INTRODUCTION
The fight against tuberculosis (TB) is entering a new era, from one of control to one of attempting to end the TB epidemic, where the international donor and policy community have embraced targets of 90–95% reductions in TB incidence and mortality by 20506. One important component of such “epidemic-ending” approaches is an increased focus on local-level strategies, which have proved instrumental in eliminating infectious diseases ranging from smallpox to polio7–10. The successful elimination of disease epidemics has typically involved two important components: (1) systematic reporting of every case and (2) identification of disease clusters or “hotspots” at the local level where ongoing transmission occurs. These components enable the documentation of disease trends in each community and the subsequent targeting of resources to where they are needed most. Local strategies for TB could, for example, tailor diagnosis and treatment of TB infection to subpopulations that are at highest risk of disease progression, or target case finding to stop transmission in high-incidence populations. Some countries are starting to use subnational trends to inform more tailored approaches12; however, to end TB in a 20-year time frame, this trend must be accelerated along with increased focus on local empowerment with centralized (national and global) support13.
Since 1993, with the adoption of a widely-accepted approach to TB treatment known as DOTS14, a standard set of clinical, demographic, bacteriological, and treatment outcome data have been collected and aggregated by national TB programs and subsequently notified to the World Health Organization (WHO)15. This approach, while essential for informing country-level and global estimates and monitoring the high-level progress of strategies such as DOTS, has not traditionally emphasized the use of existing data (or collection of additional data) to identify sites of ongoing transmission and target local responses accordingly. Local TB epidemics differ in terms of intensity, drivers, and key characteristics, and approaches that are effective in some “hotspots” (e.g., informal urban settlements) may fail in others (e.g., prisons or rural villages with poor access to care). Without high-quality data and infrastructure at the local level (and support from national and global entities) to inform a tailored response to each individual micro-epidemic, the goal of ending TB globally will not be achieved.
Awareness is building surrounding the importance of local data and capacity, but action is not being taken fast enough. The WHO has championed the need for national programs to respond to setting-specific differences, according to the scale of the epidemic in the country16. Three specific steps will accelerate this process. First, countries must better use existing data on TB notifications, risk factors, and treatment outcomes to inform local interventions. Second, national and global systems must augment the set of standard, routinely-collected data with additional data elements to better target resources, while ensuring that this additional data collection is feasible. Examples of additional data include geographic information, drug resistance, and clinical risk factors. Thirdly, programs must build capacity for the periodic, focused collection of novel data components, such as targeted surveys, contact investigations, and sequencing data, to inform local policy decisions.
In this manuscript, we describe how existing data and analysis systems could be improved to enable these three steps, highlighting the benefits and challenges in transitioning to a locally-focused agenda to end TB (Table 1). Combined with strategies to interrupt transmission, treat latent TB, and improve social conditions, empowering the use of local data and infrastructure to target interventions appropriately can form the basis for a coherent strategy to end TB, from both a top-down and a bottom-up direction.
Table 1.
Key elements of a data-driven, locally tailored approach to TB elimination
| Element | Current capacity | Potential improvements | Key challenges |
|---|---|---|---|
| Programmatic data |
|
|
|
| Additional data that could be collected programmatically |
|
|
|
| Specific surveys |
|
|
|
| Novel data |
|
|
|
| Systems for reporting and analyzing data |
|
|
|
| Empirical evidence to support local approaches |
|
|
|
IMPROVING DATA COLLECTION AND ANALYSIS TO END TB: THREE STEPS
Step 1. Bidirectional systems for accessing and linking programmatic data to policy
Routinely collected TB data varies substantially in scope and detail between countries. The WHO recommends a minimum set of variables, comprising age, sex, geographic region, previous treatment, smear microscopy result, anatomic site (pulmonary or extrapulmonary), and treatment outcome17,18, which are ideally linked to unique patient identifiers. In many settings, data on HIV and exposure to high-risk congregate settings are also routinely collected. Although the WHO recommends the use of secure, self-contained electronic systems, paper forms are still predominantly used18,19. Data analysis is thus often delayed until entry into a central country-wide database is completed19, reducing its utility to inform real-time programmatic decisions. When such data are rapidly incorporated into policy, results can be dramatic. For example, in 2008 the Lesotho TB program found that >90% of patients diagnosed with TB were HIV seropositive20. The Ministry of Health, in collaboration with Médecins Sans Frontières, rapidly scaled up and integrated decentralized TB/HIV care21 in response. As a result, the number of adults on antiretroviral therapy (ART) in the program doubled over four years, and the incidence of HIV-positive TB decreased by approximately 40%20.
Of particular importance to interrupting transmission is better detection of childhood TB, which is currently grossly under-detected22,23 and can serve as an important marker of ongoing transmission24. Better systems for the detection of pediatric TB and rapid notification when childhood cases rise above a certain level might inform not only specific interventions such as household contact tracing and preventive therapy for children25 but can also serve as an early detection system for identifying transmission hotspots more broadly.
Ultimately, centralized TB data collection and reporting systems must be designed not only to inform national policy changes but also for building local capacity to create tailored responses at the community level. Examples exist in other infectious diseases: polio surveillance in India demonstrated lower vaccine efficacy in high population density districts with poor sanitation26; thereby enabling the roll-out of a different vaccine that was better suited to these districts27. This ultimately contributed to the elimination of polio where national-level policies had failed28. Similar targeted approaches, which are often as cost-effective as broader, untargeted interventions29–31, will be required to end epidemics of TB.
Step 2. Collection of additional routine data to inform targeted responses
Though challenging in many settings, expanding the minimum set of routinely collected TB data is essential to empower more locally responsive strategies16. Additional data may include geographic information [e.g., to assist with community-based follow-up (Box 1) or transmission hotspot mapping (Figure 1)], drug resistance patterns (e.g., for region-specific drug susceptibility testing algorithms and localized treatment regimens), and risk factors such as diabetes, smoking, or previous hospitalization or imprisonment32–34 (e.g., to inform local screening strategies). For example, Japan found high diabetes mellitus rates in certain populations of elderly or homeless TB patients, thereby enabling clinics serving these individuals to perform targeted screening35. Similarly, data from China showed a dramatic increase in the proportion of patients that had recently migrated into Beijing, and that these patients rarely completed treatment36. This led to targeted case finding and counselling to be carried out by clinics serving these communities. In Table 2, we provide an illustrative list of additional data that could be collected and used for local decision-making.
