Abstract
Accurate imaging of urethral strictures is critical for preoperative staging and planning of reconstruction. The current gold standard, retrograde urethrography (RUG), allows for accurate diagnosis, staging, and delineation of urethral strictures, and remains a cornerstone in the management of urethral stricture disease. In complex situations, the RUG can be combined with voiding cystourethrogram (VCUG) in order to better visualize the posterior urethra or complex distraction defects. Direct visualization of the stricture by cystoscopy, either retrograde or antegrade, can provide additional information as to the location and appearance of stricture, as well as precise location on fluoroscopic imaging. Sonourethrography (SU) is a useful adjunct to allow for three-dimensional assessment of stricture length and location, and can be a useful intraoperative assessment tool, however, its use remains limited to a second-line setting. Cross-sectional imaging in the form of computed tomography (CT) or magnetic resonance urethrography can provide additional three-dimensional information of anatomic structures and their relations, and can serve as a useful adjunct in complex clinical scenarios.
Keywords: Urethra, stricture, imaging, urethrogram
Introduction
Accurate diagnosis and staging is a key element to successful surgical outcomes in any discipline, and is particularly relevant in the evaluation of urethral stricture. Multiple options are available in the urologist’s armamentarium to thoroughly evaluate a urethral stricture, and alongside careful history and physical exam, can allow for selection of an optimal reconstructive procedure to restore normal voiding function. Dynamic retrograde urethrography (RUG) and flexible cystoscopy remain gold standard techniques for patient evaluation in the setting of urethral stricture. Additional modalities have been applied to urethral stricture as well, including ultrasonography, magnetic resonance imaging (MRI), and computerized tomography. Proper selection of imaging modalities for preoperative evaluation of patients with urethral stricture is critical in decision making for reconstructive procedures, and correct utilization of these techniques can optimize patient outcomes.
Urethrography, cystography, and cystoscopy
Urethrography
The urethrogram is the oldest radiographic test for assessing urethral stricture disease, and remains the gold standard for diagnosis and staging (1,2). Most often, the urethrogram is performed in a retrograde fashion. The study commences with a scout film to assess bony structures as well as the presence of any calcified urinary tract pathology. Subsequently, 20-30 mL of water-soluble iodine based contrast medium is injected to the urethra under direct fluoroscopic or radiographic vision, and multiple images are obtained. This is known as a dynamic retrograde urethrogram, which allows for live assessment of the urethra as contrast is delivered (3).
The retrograde urethrogram should ideally be performed and interpreted by the treating urologist. A significant disparity has been suggested between independently reported urethrograms compared to those interpreted by the treating urologist. A concordance between independently reported length to intraoperative measured length has been shown to be 0.47 for independently reported urethrograms, compared to 0.93 for those reported by the treating urologist (4). This affirms the notion that the treating urologist should personally and carefully examine the results of the retrograde urethrogram.
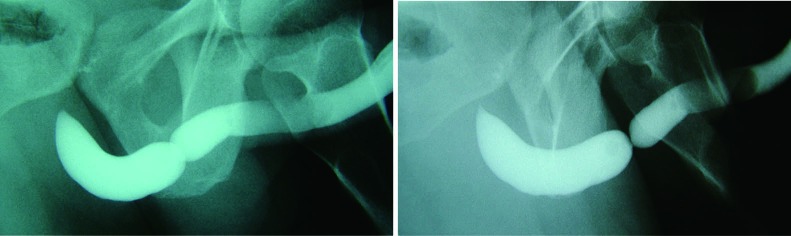
Patient positioning during retrograde urethrogram is critical. The patient should be in an oblique position (35-45 degrees) to maximize visualization of the bulbar urethra. Proper positioning can be confirmed by a closed downward oriented obturator foramen. Such a position ensures that the majority of the urethra is parallel to the radiographic film. Improper positioning will place the urethra at an angle relative to the film, and result in underestimation of stricture length (5). The penis should be placed on stretch in order to maximize complete assessment of the urethra. The use of anesthetic-impregnated lubrication can obscure the image, induce edema, and provide questionable benefit to patient comfort (6). Contrast should be visualized through the stricture and the membranous urethra to allow imaging of the urethra proximal to the stricture, in order to ensure that the full extent of urethral pathology is visualized. The importance of patient positioning is demonstrated in Figure 1.
