar]
- Arias IM, Gartner LM, Cohen M, Ezzer JB, Levi AJ. Choronic nonhemolytic unconjugated hyperbilirubinemia with Glucuronosyl transferase deficiency. Am J Med. 1969;47:395–409. doi: 10.1016/0002-9343(69)90224-1. [DOI] [PubMed] [Google Scholar]
- Arias IM, Gartner LM, Seifter S. Neonatal unconjugated hyperbilirubinemia associated with breast-feeding and a factor in milk that inhibits glucuronide formation in vitro. J. Clin. Invest. 1963;42:913. doi: 10.1172/JCI105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias IM, Gartner LM, Seifter S, Furman M. Prolonged neonatal unconjugated hyperbilirubinemia associated with breast feeding and a steriod, pregnane-3(alpha), 20(beta)-diol, in maternal milk that inhibits glucuronide formation in vitro. J. Clin. Invest. 1964;43:2037–2047. doi: 10.1172/JCI105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenat E, Gerbal-Chaloin S, Larrey D, Saric J, Fabre JM, Maurel P, Vilarem MJ, Pascussi JM. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40:951–960. doi: 10.1002/hep.20387. [DOI] [PubMed] [Google Scholar]
- Azlin I, Wong FL, Ezham M, Hafiza A, Ainoon O. Prevalence of uridine glucuronosyl transferase 1A1 (UGT1A1) mutations in Malay neonates with severe jaundice. Malays. J. Pathol. 2011;33:95–100. [PubMed] [Google Scholar]
- Babaoglu MO, Yigit S, Aynacioglu AS, Kerb R, Yurdakok M, Bozkurt A. Neonatal jaundice and bilirubin UDP-glucuronosyl transferase 1A1 gene polymorphism in Turkish patients. Basic. Clin. Pharmacol. Toxicol. 2006;98:377–380. doi: 10.1111/j.1742-7843.2006.pto_341.x. [DOI] [PubMed] [Google Scholar]
- Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA. 1998;95:8170–8174. doi: 10.1073/pnas.95.14.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan BR, Holton JB. Inhibition of bilirubin conjugation in rat liver slices by free fatty acids, with relevance to the problem of breast milk jaundice. Clin. Chim. Acta. 1972;41:101–107. doi: 10.1016/0009-8981(72)90501-3. [DOI] [PubMed] [Google Scholar]
- Bhutani VK, Donn SM, Johnson LH. Risk management of severe neonatal hyperbilirubinemia to prevent kernicterus. Clin. Perinatol. 2005;32:125–139. vii. doi: 10.1016/j.clp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bortolussi G, Zentilin L, Baj G, Giraudi P, Bellarosa C, Giacca M, Tiribelli C, Muro AF. Rescue of bilirubin-induced neonatal lethality in a mouse model of Crigler-Najjar syndrome type 1 by AAV9-mediated gene transfer. FASEB. J. 2012;26:1052–1063. doi: 10.1096/fj.11-195461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, Chowdhury NR. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. New Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- Bosma PJ, Chowdhury NR, Goldhoorn BG, Hofker MH, Oude Elferink RP, Jansen PL, Chowdhury JR. Sequence of exons and the flanking regions of human bilirubin-UDP-glucuronosyltransferase gene complex and identification of a genetic mutation in a patient with Crigler-Najjar syndrome, type I. Hepatology. 1992;15:941–947. doi: 10.1002/hep.1840150531. [DOI] [PubMed] [Google Scholar]
- Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem. 1994;269:17960–17964. [PubMed] [Google Scholar]
- Burchell B. Observations of uridine diphosphate glucuronyltransferase activity towards oestriol and xenobiotics in developing and cultured tissues from mouse and man. Biochem. Soc. Trans. 1973;1:1212–1214. doi: 10.1042/bst0011212. [DOI] [PubMed] [Google Scholar]
- Burchell B, Coughtrie M, Jackson M, Harding D, Fournel-Gigleux S, Leakey J, Hume R. Development of human liver UDP-glucuronosyltransferases. Dev. Pharmacol. Ther. 1989;13:70–77. doi: 10.1159/000457587. [DOI] [PubMed] [Google Scholar]