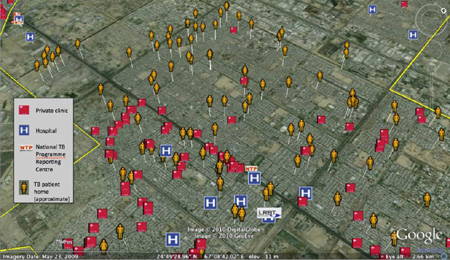
Box 1: Data for Action in Karachi, Pakistan.
Interactive Research and Development has used a range of electronic recording and reporting systems to improve access to and reporting from diagnostic and treatment sites1. For example, Geographical Positioning System (GPS) data have been used to identify the exact coordinates of private family practitioner clinics, public and private NTP reporting centers, private laboratories and pharmacies. All patients with drug-resistant tuberculosis or a high risk of loss to follow-up are mapped to approximate home locations using GPS-enabled phones, in order to inform assignment of community treatment supporters and to facilitate follow-up. For the majority of these patients, private clinics (red boxes in the Figure) are more accessible than the National TB Programme Reporting Centre (“NTP” in the Figure) for scheduling of follow-up visits. These data have informed key program decisions with regards to targeting intensified case finding, location of digital X-ray systems and GeneXpert machines, and recruitment of treatment supporters.
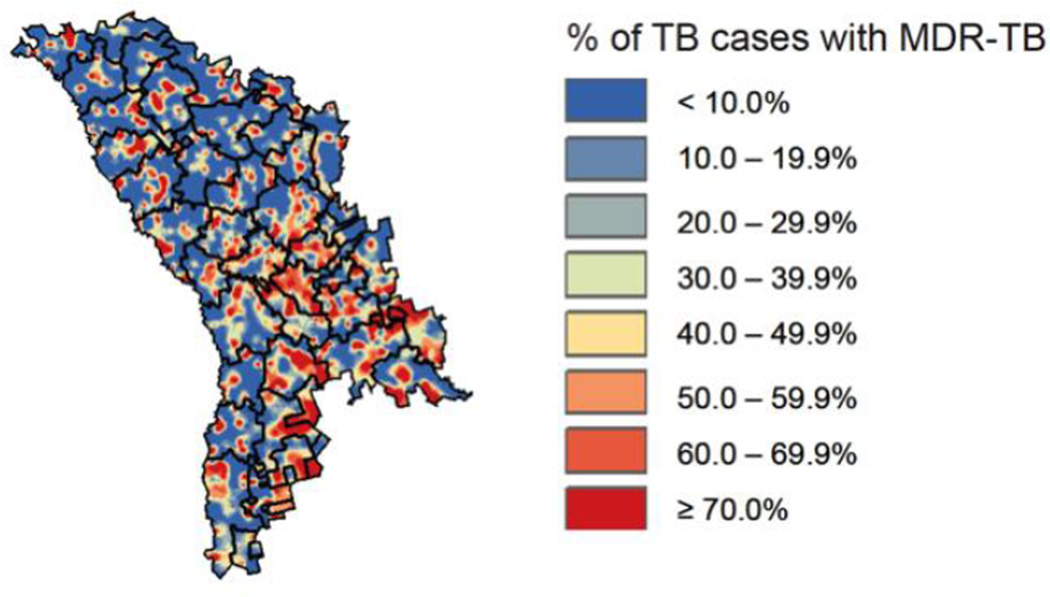
Figure 1. Geographic hot-spots of MDR-TB risk in the Republic of Moldova.
Colors represent the proportion of previously treated TB cases with drug susceptibility testing data that are multidrug-resistant by location of residence. Maps such as this – which can help target intervention efforts and direct future research – represent the product of strengthening multiple aspects of the TB surveillance system. In the early 2000s, Moldova’s TB program updated the laboratory network, revised guidelines and improved training to ensure universal drug susceptibility testing. Standardized reporting systems facilitated more complete and accurate reporting of TB incidence, outcomes and drug resistance84, and a nationwide online database was introduced with access at every national TB facility. Physicians and laboratory staff enter data on individual TB patients (including routinely collecting location of residence) in real time at the relevant points of contact. Data are then synthesized into detailed maps of TB and drug-resistant TB, such as the one presented here, which can in turn be used to focus resources and efforts on regions of likely high ongoing transmission of drug-resistant TB (such as certain locales in the southeast represented in orange and red).
Reproduced with permission of the European Respiratory Society: Eur Respir J November 2013 42:1291–1301. This material has not been reviewed by the European Respiratory Society prior to release; therefore the European Respiratory Society may not be responsible for any errors, omissions or inaccuracies, or for any consequences arising there from, in the content.
Table 2.