Figure 1.
A representative RUG demonstrating the effect of pelvic angulation on stricture appearance. With improper patient positioning, the length of the stricture may be underestimated. RUG, retrograde urethrography.
There are three key features of a stricture that a RUG must identify, including location of the stricture, the length of the stricture, and coexistent urethral pathology. In order to allow for accurate interpretation, a detailed understanding of normal urethral anatomy is critical. The urethra is typically divided into an anterior and posterior portion. The anterior urethra is comprised of the fossa navicularis, penile urethra, and bulbar urethra. The posterior urethra is composed of the membranous urethra, and prostatic urethra. The distribution of normal urethral anatomy on RUG is highlighted in Figure 2.
Figure 2.
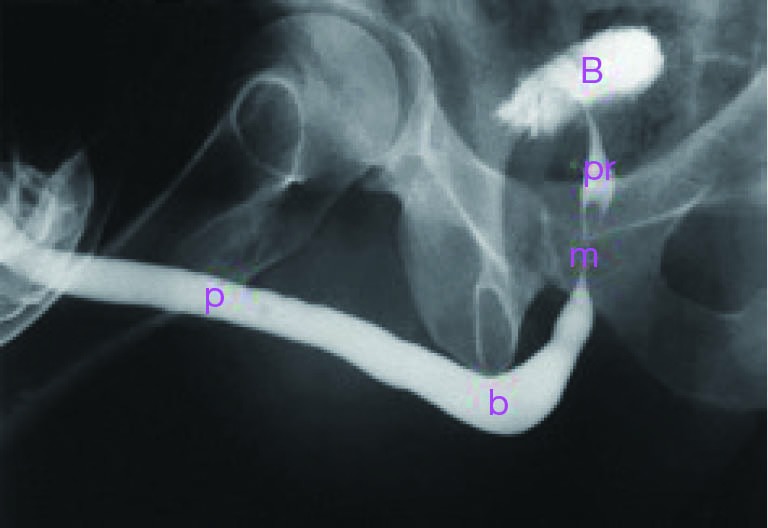
Normal RUG demonstrating the anatomy of the male urethra. p, penile urethra; b, bulbar urethra; m, membranous urethra; pr, prostatic urethra; B, bladder; RUG, retrograde urethrography.
The caliber of the anterior urethra should be smooth and uniform from the urethral meatus to the penobulbar junction. At this point, the bulbar urethra takes a slight S-shaped change in course, which typically correlates to the penoscrotal junction. Occasionally, soft tissue structures of the scrotum can be identified, assisting in the identification of this landmark. Maintaining stretch on the penis allows for maximal straightening of the urethra, which in turn assists in correctly identifying stricture length of this segment.
At the level of the bulbar urethra, a widening in caliber is observed, followed by a prompt tapering proximally to the level of the membranous urethra. Occasionally opacification of Cowper’s ducts can be seen here, especially if there is obstruction distal to the opening of these structures in the proximal bulbar urethra. In some cases, compression of the anterior leaf of the bulbospongiosus muscle (musculus compressor nuda) may be seen in the very proximal bulbar urethra. This is a normal finding that should not be mistaken for a proximal bulbar urethral stricture.
The membranous urethra opacifies as a thin wisp of contrast to the level of the apex of the prostate gland, which can be identified with the verumontanum behaving as a discrete filling defect. A normal, competent posterior urethra will be closed at rest and during RUG, and as such distension of this urethral segment is not seen.