- Burchell B, Dutton GJ, Winsnes A. Comparison of culture-induced, phenobarbital-induced and naturally-developing UDP-glucuronyltransferase. Enzyme. 1974;17:146–154. doi: 10.1159/000459323. [DOI] [PubMed] [Google Scholar]
- Burchell B, Hume R. Molecular genetic basis of Gilbert's syndrome. J. Gastroenterol. Hepatol. 1999;14:960–966. doi: 10.1046/j.1440-1746.1999.01984.x. [DOI] [PubMed] [Google Scholar]
- Crigler JF, Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169–179. [PubMed] [Google Scholar]
- Chang PF, Lin YC, Liu K, Yeh SJ, Ni YH. Identifying term breast-fed infants at risk of significant hyperbilirubinemia. Pediatr. Res. 2013;74:408–412. doi: 10.1038/pr.2013.120. [DOI] [PubMed] [Google Scholar]
- Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, Remmel RP, Trottier J, Barbier O, Ritter JK, Tukey RH. Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J. Biol. Chem. 2005;280:37547–37557. doi: 10.1074/jbc.M506683200. [DOI] [PubMed] [Google Scholar]
- Chen S, Yueh MF, Bigo C, Barbier O, Wang K, Karin M, Nguyen N, Tukey RH. Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11) Proc Natl Acad Sci USA. 2013;110:19143–19148. doi: 10.1073/pnas.1319123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yueh MF, Evans RM, Tukey RH. The Pregnane-X-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 Mice. Hepatology. 2012;56:658–667. doi: 10.1002/hep.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantopoulos A, Messaritakis J, Matsaniotis N. Breast milk jaundice; the role of lipoprotein lipase and the free fatty acids. Eur. J. Pediatr. 1980;134:35–38. doi: 10.1007/BF00442400. [DOI] [PubMed] [Google Scholar]
- Coyl DD, Roggman LA, Newland LA. Stress, maternal depression, and negative mother–infant interactions in relation to infant attachment. Infant Ment. Health J. 2002;23:145–163. [Google Scholar]
- Crowell JA, Treboux D. A Review of Adult Attachment Measures: Implications for Theory and Research. Social Development. 1995;4:294–327. [Google Scholar]
- Csala M, Marcolongo P, Lizák B, Senesi S, Margittai E, Fulceri R, Magyar JE, Benedetti A, Bánhegyi G. Transport and transporters in the endoplasmic reticulum. Biochim. Biophys. Acta. 2007;1768:1325–1341. doi: 10.1016/j.bbamem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin. Pharmacokinet. 1999;36:439–452. doi: 10.2165/00003088-199936060-00005. [DOI] [PubMed] [Google Scholar]
- Dutton GJ. Acceptor substrates of UDP glucuronosyltransferase and their assay. In: Dutton GJ, editor. Glucuronidation of Drugs and Other Compounds. Boca Raton, FL: CRC Press; 1980. pp. 69–78. [Google Scholar]
- Finel M, Kurkela M. The UDP-glucuronosyltransferases as oligomeric enzymes. Curr. Drug Metab. 2008;9:70–76. doi: 10.2174/138920008783331158. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Itoh T. Extensive protein-protein interactions involving UDP-glucuronosyltransferase (UGT) 2B7 in human liver microsomes. Drug. Metab. Pharmacokinet. 2014a;29:259–265. doi: 10.2133/dmpk.dmpk-13-rg-096. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Itoh T. Extensive protein interactions involving cytochrome P450 3A4: a possible contributor to the formation of a metabolosome. Pharma. Res. Per. 2014b;2:e00053. doi: 10.1002/prp2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R, Nakajima M, Oda S, Yamanaka H, Ikushiro S, Sakaki T, Yokoi T. Interactions between human UDP-glucuronosyltransferase (UGT) 2B7 and UGT1A enzymes. J. Pharm. Sci. 2010;99:442–454. doi: 10.1002/jps.21830. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nakajima M, Yamanaka H, Katoh M, Yokoi T. Interactions between human UGT1A1, UGT1A4, and UGT1A6 affect their enzymatic activities. Drug Metab. Dispos. 2007a;35:1781–1787. doi: 10.1124/dmd.107.016402. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nakajima M, Yamanaka H, Nakamura A, Katoh M, Ikushiro S, Sakaki T, Yokoi T. Effects of coexpression of UGT1A9 on enzymatic activities of human UGT1A isoforms. Drug Metab. Dispos. 2007b;35:747–757. doi: 10.1124/dmd.106.014191. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nguyen N, Chen S, Tukey RH. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci U S A. 2010;107:5024–5029. doi: 10.1073/pnas.0913290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R, Chen S, Karin M, Tukey RH. Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia. Gastroenterology. 2012;142:109–118. doi: 10.1053/j.gastro.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney PT, Buttenshaw RL, Ward M, Diplock RD. Breast milk β-glucuronidase and neonatal jaundice. Lancet. 1986;1:1161–1162. doi: 10.1016/s0140-6736(86)91879-9. [DOI] [PubMed] [Google Scholar]
- Gilbert A, Lereboullet P. La cholémie simple familiale. Semaine Med. 1901;21:241–243. [Google Scholar]
- Gourley GR. Pathophysiology of breast-milk jaundice. Fetal and Neonatal Physiology. (2nd Edition) 1998:1499–1505. [Google Scholar]
- Gourley GR. Breast-feeding, neonatal jaundice and kernicterus. Semin. Neonatol. 2002;7:135–141. doi: 10.1053/siny.2002.0101. [DOI] [PubMed] [Google Scholar]
- Gourley GR, Arend RA. β-Glucuronidase and hyperbilirubinemia in breast-fed and formula-fed babies. Lancet. 1986;1:644–646. doi: 10.1016/s0140-6736(86)91724-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves T. Effect of fatty acids on bilirubin conjugation. Arch. Dis. Child. 1973;48:446–450. doi: 10.1136/adc.48.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann F, Bissell DM. Metabolism of heme and bilirubin in rat and human small intestinal mucosa. J. Clin. Invest. 1982;70:23–29. doi: 10.1172/JCI110598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS. Risk factors for severe hyperbilirubinemia in neonates. Pediatr. Res. 2004;56:682–689. doi: 10.1203/01.PDR.0000141846.37253.AF. [DOI] [PubMed] [Google Scholar]
- Ip S, Chung M, Kulig J, O'Brien R, Sege R, Glicken S, Maisels MJ, Lau J. An Evidence-Based Review of Important Issues Concerning Neonatal Hyperbilirubinemia. Pediatrics. 2004;114:e130–e153. doi: 10.1542/peds.114.1.e130. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Iwanaga M, Nishimura Y, Takeda S, Ikushiro S, Nagata K, Yamazoe Y, Mackenzie PI, Yamada H. Protein-protein interactions between rat hepatic cytochromes P450 (P450s) and UDP-glucuronosyltransferases (UGTs): evidence for the functionally active UGT in P450-UGT complex. Drug Metab. Pharmacokinet. 2007;22:367–376. doi: 10.2133/dmpk.22.367. [DOI] [PubMed] [Google Scholar]
- Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T. Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab. Dispos. 2009;37:1759–1768. doi: 10.1124/dmd.109.027227. [DOI] [PubMed] [Google Scholar]
- Kamisako T, Kobayashi Y, Takeuchi K, Ishihara T, Higuchi K, Tanaka Y, Gabazza EC, Adachi Y. Recent advances in bilirubin metabolism research: the molecular mechanism of hepatocyte bilirubin transport and its clinical relevance. J. Gastroenterol. 2000;35:659–664. doi: 10.1007/s005350070044. [DOI] [PubMed] [Google Scholar]
- Keppler D, Konig J, Buchler M. The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv. Enzyme Regul. 1997;37:321–333. doi: 10.1016/s0065-2571(96)00013-1. [DOI] [PubMed] [Google Scholar]