Possible data items to be collected on individual TB cases, in addition to the WHO minimum set of variables18
| Purpose | Data type | Items |
|---|---|---|
| Drug resistance Surveys |
Drug resistance diagnoses |
Genotypic (e.g., Xpert MTB/RIF) and phenotypic (e.g., liquid culture) drug susceptibility testing result, mutational analyses |
| Monitoring of disease severity |
Bacterial load | Smear grade, culture time-to-positivity, Xpert MTB/RIF cycle threshold values, LAM strip grade |
| Clinical test data | Chest radiograph, BMI, haemoglobin levels | |
| Transmission mapping |
Strain genotype | MIRU-VNTR, spoligotype, RFLP pattern, WGS |
| Geospatial, location, and contact data |
Administrative region (e.g. district, city, and suburb), residential address, or GPS co-ordinates of residence. Recent hospital admissions (name of hospital, duration, and reason for treatment) or incarcerations or known TB contacts. |
|
| Risk factor analysis | Comorbidities | HIV, diabetes, chronic obstructive pulmonary disease, pneumonia, diabetes testing, |
| Occupational exposure | Health care workers, miners | |
| Substance use | Cigarette pack years, AUDIT alcohol use scores, illicit narcotic usage |
In routine practice, TB programs must weigh data quantity against quality and may therefore focus additional data collection in particular patient groups or during the roll-out of new initiatives. To encourage the collection and use of relevant data, policymakers and TB programs could promote new frameworks that use local data collection as benchmarks for clinic performance. Local clinics must have sufficient autonomy, funding, and also oversight to collect the data and implement interventions that will be most responsive to their unique epidemics. Examples of strategies for additional TB data collection and linkage to tailored interventions are multi-country projects such as ENGAGE-TB37 and TB-REACH38. Importantly, the implementation of local interventions may reveal other issues (e.g., comorbidities) that traditionally would have been centrally managed. Thus, the better collection of local data will likely drive organizational and operational changes within healthcare systems, which can be facilitated by better integration of care.
Step 3: Targeted collection of novel data: surveys, spatial data, and strains/sequences
Routine data will always be limited to elements that can be collected during busy clinical practice, with limited programmatic budgets, and from patients who actually present to care. To take a more comprehensive step toward ending TB, these data must be occasionally augmented by additional investment in collecting non-routine information that can improve understanding of transmission and drug resistance patterns.
Prevalence surveys estimate how many people have TB in a representative population sample13. Between 2009 and 2015, 23 countries are expected to have conducted TB prevalence surveys39. These surveys, with WHO guidance40, can produce national (or occasionally subnational) estimates of the fraction of new cases with drug resistance17, characterize broader patterns of transmission, and identify gaps in current control efforts41. Because surveys are expensive, logistically complex, and have relatively small sample sizes at the sub-national level, they generally lack the resolution to inform local decisions. Innovative approaches to representative survey designs must therefore be considered.
One example of an alternative design in the case of drug resistance surveys is lot quality-assurance sampling (LQAS)42,43. LQAS can classify the risk of drug resistance among TB patients at a sub-national level using pre-defined thresholds of TB drug resistance44. Unlike traditional national-level drug resistance surveys, LQAS surveys do not attempt to precisely estimate the prevalence of resistance. Instead, LQAS surveys classify areas as likely being above or below a threshold selected to guide local interventions. LQAS has shown, for example, that although Tanzania and Vietnam appear to have low MDR-TB prevalence amongst new cases45,46 nationally, Vietnam may have considerably more subnational heterogeneity44. In particular, one province (Tay Ninh) was classified as having high MDR-TB prevalence, focusing attention on areas closer to Cambodia, where MDR-TB is more prevalent. Targeted surveys have also shown unusually high rates of MDR-TB in certain ART clinics and Tibetan refugee communities in India47,48. Similar methods, such as sentinel surveillance, have identified large numbers of MDR-TB patients from Somalia seeking treatment in Kenya49.
Other potentially useful data sources include molecular data on strain types, transmission, and drug resistance50. Currently, such data are only collected broadly and systematically in resource-rich settings. For example, an analysis of United States national surveillance identified which racial minorities are most likely to develop TB from recent transmission,51 and the United Kingdom has used molecular typing prospectively since 2010 to identify outbreaks52 and to estimate the proportion and identity of MDR-TB cases attributable to transmission53. Locally, such data can also be used to improve both contact investigations (which may be complemented by online social network data54) and the laboratory methods used to diagnose drug-resistant TB (Box 2). Newer technologies, such as whole genome sequencing (WGS), can identify strains responsible for major outbreaks50,55–57, uncover highly infectious “super-spreaders”50, and help understand the completeness of contact investigations58.
Box 2: Illustrative Example: Strain Typing to Inform the Local Scale-up of Drug Susceptibility Testing (DST) in South Africa.
The Western Cape Province in South Africa, which has relatively strong drug-resistant TB surveillance infrastructure, has seen a change in DR-TB strain diversity. Historically scarce atypical Beijing genotype strains have become dominant and are associated with clustered outbreaks of extensively drug-resistant TB (XDR-TB)2. A series of molecular epidemiological studies demonstrated that these strains likely originated from an adjacent province (Eastern Cape), which has relatively weak DST surveillance infrastructure3–5. These atypical Beijing strains had an unusually high prevalence of inhA promoter mutations which, in addition to conferring low-level resistance to isoniazid (a key drug in the first line regimen), also confer resistance to ethionamide (a key drug in the second line regimen used to treat MDR-TB, but for which resistance was not routinely tested). The effectiveness of the second-line drug regimen was thus substantially weakened, and atypical Beijing strains were programmatically selected to evolve into XDR-TB, which subsequently entered the Western Cape, likely via the large migrant work population. Molecular tests are now used to identify inhA promoter mutations in the Eastern Cape. An alternative drug can thus be potentially be substituted for ethionamide in order to limit the emergence of XDR-TB, however, in practice, this is not yet widely adopted11.
Although not widely implemented, the BRICS and other middle income countries have capacity to collect and analyze molecular data, and guidance exists regarding strain genotyping for TB surveillance16. While WGS may be more challenging to implement, it can inform the development of simpler tests, which have been used in preliminary studies to infer transmission patterns59. Mobile technology may also facilitate the collection of novel geospatial information. For example, human movement (measured via cellphone towers) has been combined with high resolution malaria prevalence data in Kenya to show that migration from less-developed residential areas accounts for the majority of malaria within urban centres60. Importantly, the usefulness of these additional data will always be limited if it cannot also be easily captured and integrated into existing data systems.
ENHANCING DATA SYSTEMS
Systems for reporting and analyzing data
Achieving more local control requires an investment in TB surveillance systems, including a strengthening of WHO-supported electronic data collection systems16,19. Maintaining a system that is sufficiently agile to be useful for heterogeneous patient populations and levels of resource availability (e.g., internet access) across all localities can be difficult. This is compounded by the long-term use of proprietary systems for which support may have ceased61, and the requirement by governments for a lengthy public tender process. Implementing similarly flexible systems for a locally tailored TB response – especially in high-burden countries that often have extreme resource limitations, little political will and the highest need for such systems among disenfranchised populations – will be no easier.