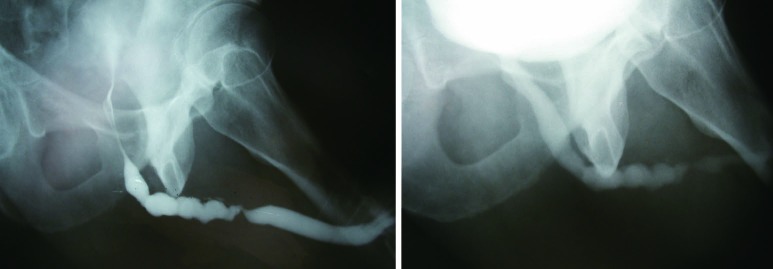
The sensitivity and specificity of RUG for the diagnosis of a urethral stricture has been reported in current literature. Sensitivities between 75% and 100% have been observed, with specificities of 72-97%. Typically, imaging is compared to cystoscopy and intraoperative measurements as a comparison. Positive predictive values have been reported from 50-93%, with negative predictive values varying in the 76-100% range (7-9). As such, retrograde urethrogram is considered to be strong in its ability to diagnose stricture, and further characterize its length, location, and number with a high degree of accuracy. Figure 3 demonstrates proper delineation of a long-segment penile urethral stricture related to lichen sclerosus which was initially assessed as a “meatal stenosis”. A long-segment (5 cm) idiopathic bulbar urethral stricture is demonstrated in Figure 4.
Figure 3.
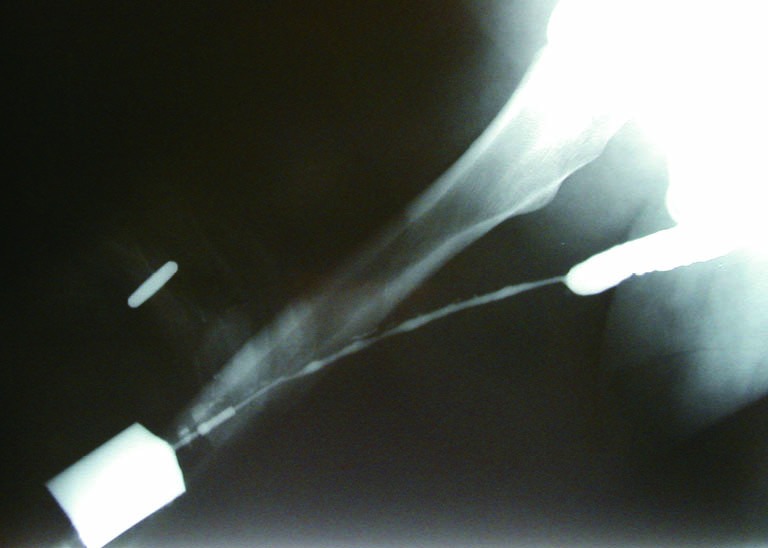
A long-segment penile urethral stricture due to lichen sclerosus.
Figure 4.
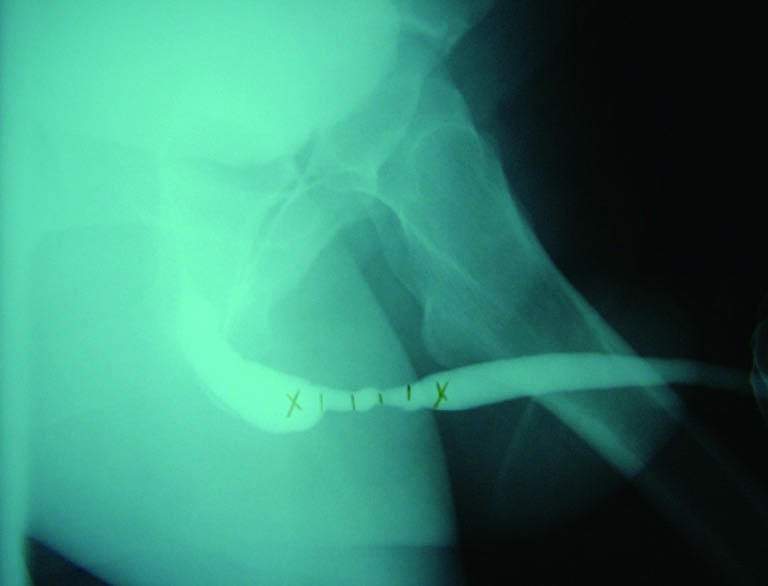
A long-segment idiopathic bulbar urethral stricture.