- Kumral A, Ozkan H, Duman N, Yesilirmak DC, Islekel H, Ozalp Y. Breast milk jaundice correlates with high levels of epidermal growth factor. Pediatr. Res. 2009;66:218–221. doi: 10.1203/PDR.0b013e3181ac4a30. [DOI] [PubMed] [Google Scholar]
- Kutsuno Y, Itoh T, Tukey RH, Fujiwara R. Glucuronidation of drugs and drug-induced toxicity in humanized UDP-glucuronosyltransferase 1 mice. Drug Metab. Dispos. 2014;42:1146–1152. doi: 10.1124/dmd.114.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuno Y, Sumida K, Itoh T, Tukey RH, Fujiwara R. Glucuronidation of drugs in humanized UDP-glucuronosyltransferase 1 mice: Similarity with glucuronidation in human liver microsomes. Pharma. Res. Per. 2013;1:e00002. doi: 10.1002/prp2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforgia N, Faienza MF, Rinaldi A, D'Amato G, Rinaldi G, Iolascon A. Neonatal hyperbilirubinemia and Gilbert's syndrome. J. Perinat. Med. 2002;30:166–169. doi: 10.1515/JPM.2002.021. [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet. Genomics. 2005;15:677–685. doi: 10.1097/01.fpc.0000173483.13689.56. [DOI] [PubMed] [Google Scholar]
- Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks' gestation: an update with clarifications. Pediatrics. 2009;124:1193–1198. doi: 10.1542/peds.2009-0329. [DOI] [PubMed] [Google Scholar]
- Maruo Y, Nishizawa K, Sato H, Sawa H, Shimada M. Prolonged unconjugated hyperbilirubinemia associated with breast milk and mutations of the bilirubin uridine diphosphate- glucuronosyltransferase gene. Pediatrics. 2000;106:E59. doi: 10.1542/peds.106.5.e59. [DOI] [PubMed] [Google Scholar]
- Maruo Y, Nishizawa K, Sato H, Doida Y, Shimada M. Association of neonatal hyperbilirubinemia with bilirubin UDP-glucuronosyltransferase polymorphism. Pediatrics. 1999;103:1224–1227. doi: 10.1542/peds.103.6.1224. [DOI] [PubMed] [Google Scholar]
- Maruo Y, D'Addario C, Mori A, Iwai M, Takahashi H, Sato H, Takeuchi Y. Two linked polymorphic mutations (A(TA)7TAA and T-3279G) of UGT1A1 as the principal cause of Gilbert syndrome. Hum. Genet. 2004;115:525–526. doi: 10.1007/s00439-004-1183-x. [DOI] [PubMed] [Google Scholar]
- Maruo Y, Morioka Y, Fujito H, Nakahara S, Yanagi T, Matsui K, Mori A, Sato H, Tukey RH, Takeuchi Y. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J. Pediatr. 2014;165:36–41. doi: 10.1016/j.jpeds.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley MM, Hooker RL, Rabinowitz S, Holton R, Jaffe BM. Correction of congenital indirect hyperbilirubinemia by small intestinal transplantation. Am. J. Surg. 1995;169:20–27. doi: 10.1016/s0002-9610(99)80105-6. [DOI] [PubMed] [Google Scholar]
- Minekawa R, Takeda T, Sakata M, Hayashi M, Isobe A, Yamamoto T, Tasaka K, Murata Y. Human breast milk suppresses the transcriptional regulation of IL-1beta-induced NF-kappaB signaling in human intestinal cells. Am. J. Physiol. Cell Physiol. 2004;287:C1404–C1411. doi: 10.1152/ajpcell.00471.2003. [DOI] [PubMed] [Google Scholar]
- Moghrabi N, Clarke DJ, Boxer M, Burchell B. Identification of an A-to-G missense mutation in exon 2 of the UGT1 gene complex that causes Crigler-Najjar syndrome type 2. Genomics. 1993;18:171–173. doi: 10.1006/geno.1993.1451. [DOI] [PubMed] [Google Scholar]
- Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert's syndrome. Lancet. 1996;347:578–581. doi: 10.1016/s0140-6736(96)91273-8. [DOI] [PubMed] [Google Scholar]
- Monaghan G, McLellan A, McGeehan A, Li Volti S, Mollica F, Salemi I, Din Z, Cassidy A, Hume R, Burchell B. Gilbert's syndrome is a contributory factor in prolonged unconjugated hyperbilirubinemia of the newborn. J. Pediatr. 1999;134:441–446. doi: 10.1016/s0022-3476(99)70201-5. [DOI] [PubMed] [Google Scholar]