Benchmarks and performance indicators can facilitate the collection of standardized data and identification of surveillance gaps16,18,19. These benchmarks encourage TB programs to assess the consistency of case definitions and national data in interactive workshops with stakeholders. Such benchmarks can be internal (e.g., subtotals by age group equal the total number of reported cases) or external (e.g., the percentage of new TB cases in subgroups, such as children, is comparable to similar countries). Although linking data across disparate electronic databases (e.g., laboratory results and treatment information) is challenging, guidelines for the development of national electronic TB data systems19 are potentially useful for local system development.
Potential improvements to existing systems
Existing systems may be improved by: (a) incorporating more local data, (b) facilitating the easy capture of additional setting-specific data, (c) integrating with other disease databases, and (d) implementing features that facilitate rapid data analysis and linkage to intervention.
Systems incorporating local data should permit the timely collection, reporting, and analysis of those data at all levels of the healthcare system (Figure 2). Critically, this must be done while maintaining the capacity of existing systems to facilitate country-level reporting. This will require substantial new investments into human resource capacity (particularly epidemiological expertise) and IT infrastructure. These data can be inputted directly using mobile devices. Countries62 and cities63,64 are increasingly developing individual-based electronic data systems. Mobile technology can also be combined with innovative methods to maximize case finding by reimbursing TB control officers promptly or providing appropriate incentives for finding additional cases62.
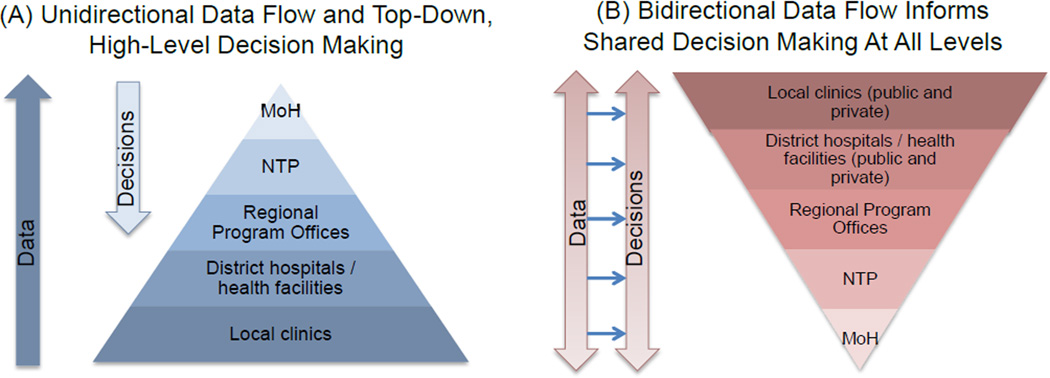
Figure 2. Structuring Data and Decision Making for TB Elimination.
Existing structures largely consist of data that are sent from the local level and aggregated at the central level for purposes of reporting and broad target-setting, with decisions made in top-down fashion and rarely involving individuals below the regional or district level (Panel A). In order to achieve TB elimination, data structures and decision-making should arguably be centered on activities the local level, which is the level at which TB transmission occurs. Such structures should support data and decision-making that is bidirectional and mutually informative in nature, involving all levels of the TB control system (Panel B). This flow of information should not only occur between healthcare system tiers, but also between localities, in order to disseminate information about what works in different settings. NTP = National Tuberculosis Program, MoH = Ministry of Health.
Importantly, these improved systems for local data should not only integrate with national systems but also allow for bidirectional data flow, facilitating the direct transfer of data from national to local level and between local control programs. This information can also link into systems used in other sectors. For example, the INDEPTH Network provides support and guidance for the collection of community-level demographic and healthcare information65, which supplements the surveillance of non-communicable diseases in high burden countries and are subsequently fed into national databases66. Including data from both public and private sectors is also an important consideration67.
If locally important data are to be effectively analyzed, improved quality control and standardized “best practice” guidelines are required, especially for new types of data. Open-source tools are available to assist in the analysis of these data, whether, for example, it is to project the local impact and cost of diagnostic tests68, or detect drug resistance mutations from WGS data69. Wider availability and adoption of such tools could encourage the collection of local data and improve the analytical capacity of TB programs; however, data might also need to be analyzed at a more centralized level where analytical capacity is likely to be greater.
Unique patient identifiers are essential. Without these, it will be challenging to link routine clinical and laboratory data with those from targeted surveys, sentinel surveillance systems, and other novel data collection efforts. This data linkage can facilitate pragmatic studies on the impact of interventions at a sub-district-level. In Brazil, data collected before and after the roll-out of Xpert MTB/RIF (a molecular test for TB and rifampin resistance) allowed for Xpert MTB/RIF’s effect on local case notification rates to be quantified and for poor-performing sites to be identified and targeted for further strengthening70. However, because the laboratory and treatment databases used their own internal identifiers, linking specific results with specific outcomes was a challenge. Weak existing data structures have also made it difficult to generate empirical evidence for locally targeted approaches to TB control.
Despite their clear benefits and potential cost savings71–73, improvements to these systems will require substantial investment. To justify such investment, it is essential to strengthen the empirical evidence base to support locally tailored approaches to ending TB.
EMPIRICAL EVIDENCE FOR LOCAL APPROACHES TO END TB
At present, there is limited evidence describing the effectiveness of the types of locally targeted approaches described above. Nevertheless, targeting high-risk populations (e.g., homeless populations, HIV-infected people, or drug users) has been a critical component of nearly every major success in TB control74,75. Mathematical models based on empirical data provide indirect support for targeted TB elimination strategies. Data from Rio de Janeiro suggest that, as with other diseases8,31,76,77, targeting hotspots containing 6% of the population on a district level (identified from local notification rates) could reduce city-wide TB incidence to a similar degree as an intervention of equal intensity covering the remaining 94% of the population78.