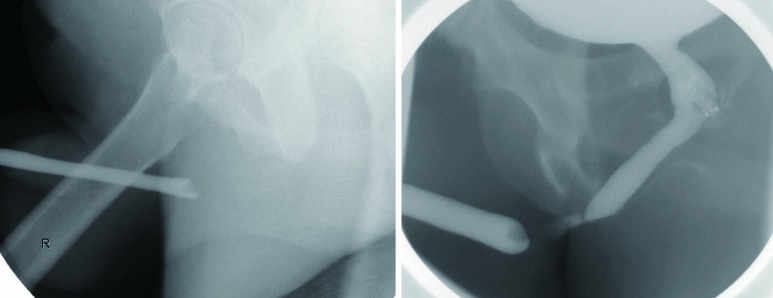
Given that retrograde urethrogram relies on an intraluminal opacification of the urethra, it provides minimal direct assessment of periurethral pathology. Retrograde urethrogram is limited in its assessment of spongiofibrosis, which can be interpolated through the examiners diagnostic suspicion and experience. In most situations, however, complicating features such as fistula, false passage, and significant ductal reflux can be readily identified (10) (Figure 5).
Figure 5.
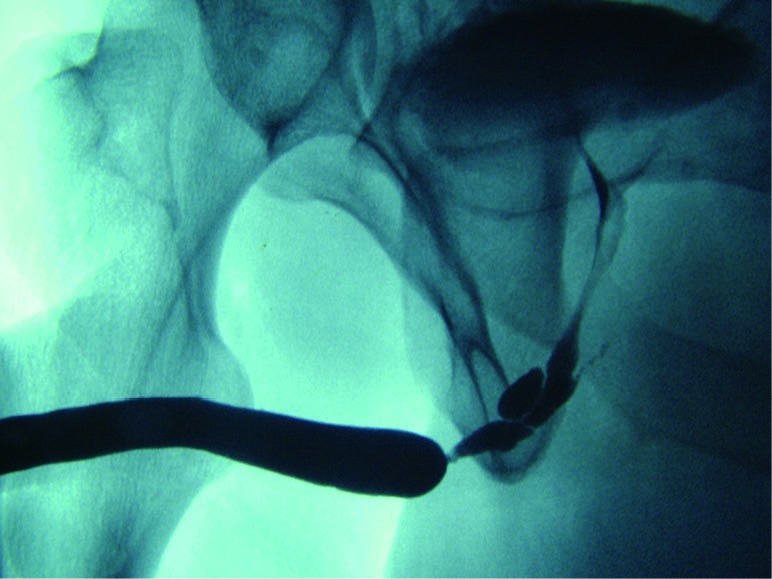
False passages associated with a complex bulbar urethral stricture. Note the filling of Cowper’s duct.
In the setting of traumatic urethral disruption, retrograde urethrogram plays a central role. Not only is it indicated in acute diagnosis and assessment of these injuries, it carries a central role in operative planning for reconstruction of these defects. The degree of urethral distraction and measurement of bulbar length, or urethrometry, can be easily performed with this study (11). Such measurements are critical to planning operative approaches for repair of these injuries (12). Severe urethral occlusion may lead to inadequate visualization, which can require combination antegrade urethrography in order to fully delineate the stenosis (13).
While retrograde urethrogram is a minimally invasive investigation, adverse effects may occur. Most frequently reported complications of retrograde urethrogram include patient discomfort, urinary tract infection, and contrast agent reaction. There is also a significant radiation exposure with this procedure, which may be relevant in young patients or those requiring multiple urethrograms over the course of their illness.
In summary, retrograde urethrogram is a reliable means for establishing the diagnosis of a suspected urethral stricture but also provides accurate staging information with regard to stricture number, length, location, and coexistent urethral pathology. The combination of retrograde urethrogram with other modalities can further improve diagnostic accuracy in the evaluation of urethral stricture, and allow for optimal preoperative assessment and planning.