- Murphy JF, Hughes I, Jones EV, Gaskell S, Pike AW. Pregnanediols and breast milk jaundice. Arch. Dis. Child. 1981;56:474–476. doi: 10.1136/adc.56.6.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Yamanaka H, Fujiwara R, Katoh M, Yokoi T. Stereoselective glucuronidation of 5-(4'-hydroxyphenyl)-5-phenylhydantoin by human UDP-glucuronosyltransferase (UGT) 1A1, UGT1A9, and UGT2B15: effects of UGT-UGT interactions. Drug Metab. Dispos. 2007;35:1679–1686. doi: 10.1124/dmd.107.015909. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab. Dispos. 2008;36:1461–1464. doi: 10.1124/dmd.108.021428. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Gross S. Hyperbilirubinemia in breast-fed infants. Pediatrics. 1963;32:995–1001. [PubMed] [Google Scholar]
- Newman TB, Easterling MJ, Goldman ES, Stevenson DK. Laboratory evaluation of jaundice in newborns. Frequency, cost, and yield. Am. J. Dis. Child. 1990;144:364–368. doi: 10.1001/archpedi.1990.02150270114039. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner MJ, Hardiman G, Bélanger A, Tukey RH. Disruption of the ugt1 locus in mice resembles human Crigler-Najjar type I disease. J. Biol. Chem. 2008;283:7901–7911. doi: 10.1074/jbc.M709244200. [DOI] [PubMed] [Google Scholar]
- Ohno S, Nakajin S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab. Dispos. 2009;37:32–40. doi: 10.1124/dmd.108.023598. [DOI] [PubMed] [Google Scholar]
- Onishi S, Kawade N, Itoh S, Isobe K, Sugiyama S. Postnatal development of uridine diphosphate glucuronosyltransferase activity toward bilirubin and 2-aminophenol in human liver. Biochem. J. 1979;184:705–707. doi: 10.1042/bj1840705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozawa 11246096. [Google Scholar]
- Ota Y, Maruo Y, Matsui K, Mimura Y, Sato H, Takeuchi Y. Inhibitory effect of 5β-pregnane-3α,20β-diol on transcriptional activity and enzyme activity of human bilirubin UDP-glucuronosyltransferase. Pediatr. Res. 2011;70:453–457. doi: 10.1203/PDR.0b013e31822f242e. [DOI] [PubMed] [Google Scholar]
- Owens D, Evans J. Population study on Gilbert's syndrome. J. Med. Genet. 1975;12:152–156. doi: 10.1136/jmg.12.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters WH, Allebes WA, Jansen PL, Poels LG, Capel PJ. Characterization and tissue specificity of a monoclonal antibody against human uridine 5'-diphosphate-glucuronosyltransferase. Gastroenterology. 1987;93:162–169. doi: 10.1016/0016-5085(87)90329-5. [DOI] [PubMed] [Google Scholar]
- Ramos A, Silverberg M, Stern M. Pregnanediol and neonatal hyperbilirubinemia. Am. J. Dis. Child. 1966;111:353–356. doi: 10.1001/archpedi.1966.02090070051003. [DOI] [PubMed] [Google Scholar]
- Ritter JK, Kessler FK, Thompson MT, Grove AD, Auyeung DJ, Fisher RA. Expression and inducibility of the human bilirubin UDP-glucuronosyltransferase UGT1A1 in liver and cultured primary hepatocytes: evidence for both genetic and environmental influences. Hepatology. 1999;30:476–484. doi: 10.1002/hep.510300205. [DOI] [PubMed] [Google Scholar]
- Ritter JK, Yeatman MT, Kaiser C, Gridelli B, Owens IS. A phenylalanine codon deletion at the UGT1 gene complex locus of a Crigler-Najjar type I patient generates a pH-sensitive bilirubin UDP-glucuronosyltransferase. J. Biol. Chem. 1993;268:23573–23579. [PubMed] [Google Scholar]