Local control officials undoubtedly target high-risk patient groups intuitively, but to demonstrate the effectiveness of these approaches, data must be collected and compared against standardized benchmarks. Ideally, these benchmarks should be agreed upon at the local and national level, accounting for local epidemiology and existing trends (Panel). Guidance regarding these measures of success could come from global agencies such as the WHO, and implementation of these standards could drive the improvement of local data collection efforts. Targeted approaches become increasingly important as TB incidence declines and TB becomes more concentrated within specific subpopulations79; thus, collection of empirical evidence to inform such approaches against standardized benchmarks should become a higher priority.
Encouraging parallels exist for other diseases. The Tanzanian ART program’s “Know your CD4 count campaign” used a consultation process to identify clinic, patient, and infrastructural factors that limited the number of HIV-infected patients with a known CD4 count80. After data for each clinic were reviewed in conjunction with local staff, site-specific interventions were implemented that involved addressing administrative and laboratories barriers, strengthening staff training, and educating patients. After the roll-out of the intervention, ART enrolment increased by an average of 62% at each clinic.
Evidence for the effectiveness of local interventions could also be collected using pragmatic trials embedded within the implementation of locally tailored responses, or before-after comparisons of communities that adopt tailored strategies for TB control. A study in Karachi showed that when community members screened patients in private healthcare facilities, the number of detected TB cases doubled, compared to areas without the intervention1.
PANEL
Examples of settings and potential benchmarks for success of locally targeted strategies or interventions to end TB.
| Illustrative Setting | Examples of potential benchmarks* | Improvements in data systems and structures required to assess progress |
|---|---|---|
| High HIV, low MDR, urban setting (e.g., African city) |
Percent decline in notified TB incidence in the five highest- incidence neighborhoods |
Ability to measure TB incidence by neighborhood or postal code |
| Diffuse, private- sector driven, peri- urban setting (e.g., Indian informal settlement) |
Percent increase in patients notified and successfully treated (including referrals) among patients diagnosed with TB in the private sector |
Integration of private care notification data with routine public systems |
| Low HIV, moderate incidence, high MDR (e.g., town in Former Soviet Union) |
Absolute decline in incidence of MDR- TB among treatment-naive individuals |
Repeat, targeted surveys to measure and stratify MDR-TB according to previous TB history |
| Rural sub-district with poor access to laboratory testing facilities (e.g., in Southeast Asia) |
Absolute reductions in average time to diagnosis and the proportion of patients who test positive but do not start treatment |
Integration of laboratory results with treatment initiation (yes/no, and date- stamped) data |
| Well-resourced city with large migrant community (e.g., in Western Europe) |
Absolute reduction in proportion of new cases due to recent infection informed by molecular epidemiology |
Inclusion of strain type data into routine notification systems |
The specific change targeted, and the duration of time provided to meet the benchmark, would depend on the current rate of TB, existing trends, and anticipated costs.
ETHICAL CONSIDERATIONS
In designing targeted approaches to ending TB locally, ethical considerations are an important challenge. TB programs routinely collect anonymized data and are working increasingly closely with patient advocacy groups, but local-level collection requires additional engagement with the targeted communities. TB officers may therefore wish to consult with community organizations to ensure that data are used to address local public health priorities. For example, community consultation is a core component of the “Reaching Every District” approach for childhood vaccination,81 and many countries with the most successful vaccination programs also have high levels of outreach and community engagment82. There are also ethical considerations when prioritizing interventions such as ART83 to specific groups; targeting one region or population over another may be perceived as inequitable. Finally, with regard to security, data can be anonymized, but sufficient IT infrastructure is still required to protect patient privacy, especially in high-burden settings where such systems may be weaker. Systems to protect privacy need not be TB-specific, however, and cross-sector initiatives should be encouraged.
CONCLUSION
Traditionally, interventions to control TB have focused on providing a basic level of care to a large number of people. As global priorities shift from controlling to ending TB, we must rapidly develop new systems that empower interventions tailored to heterogeneous epidemics. Locally targeted approaches have been successful in other diseases but require routine collection of local data, bidirectional flow of information and capacity between local and central level, augmentation of existing data collection efforts, and investment in the systems needed to collect and analyze disaggregated data.
In many settings, the focus of data collection is already shifting from national reporting to informing local strategy. Accelerating this expansion will require stronger links between local clinics, national TB programs, in-country and regional institutions with specialized expertise, and global bodies such as the WHO. A political commitment to increase human and information technology resources at all levels, and to collect empiric data to demonstrate the effectiveness of locally targeted strategies, will also be essential. To stop TB globally, we must address variation in TB epidemics locally – meaning that we must modernize data, systems, and ethical structures at all levels to empower communities to better understand their TB epidemics, and ultimately to end them.
KEY MESSAGES.
TB epidemics, like those of other infectious diseases, vary dramatically across different geographical regions; to end TB epidemics in high-burden areas, control efforts will need to be tailored to local conditions.
To design interventions that effectively combat TB, national control programs should shift from a centralized approach where local data is deposited into national databases for aggregated analyses, to a bidirectional one in which local partners have the capacity to collect and analyse data, using those data to design locally-responsive interventions.
This shift requires local TB programs to make better use of existing data, expand routine data collection, and make informed use of targeted surveys.
These efforts also require the modernization of data collection and storage systems, substantial financial investment in infrastructure and human resources (including the use of mobile technology and social media), and the reallocation of resources to support local decision making.
TB control programs will need to develop the necessary analytical and support infrastructure to measure the impact of local interventions and disseminate these findings within the national program.
Acknowledgments
Funding source
This work was supported by the Wellcome Trust (WT099854MA) and a South African Medical Research Council Career Development Award (GT); the U.S. National Institutes of Health (US NIH K01AI102944 award to HEJ, US NIH AI112438-01 award to TC); the B. Frank and Kathleen Polk Assistant Professorship in Epidemiology (DWD); and the UK National Institute of Health Research, Medical Research Council and Public Health England (IA). FC and AJK have no external funding sources to disclose. The funders had no role in the conception, preparation, review, approval or submission of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the views of the U.S. National Institute of Allergy and Infectious Diseases or the U.S. National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
GT, HEJ, TC, and DWD conceived the idea for this manuscript. GT and HEJ wrote the first draft, and all authors revised it for important intellectual content. All authors approved the final version as submitted for publication.