Cystography
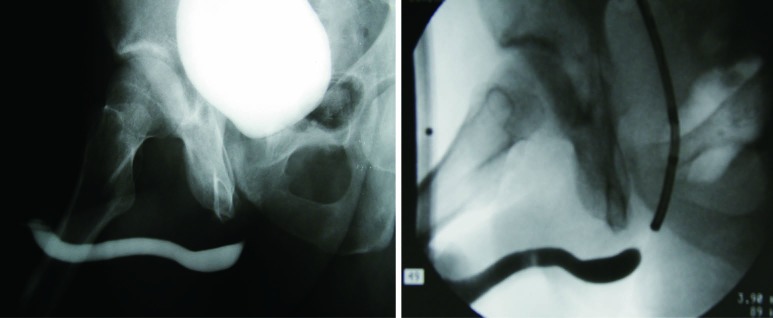
Cystography can stage urethral strictures both in a dynamic and static fashion. Voiding cystourethrogram (VCUG) can provide excellent assessment of the posterior urethra. Contrary to RUG, the bladder neck and prostatic urethra are distended during a VCUG assessment (Figure 6). Because of this physiologic feature, VCUG can allow for better proximal assessment of an obliterated or near-obliterated stricture that fails to be adequately staged with RUG alone (Figure 7). Furthermore, VCUG can give indication of the degree of functional impairment that the stricture imparts, by examining the hydrodistension effect of dilation of the urethra proximal to the stricture (13).
Figure 6.
The combined use of RUG and VCUG to stage a lengthy bulbar urethral stricture and evaluate the posterior urethra. RUG, retrograde urethrography; VCUG, voiding cystourethrogram.
Figure 7.
The combined use of retrograde and antegrade urethrography (through a flexible cystoscope) to stage an obliterated traumatic bulbar urethral stricture.
The option of performing VCUG exists immediately following contrast instillation at time of RUG. Alternatively, a small-bore ureteral access catheter can be passed through the stricture to instill contrast into the bladder. Occasionally, patients will have a suprapubic catheter in-situ during radiographic assessment, which greatly simplifies the performance of VCUG. In rare instances, VCUG can be performed following administration of intravenous contrast. Such a technique is uncommon, owing to its lengthy nature and risk of contrast reaction. When VCUG is performed to assess urethral stricture, patient positioning should be maintained in the same manner as RUG, with the patient in an oblique position as previously described.
Static cystography has a minor role in the evaluation of anterior urethral stricture. In the setting of a pelvic fracture urethral injury (PFUI), static cystography can provide an estimate of the length of distraction defect. Furthermore, static cystography can provide an assessment of the competency of the bladder neck. Pelvic fracture patients carry a significant risk of neurologic injury, and subsequent incontinence following attempts at reconstruction. A study by Iselin et al. have associated the presence of an open bladder neck on static cystogram prior to reconstruction to carry a 53% rate of incontinence (14). As such, static cystogram can provide useful information prior to surgical management of a urethra defect related to PFUI.
Cystoscopy
While cystoscopy is a ubiquitous examination in urology and not technically an imaging modality, its use in the assessment of urethral stricture remains underreported. Cystoscopy remains the de facto gold standard for most urologists in determining the presence or absence of a urethral stricture and is very helpful in staging urethral strictures in combination with other imaging modalities. Urethral pathology can also be identified with cystoscopy before functional impairment is seen on uroflowmetry or symptom score indices (15).
The chief limitation of cystoscopy is the inability of the instrument to pass through a significant stricture, which can compromise more proximal assessment of stricture length, number, and location. Attempts to overcome this limitation include the use of smaller caliber instruments, such as a pediatric cystoscope, a ureteroscope, or a flexible hysteroscope. Utilization of such devices is particularly useful to assess the urethra proximal to a distal stricture.