- Ritter JK, Yeatman MT, Ferreira P, Owens IS. Identification of a genetic alteration in the code for bilirubin UDP-glucuronosyltransferase in the UGT1 gene complex of a Crigler-Najjar type I patient. J. Clin. Invest. 1992;90:150–155. doi: 10.1172/JCI115829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KJ, Clarke D, Sutherland L, Wooster R, Coughtrie MW, Burchell B. Investigation of the molecular basis of the genetic deficiency of UDP-glucuronosyltransferase in Crigler-Najjar syndrome. J. Inherit. Metab. Dis. 1991;14:563–579. doi: 10.1007/BF01797927. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Rice M. Low birth weight and subsequent poor weight gain. J. Pediatr. Health Care. 2014;28:350–356. doi: 10.1016/j.pedhc.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013;45:1121–1132. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Sato H, Uchida T, Toyota K, Kanno M, Hashimoto T, Watanabe M, Nakamura T, Tamiya G, Aoki K, Hayasaka K. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G>A (G71R) mutation becomes a risk factor under inadequate feeding. J. Hum. Genet. 2013;58:7–10. doi: 10.1038/jhg.2012.116. [DOI] [PubMed] [Google Scholar]
- Salih FM. Can sunlight replace phototherapy units in the treatment of neonatal jaundice? An in vitro study. Photodermatol. Photoimmunol. Photomed. 2001;17:272–277. doi: 10.1034/j.1600-0781.2001.170605.x. [DOI] [PubMed] [Google Scholar]
- Schneider AP. Breast milk jaundice in the newborn. A real entity. JAMA. 1986;255:3270–3274. [PubMed] [Google Scholar]
- Sieg A, Arab L, Schlierf G, Stiehl A, Kommerell B. Prevalence of Gilbert's syndrome in Germany. Dtsch. Med. Wochenschr. 1987;112:1206–1208. doi: 10.1055/s-2008-1068222. [DOI] [PubMed] [Google Scholar]
- Shapiro SM. Bilirubin toxicity in the developing nervous system. Pediatr. Neurol. 2003;29:410–421. doi: 10.1016/j.pediatrneurol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND) J. Perinatol. 2005;25:54–59. doi: 10.1038/sj.jp.7211157. [DOI] [PubMed] [Google Scholar]
- Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin. Fetal. Neonatal. Med. 2010;15:157–163. doi: 10.1016/j.siny.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Shepherd SR, Baird SJ, Hallinan T, Burchell B. An investigation of the transverse topology of bilirubin UDP-glucuronosyltransferase in rat hepatic endoplasmic reticulum. Biochem. J. 1989;259:617–620. doi: 10.1042/bj2590617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby MK, Cherrington NJ, Vansell NR, Klaassen CD. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab. Dispos. 2003;31:326–333. doi: 10.1124/dmd.31.3.326. [DOI] [PubMed] [Google Scholar]
- Shibuya A, Itoh T, Tukey RH, Fujiwara R. Impact of fatty acids on human UDP-glucuronosyltransferase 1A1 activity and its expression in neonatal hyperbilirubinemia. Sci. Rep. 2013;3:2903. doi: 10.1038/srep02903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson IH, Dutton GJ. Glucuronide synthesis in kidney and gastrointestinal tract. Biochem. J. 1962;82:330–340. doi: 10.1042/bj0820330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm ER, Ryan J. Breast-milk Jaundice. Report of eight cases and effect of breast feeding on incidence and severity of unexplained hyperbilirubinemia. Am. J. Dis. Child. 1965;109:212–216. [PubMed] [Google Scholar]
- Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, Manns MP. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–265. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburg CP. Pharmacogenetics of Gilbert's syndrome. Pharmacogenomics. 2008;9:703–715. doi: 10.2217/14622416.9.6.703. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Oldhafer K, Manns MP, Tukey RH. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol. Pharmacol. 1997;52:212–220. doi: 10.1124/mol.52.2.212. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Nguyen N, Manns MP, Tukey RH. Polymorphic expression of the UDP-glucuronosyltransferase UGT1A gene locus in human gastric epithelium. Mol. Pharmacol. 1998a;54:647–654. [PubMed] [Google Scholar]