References
- 1.Khan AJ, Khowaja S, Khan FS, et al. Engaging the private sector to increase tuberculosis case detection: an impact evaluation study. The Lancet Infectious Diseases. 2012;12(8):608–616. doi: 10.1016/S1473-3099(12)70116-0. [DOI] [PubMed] [Google Scholar]
- 2.Chihota VN, Müller B, Mlambo CK, et al. The population structure of multi-and extensively drug-resistant tuberculosis in South Africa. J Clin Microbiol 2011: JCM. :05832–05811. doi: 10.1128/JCM.05832-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller B, Chihota VN, Pillay M, et al. Programmatically Selected Multidrug-Resistant Strains Drive the Emergence of Extensively Drug-Resistant Tuberculosis in South Africa. PloS one. 2013;8(8):e70919. doi: 10.1371/journal.pone.0070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller B, Streicher E, Hoek K, et al. inhA promoter mutations: a gateway to extensively drug-resistant tuberculosis in South Africa? The International Journal of Tuberculosis and Lung Disease. 2011;15(3):344–351. [PubMed] [Google Scholar]
- 5.Klopper M, Warren RM, Hayes C, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2013;19(3):449. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stop TB Partnership. The Global Plan to Stop TB 2011–2015 Progress Report 2006–2008. Geneva, Switzerland: World Health Organization; 2009. [3 September 2014]. Retrieved from http://www.stoptb.org/assets/documents/global/plan/The_global_plan_progress_report1.pdf. [Google Scholar]
- 7.Guerra CA, Gikandi PW, Tatem AJ, et al. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS medicine. 2008;5(2):e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The Neglected Tropical Diseases of Latin America and the Caribbean: A Review of Disease Burden and Distribution and a Roadmap for Control and Elimination. PLoS Negl Trop Dis. 2008;2(9):e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control Prevention. Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children--United States, 1998–2000. MMWR Morb Mortal Wkly Rep. 2002;51(11):234. [PubMed] [Google Scholar]
- 10.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organisation; 1988. [Google Scholar]
- 11.Bateman C. Eastern Cape treatment dysfunction boosts virulent new XDR-TB strain. 2015 doi: 10.7196/samj.9475. [DOI] [PubMed] [Google Scholar]
- 12.Nanoo A, Izu A, Ismail NA, et al. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004–12: a time series analysis. The Lancet Infectious Diseases. 2015 doi: 10.1016/S1473-3099(15)00147-4. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Global Tuberculosis Control 2014. Publication no. WHO/HTM/TB/2014.08. Geneva, Switzerland: 2014. [Google Scholar]
- 14.World Health Organization. What is DOTS?: A guide to understanding the WHO-recommended TB Control Strategy Known as DOTS. 1999
- 15.World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision. Publication no. WHO/HTM/TB/2013.2. Geneva, Switzerland: 2013. [Google Scholar]
- 16.World Health Organization. Understanding and Using Tuberculosis Data. Geneva: 2014. [Google Scholar]
- 17.World Health Organization. Global Tuberculosis Report 2014. Geneva: 2014. [Google Scholar]
- 18.World Health Organization. Standards and benchmarks for tuberculosis surveillance and vital registration systems: Checklist and User Guide. WHO/JTM/TB/2014.02. Geneva, Switzerland: 2014. [Google Scholar]
- 19.World Health Organization. Electronic recording and reporting for tuberculosis care and control. WHO/HTM/TB/2011.22. Geneva, Switzerland: 2012. [Google Scholar]
- 20.World Health Organization. TB Country Profile: Lesotho. Geneva, Switzerland: 2014. ( https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=LS&LAN=EN&outtype=html). [Google Scholar]
- 21.Cohen R, Lynch S, Bygrave H, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. Journal of the International AIDS Society. 2009;12(1):23. doi: 10.1186/1758-2652-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. The lancet global health. 2014:e453–e459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. The Lancet. 2014;383(9928):1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloch A, Snider D., Jr How much tuberculosis in children must we accept? Am J Public Health. 1986;1986;76(1):14. doi: 10.2105/ajph.76.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Roadmap for Childhood Tuberculosis. WHO/HTM/TB/2013.12. Geneva, Switzerland: 2013. [Google Scholar]
- 26.Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314(5802):1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 27.Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007;369(9570):1356–1362. doi: 10.1016/S0140-6736(07)60531-5. [DOI] [PubMed] [Google Scholar]
- 28.Bahl S, Kumar R, Menabde N, et al. Polio-free certification and lessons learned--South-East Asia region, March 2014. MMWR Morb Mortal Wkly Rep. 2014;63(42):941–946. [PMC free article] [PubMed] [Google Scholar]
- 29.Gerberry DJ, Wagner BG, Garcia-Lerma JG, Heneine W, Blower S. Using geospatial modelling to optimize the rollout of antiretroviral-based pre-exposure HIV interventions in Sub-Saharan Africa. Nat Commun. 2014:5. doi: 10.1038/ncomms6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azman AS, Luquero FJ, Rodrigues A, et al. Urban Cholera Transmission Hotspots and Their Implications for Reactive Vaccination: Evidence from Bissau City, Guinea Bissau. PLoS Negl Trop Dis. 2012;6(11):e1901. doi: 10.1371/journal.pntd.0001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bousema T, Griffin JT, Sauerwein RW, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9(1):e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuckler D, Basu S, McKee M, King L. Mass incarceration can explain population increases in TB and multidrug-resistant TB in European and central Asian countries. Proc Natl Acad Sci U S A. 2008;105(36):13280–13285. doi: 10.1073/pnas.0801200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bantubani N, Kabera G, Connolly C, et al. High rates of potentially infectious tuberculosis and multidrug-resistant tuberculosis (MDR-TB) among hospital inpatients in KwaZulu Natal, South Africa indicate risk of nosocomial transmission. PLoS One. 2014;9(3):e90868. doi: 10.1371/journal.pone.0090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Archives of internal medicine. 2007;167(4):335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 35.Uchimura K, Ngamvithayapong-Yanai J, Kawatsu L, et al. Characteristics and treatment outcomes of tuberculosis cases by risk groups, Japan, 2007–2010. Western Pacific Surveillance and Response Journal : WPSAR. 2013;4(1):11–18. doi: 10.5365/WPSAR.2012.3.4.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Tu D, An Y, Enarson D. The impact of migrants on the epidemiology of tuberculosis in Beijing, China. The International Journal of Tuberculosis and Lung Disease. 2006;10(9):959–962. [PubMed] [Google Scholar]