The presence of a urethral distraction after PFUI presents a unique utilization of cystoscopy. In these cases, cystoscopy is frequently required to accurately assess the length of a stricture. These patients often have a completely obliterated segment, and are unable to sufficiently relax the bladder neck during voiding studies to visualize the posterior urethra. As such, in conjunction with retrograde urethrogram, contrast-based assessment alone can result in significant overestimation of the length of the distraction defect. In these instances, antegrade cystoscopy can be performed through the suprapubic tract, and the cystoscope can be advanced through the bladder neck to the level of the stricture. In combination with RUG, this maneuver can assist in accurate identification of distraction length (Figure 8). Correct identification of distraction length can be useful in planning surgical intervention to restore urethral patency, and can assist in predicting outcomes during the reconstruction of these defects.
Figure 8.
A demonstration of overestimation of urethral distraction defect length by RUG and cystogram alone. The top image shows the RUG and VCUG, and the bottom demonstrates combined RUG and flexible suprapubic tract cystoscopy to more accurately delineate urethral distraction length. RUG, retrograde urethrography; VCUG, voiding cystourethrogram.
Suprapubic tract cystoscopy can allow for direct visual assessment of bladder neck competence, which cystography alone may not provide. Furthermore, cystoscopy can provide information on bladder neck fibrosis and tethering, which when present may indicate an increased risk of incontinence following urethral reconstruction. This can be a significant addition to preoperative patient counselling (16).
Ultrasonography
Urethral ultrasound, or sonourethrography (SU), was first described by McAninch in 1988. Currently, SU is used primarily as an adjunctive technique in the evaluation of urethral stricture disease with the main advantage being an enhanced assessment of associated spongiofibrosis. For anterior strictures 3-5 cm in length, SU has been shown to have sensitivities and specificities of 66-100% and 97-98%, with corresponding positive and negative predictive values of 50-80% and 96-98%, respectively (17-19).
The benefits of SU include a three-dimensional anatomic assessment of stricture length and location. SU is considerably more sensitive in identifying strictures in the penile urethra as opposed to the bulbar urethra. Other drawbacks include operator dependency and a semi-invasive nature, and the requirement of local or general anesthesia for full urethral distension to maximize visualization.
Most studies reported use SU as an adjunct following a primary screen for urethral stricture, and only 8% of studies used SU as a tool to evaluate for stricture recurrence (20). Prospective data comparing RUG and high-resolution SU has demonstrated that SU is as effective as RUG for identifying an anterior urethral stricture, with a greater sensitivity to characterize length (73.3-100%), and associated spongiofibrosis. The investigators reported less discomfort and bleeding with SU compared to RUG (8). Other prospective series have shown that SU and RUG are each effective in characterizing strictures once limitations of radiographic magnification are taken into consideration, suggesting that a combined modality approach is optimal for staging of urethral stricture disease (10).
SU has taken a role in some centers as an intraoperative tool for assessment of urethral stricture and choice of reconstructive approach. In a retrospective review of 232 patients, Buckley et al. found that intraoperative SU changed surgical approach in 19% of patients, and influenced decision-making in 26% of patients. SU identified longer strictures than RUG in patients whose operative approach was changed from anastomotic to onlay urethroplasty, whereas SU identified shorter strictures than RUG in patients whose operative approach was changed from onlay to anastomotic urethroplasty (21).
Overall, SU remains a useful adjunct for the assessment of urethral stricture disease particularly associated spongiofibrosis, and may serve an additional role as an intraoperative assessment tool.
Cross-sectional imaging
Conventional imaging of the urethra in the setting of an obliterative urethral stricture secondary to a distraction defect typically relies on RUG and VCUG. These modalities, however, carry several important limitations in this setting. Failure of bladder neck opening on VCUG can lead to incorrect estimation of the length of distraction defect. Prostatic displacement on the horizontal or vertical axis may not be identified. Furthermore, complicating features such as fistulae, cavitation, diverticula, and false passages may be overlooked with conventional imaging modalities.