- Strassburg CP, Manns MP, Tukey RH. Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J. Biol. Chem. 1998b;273:8719–8726. doi: 10.1074/jbc.273.15.8719. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Nguyen N, Manns MP, Tukey RH. UDP-glucuronosyltransferase activity in human liver and colon. Gastroenterology. 1999a;116:149–160. doi: 10.1016/s0016-5085(99)70239-8. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Strassburg A, Nguyen N, Li Q, Manns MP, Tukey RH. Regulation and function of family 1 and family 2 UDP- glucuronosyltransferase genes (UGT1A, UGT2B) in human oesophagus. Biochem. J. 1999b;338(Pt 2):489–498. [PMC free article] [PubMed] [Google Scholar]
- Sumida K, Kawana M, Kouno E, Itoh T, Takano S, Narawa T, Tukey RH, Fujiwara R. Importance of UDP-glucuronosyltransferase 1A1 expression in skin and its induction by UVB in neonatal hyperbilirubinemia. Mol. Pharmacol. 2013;84:679–686. doi: 10.1124/mol.113.088112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Nozawa M, Sugitani K, Ishida K. Effect of small bowel transplantation in the congenitally enzyme-deficient Gunn rat. Transplant. Proc. 1994;26:1675–1678. [PubMed] [Google Scholar]
- Travan L, Lega S, Crovella S, Montico M, Panontin E, Demarini S. Severe Neonatal Hyperbilirubinemia and UGT1A1 Promoter Polymorphism. J. Pediatr. 2014;165:42–45. doi: 10.1016/j.jpeds.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- Vanstapel F, Blanckaert N. Topology and regulation of bilirubin UDP-glucuronyltransferase in sealed native microsomes from rat liver. Arch. Biochem. Biophys. 1988;263:216–225. doi: 10.1016/0003-9861(88)90630-3. [DOI] [PubMed] [Google Scholar]
- Victora CG, Vaughan JP, Martines JC, Barcelos LB. Is prolonged breast-feeding associated with malnutrition? Am. J. Clin. Nutr. 1984;39:307–314. doi: 10.1093/ajcn/39.2.307. [DOI] [PubMed] [Google Scholar]
- Waddell ID, Robertson K, Burchell A, Hume R, Burchell B. Evidence for glucuronide (small molecule) sorting by human hepatic endoplasmic reticulum. Mol. Membr. Biol. 1995;12:283–288. doi: 10.3109/09687689509072429. [DOI] [PubMed] [Google Scholar]
- Watchko JF, Oski FA. Kernicterus in preterm newborns: past, present, and future. Pediatrics. 1992;90:707–715. [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab. Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J. Biol. Chem. 2009;284:2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- Yueh MF, Chen S, Nguyen N, Tukey RH. Developmental Onset of Bilirubin-induced Neurotoxicity Involves Toll-like Receptor 2-dependent Signaling in Humanized UDP-glucuronosyltransferase1 Mice. J. Biol. Chem. 2014;289:4699–4709. doi: 10.1074/jbc.M113.518613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J. Biol. Chem. 2003;278:15001–15006. doi: 10.1074/jbc.M300645200. [DOI] [PubMed] [Google Scholar]
- Zanardo V, Golin R, Amato M, Trevisanuto D, Favaro F, Faggian D, Plebani M. Cytokines in human colostrum and neonatal jaundice. Pediatr. Res. 2007;62:191–194. doi: 10.1203/PDR.0b013e31809871c9. [DOI] [PubMed] [Google Scholar]
- Zhang W, He YJ, Gan Z, Fan L, Li Q, Wang A, Liu ZQ, Deng S, Huang YF, Xu LY, Zhou HH. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin. Exp. Pharmacol. Physiol. 2007;34:1240–1244. doi: 10.1111/j.1440-1681.2007.04798.x. [DOI] [PubMed] [Google Scholar]