- 37.World Health Organization. The ENGAGE-TB Approach: Integrating community-based TB activities into the work of NGOs and other CSOs. Geneva, Switzerland: World Health Organization; 2014. [Accessed 23 December 2014]. http://www.who.int/tb/people_and_communities/commcare/background/en/. [Google Scholar]
- 38.Stop TB Partnership. [Accesssed 13 March 2015];TB Reach. 2015 http://www.stoptb.org/global/awards/tbreach/
- 39.Floyd S, Sismanidis C, Yamada N, et al. Analysis of tuberculosis prevalence surveys: new guidance on best-practice methods. Emerging themes in epidemiology. 2013;10(1):10. doi: 10.1186/1742-7622-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Tuberculosis Prevalence Surveys: a handbook. WHO/HTM/TB/2010.17. Geneva, Switzerland: 2011. [Google Scholar]
- 41.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis - 5th edition. WHO/HQ/TB/2014.12. Geneva, Switzerland: 2014. [Google Scholar]
- 42.Robertson SE, Valadez JJ. Global review of health care surveys using lot quality assurance sampling (LQAS), 1984–2004. Soc Sci Med. 2006;63(6):1648–1660. doi: 10.1016/j.socscimed.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Lanata CF, Black RE. Lot quality assurance sampling techniques in health surveys in developing countries: advantages and current constraints. World Health Stat Q. 1990;44(3):133–139. [PubMed] [Google Scholar]
- 44.Lynn Hedt B, van Leth F, Zignol M, et al. Multidrug Resistance among New Tuberculosis Cases: Detecting Local Variation through Lot Quality-Assurance Sampling. Epidemiology (Cambridge, Mass) 2012;23(2):293–300. doi: 10.1097/EDE.0b013e3182459455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chonde TM, Doulla B, van Leth F, et al. Implementation of a national anti-tuberculosis drug resistance survey in Tanzania. BMC Public Health. 2008;8:427. doi: 10.1186/1471-2458-8-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huong NT, Lan NT, Cobelens FG, et al. Antituberculosis drug resistance in the south of Vietnam: prevalence and trends. J Infect Dis. 2006;194(9):1226–1232. doi: 10.1086/507906. [DOI] [PubMed] [Google Scholar]
- 47.Salvo F, Dorjee K, Dierberg K, et al. Survey of tuberculosis drug resistance among Tibetan refugees in India. The International Journal of Tuberculosis and Lung Disease. 2014;18(6):655–662. doi: 10.5588/ijtld.13.0516. [DOI] [PubMed] [Google Scholar]
- 48.Isaakidis P, Das M, Kumar AMV, et al. Alarming Levels of Drug-Resistant Tuberculosis in HIV-Infected Patients in Metropolitan Mumbai, India. PLoS ONE. 2014;9(10):e110461. doi: 10.1371/journal.pone.0110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cain KP, Marano N, Kamene M, et al. The Movement of Multidrug-Resistant Tuberculosis across Borders in East Africa Needs a Regional and Global Solution. PLoS Med. 2015;12(2):e1001791. doi: 10.1371/journal.pmed.1001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker TM, Ip CL, Harrell RH, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13(2):137–146. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moonan PK, Ghosh S, Oeltmann JE, Kammerer JS, Cowan LS, Navin TR. Using genotyping and geospatial scanning to estimate recent Mycobacterium tuberculosis transmission, United States. Emerg Infect Dis. 2012;18(3):458–465. doi: 10.3201/eid1803.111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mears J, Abubakar I, Crisp D, et al. Prospective evaluation of a complex public health intervention: lessons from an initial and follow-up cross-sectional survey of the tuberculosis strain typing service in England. BMC Public Health. 2014;14(1):1023. doi: 10.1186/1471-2458-14-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson LF, Tamne S, Brown T, et al. Transmission of multidrug-resistant tuberculosis in the UK: a cross-sectional molecular and epidemiological study of clustering and contact tracing. The Lancet Infectious Diseases. 2014 doi: 10.1016/S1473-3099(14)70022-2. [DOI] [PubMed] [Google Scholar]
- 54.Mandeville KL, Harris M, Thomas HL, Chow Y, Seng C. Using Social Networking Sites for Communicable Disease Control: Innovative Contact Tracing or Breach of Confidentiality? Public Health Ethics. 2014;7(1):47–50. doi: 10.1093/phe/pht023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364(8):730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 56.Walker TM, Lalor MK, Broda A, et al. Assessment of< i> Mycobacterium tuberculosis</i> transmission in Oxfordshire, UK, 2007–12, with whole pathogen genome sequences: an observational study. The Lancet Respiratory Medicine. 2014;2(4):285–292. doi: 10.1016/S2213-2600(14)70027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Török ME, Reuter S, Bryant J, et al. Rapid whole-genome sequencing for investigation of a suspected tuberculosis outbreak. J Clin Microbiol. 2013;51(2):611–614. doi: 10.1128/JCM.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker TM, Ip CL, Harrell RH, et al. Whole-genome sequencing to delineate< i> Mycobacterium tuberculosis</i> outbreaks: a retrospective observational study. The Lancet infectious diseases. 2013;13(2):137–146. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pérez-Lago L, Lirola MM, Herranz M, Comas I, Bouza E, García-de-Viedma D. Fast and low-cost decentralized surveillance of transmission of tuberculosis based on strain-specific PCRs tailored from whole genome sequencing data: a pilot study. Clinical Microbiology and Infection. 2014 doi: 10.1016/j.cmi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Wesolowski A, Eagle N, Tatem AJ, et al. Quantifying the Impact of Human Mobility on Malaria. Science. 2012;338(6104):267–270. doi: 10.1126/science.1223467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sankoh O, Byass P. The INDEPTH Network: filling vital gaps in global epidemiology. Int J Epidemiol. 2012;41(3):579–588. doi: 10.1093/ije/dys081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.TIBU: Use of Innovative Technology to Improve Kenya TB Program Management - The first in Africa! [accessed 3 February 2015]; Available from: www.tbcare1.org/pdfs/download.php?file=TIBU_Factsheet.pdf.