MRI was first described in 1992 in an attempt to overcome these limitations (22). In this series, a total of 18 patients with complete prostatomembranous occlusion secondary to pelvic crush injuries underwent MRI prior to surgical repair. All patients had standard evaluation with RUG and cystography, and the results were correlated with MRI. In this cohort, MRI was able to correctly identify degree and direction of prostatic displacement, alongside an accurate determination of defect length. Additionally, fractures and avulsions of the corporal bodies were identified. As such, MRI was shown to be a potentially useful adjunct in preoperative assessment of these patients, where cross sectional imaging can provide information that conventional planar radiography cannot.
Oh et al. performed a similar study, where a total of 25 men with obliterative posterior urethral strictures underwent conventional imaging alongside MRI. The authors found that MRI measurements correlated better to intraoperative measurements than combined RUG/VCUG, and that conventional imaging underestimated true stricture length (23). Similar results have been reported in other studies (24,25).
MRI has been less studied in patients with anterior urethral strictures. One report by Osman et al. compared RUG and MRI in 20 patients with urethral strictures, of which 18 were anterior. The accuracy of diagnosis was equal in both modalities, but MRI was able to provide information about degree of spongiofibrosis. As such, MRI may provide additional guidance in treatment planning in selected patients with anterior urethral stricture (26).
Three-dimensional spiral computed tomography (CT) cystourethrography (CTCUG) was reported as a novel technique for evaluating post-traumatic posterior urethral defects in 2003. In this study, 27 patients underwent conventional evaluation followed by CTCUG using a technique to maximize high-density images (bony and contrast-filled structures). Three-dimensional reconstruction of the CT-acquired data allowed for multi-plane assessment, and more precise definition of pelvic anatomy. CTCUG was able to evaluate location and length of the distraction defect, the alignment of the urethral ends, relationship of bone to the urethra, and associated pathology such as fistula, diverticula, and false passage. The authors concluded that static and dynamic CTCUG images may allow improved staging of a pelvic fracture-related urethral injury, leading to better surgical planning (27).
Overall, cross sectional imaging can provide important data that two-dimensional studies cannot. These studies may be incorporated as useful adjuncts in the preoperative evaluation of patients with urethral stricture disease, pelvic fracture associated injuries, or where high clinical suspicion of additional pathology exists.
Conclusions
Urethral imaging is a critical step in the preoperative patient evaluation prior to definitive surgical management. RUG remains the current gold standard of imaging, providing reliable and accurate diagnosis and staging of urethral stricture disease. Combination of RUG with other imaging modalities can improve and facilitate diagnosis in complex situations. VCUG can provide insight to the degree of functional impairment of the bladder neck and urethra, and can provide critical staging information in combination with RUG in complex pelvic fracture associated urethral injuries. Flexible cystoscopy is a useful adjunct as well, allowing for direct visualization of the stricture and potential complicating features, as well as improved measurement of distraction length. SU remains an adjunctive technique, and may play a role in intraoperative decision-making. Cross sectional imaging via MRI and CT may provide additional information for complicating features of structures, and can provide accurate assessment of stricture length, and is most useful in situations where additional pathology is suspected. Overall, multiple imaging modalities are available to the urologist for the diagnosis and staging of urethral stricture, and in combination can provide a comprehensive assessment of disease that can lead to optimal preoperative planning.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cunningham JH. The diagnosis of stricture of the urethra by Roentgen rays. Trans Am Assoc Genitourin Surg 1910;5:369-71. [Google Scholar]