- 63.Creswell J, Khowaja S, Codlin A, et al. An evaluation of systematic tuberculosis screening at private facilities in Karachi, Pakistan. PLoS One. 2014;9(4):e93858. doi: 10.1371/journal.pone.0093858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorent N, Choun K, Thai S, et al. Community-Based Active Tuberculosis Case Finding in Poor Urban Settlements of Phnom Penh, Cambodia: A Feasible and Effective Strategy. PLoS ONE. 2014;9(3):e92754. doi: 10.1371/journal.pone.0092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bangha M, Diagne A, Bawah A, Sankoh O. Monitoring the millennium development goals: the potential role of the INDEPTH Network. Global Health Action. 2010;3 doi: 10.3402/gha.v3i0.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng N, Van Minh H, Juvekar S, et al. Using the INDEPTH HDSS to build capacity for chronic non-communicable disease risk factor surveillance in low and middle-income countries. Global Health Action. 2009;2 doi: 10.3402/gha.v2i0.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells WA, Uplekar M, Pai M. Achieving Systemic and Scalable Private Sector Engagement in Tuberculosis Care and Prevention in Asia. PLoS Med. 2015;12(6):e1001842. doi: 10.1371/journal.pmed.1001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dowdy DW, Andrews JR, Dodd PJ, Gilman RH. A user-friendly, open-source tool to project impact and cost of diagnostic tests for tuberculosis. eLife. 2014:3. doi: 10.7554/eLife.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coll F, McNerney R, Preston MD, et al. Rapid determination of anti-tuberculosis drug resistance from whole-genome sequences. Genome Med. 2015;7(1):51. doi: 10.1186/s13073-015-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durovni B, Saraceni V, van den Hof S, et al. Impact of Replacing Smear Microscopy with Xpert MTB/RIF for Diagnosing Tuberculosis in Brazil: A Stepped-Wedge Cluster-Randomized Trial. PLoS Med. 2014;11(12):e1001766. doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blaya JA, Cohen T, Rodríguez P, Kim J, Fraser HS. Personal digital assistants to collect tuberculosis bacteriology data in Peru reduce delays, errors, and workload, and are acceptable to users: cluster randomized controlled trial. Int J Infect Dis. 2009;13(3):410–418. doi: 10.1016/j.ijid.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blaya J, Shin S, Yale G, et al. Electronic laboratory system reduces errors in National Tuberculosis Program: a cluster randomized controlled trial. The International Journal of Tuberculosis and Lung Disease. 2010;14(8):1009–1015. [PMC free article] [PubMed] [Google Scholar]
- 73.Chapman AL, Darton TC, Foster RA. Managing and monitoring tuberculosis using web-based tools in combination with traditional approaches. Clinical epidemiology. 2013;5:465. doi: 10.2147/CLEP.S37072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333(4):229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 75.Partners in Health. PIH/Russia recognized for TB achievements. Geneva: UNAIDS; 2014. [Accessed 23 December 2014]. http://www.pih.org/blog/pih-russia-recognized-for-tb-achievements. [Google Scholar]
- 76.Wand H, Ramjee G. Targeting the hotspots: investigating spatial and demographic variations in HIV infection in small communities in South Africa. Journal of the International AIDS Society. 2010;13(1):41. doi: 10.1186/1758-2652-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gurarie D, Seto EY. Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities. Journal of the Royal Society Interface. 2009;6(35):495–508. doi: 10.1098/rsif.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci U S A. 2012;109(24):9557–9562. doi: 10.1073/pnas.1203517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colijn C, Cohen T, Murray M. Emergent heterogeneity in declining tuberculosis epidemics. J Theor Biol. 2007;247(4):765–774. doi: 10.1016/j.jtbi.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Memiah P, Shumba C, Henley Y, et al. "Know your CD4 campaign": 6-year outcomes from a quality improvement initiative to promote earlier initiation of antiretroviral therapy in Tanzania. International Journal of Medicine and Public Health. 2014;4(3):194. [Google Scholar]
- 81.Vandelaer J, Bilous J, Nshimirimana D. Reaching Every District (RED) approach: a way to improve immunization performance. Bull World Health Organ. 2008;86 doi: 10.2471/BLT.07.042127. A-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Health Organization. In-Depth Evaluation of the REACHING EVERY DISTRICT APPROACH in the African Region. Geneva, Switzerland: 2007. [accessed 6 January 2015]. http://www.immunizationbasics.jsi.com/Docs/AFRO_RED_Eval_Dec07.pdf. [Google Scholar]
- 83.Gerberry DJ, Wagner BG, Garcia-Lerma JG, Heneine W, Blower S. Using geospatial modelling to optimize the rollout of antiretroviral-based pre-exposure HIV interventions in Sub-Saharan Africa. Nature communications. 2014;5:5454. doi: 10.1038/ncomms6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soltan V, Henry AK, Crudu V, Zatusevski I. Increasing tuberculosis case detection: lessons from the Republic of Moldova. Bull World Health Organ. 2008;86(1):71–76. doi: 10.2471/BLT.06.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]