- 2.McCallum RW, Colapinto V. The role of urethrography in urethral disease. Part I. Accurate radiological localization of the membranous urethra and distal sphincters in normal male subjects. J Urol 1979;122:607-11. [DOI] [PubMed] [Google Scholar]
- 3.Jordan GH, McCammon KA. Surgery of the Penis and Urethra. In: Wein AJ, Kavoussi LR, Novick AC, et al. eds. Campbell-Walsh Urology, 9th Edition. Philadelphia: Saunders, 2007:956-1000. [Google Scholar]
- 4.Bach P, Rourke K. Independently interpreted retrograde urethrography does not accurately diagnose and stage anterior urethral stricture: the importance of urologist-performed urethrography. Urology 2014;83:1190-3. [DOI] [PubMed] [Google Scholar]
- 5.Gallentine ML, Morey AF. Imaging of the male urethra for stricture disease. Urol Clin North Am 2002;29:361-72. [DOI] [PubMed] [Google Scholar]
- 6.Colapinto V, McCallum RW. The role of urethrography in urethral disease. Part II. Indications for transphincter urethroplasty in patients with primary bulbous strictures. J Urol 1979;122:612-8. [DOI] [PubMed] [Google Scholar]
- 7.Mahmud SM, El KS, Rana AM, et al. Is ascending urethrogram mandatory for all urethral strictures? J Pak Med Assoc 2008;58:429-31. [PubMed] [Google Scholar]
- 8.Choudhary S, Singh P, Sundar E, et al. A comparison of sonourethrography and retrograde urethrography in evaluation of anterior urethral strictures. Clin Radiol 2004;59:736-42. [DOI] [PubMed] [Google Scholar]
- 9.Andersen J, Aagaard J, Jaszczak P. Retrograde urethrography in the postoperative control of urethral strictures treated with visual internal urethrotomy. Urol Int 1987;42:390-1. [DOI] [PubMed] [Google Scholar]
- 10.Babnik Peskar D, Visnar Perovic A. Comparison of radiographic and sonographic urethrography for assessing urethral strictures. Eur Radiol 2004;14:137-44. [DOI] [PubMed] [Google Scholar]
- 11.Koraitim MM. Gapometry and anterior urethrometry in the repair of posterior urethral defects. J Urol 2008;179:1879-81. [DOI] [PubMed] [Google Scholar]
- 12.Figler BD, Hoffler CE, Reisman W, et al. Multi-disciplinary update on pelvic fracture associated bladder and urethral injuries. Injury 2012;43:1242-9. [DOI] [PubMed] [Google Scholar]
- 13.Goel A, Gupta A, Dalela D. Antegrade urethrogram: A technique to visualize the proximal bulbous urethral segment in anterior urethral stricture. Indian J Urol 2009;25:415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iselin CE, Webster GD. The significance of the open bladder neck associated with pelvic fracture urethral distraction defects. J Urol 1999;162:347-51. [PubMed] [Google Scholar]
- 15.Heyns CF. Follow-Up Strategies After Urethral Stricture Treatment. In: Brandes SB, Morey AF. eds. Advanced Male Urethral and Genital Reconstructive Surgery, 2nd Edition. New York: Humana Press, 2014:413-25. [Google Scholar]
- 16.Hosseini SJ, Kaviani A, Jabbari M, et al. Diagnostic application of flexible cystoscope in pelvic fracture urethral distraction defects. Urol J 2006;3:204-7. [PubMed] [Google Scholar]
- 17.Morey AF, McAninch JW. Sonographic staging of anterior urethral strictures. J Urol 2000;163:1070-5. [PubMed] [Google Scholar]
- 18.Morey AF, McAninch JW. Role of preoperative sonourethrography in bulbar urethral reconstruction. J Urol 1997;158:1376-9. [PubMed] [Google Scholar]
- 19.McAninch JW, Laing FC, Jeffrey RB, Jr. Sonourethrography in the evaluation of urethral strictures: a preliminary report. J Urol 1988;139:294-7. [DOI] [PubMed] [Google Scholar]
- 20.Meeks JJ, Erickson BA, Granieri MA, et al. Stricture recurrence after urethroplasty: a systematic review. J Urol 2009;182:1266-70. [DOI] [PubMed] [Google Scholar]
- 21.Buckley JC, Wu AK, McAninch JW. Impact of urethral ultrasonography on decision-making in anterior urethroplasty. BJU Int 2012;109:438-42. [DOI] [PubMed] [Google Scholar]
- 22.Dixon CM, Hricak H, McAninch JW. Magnetic resonance imaging of traumatic posterior urethral defects and pelvic crush injuries. J Urol 1992;148:1162-5. [DOI] [PubMed] [Google Scholar]
- 23.Oh MM, Jin MH, Sung DJ, et al. Magnetic resonance urethrography to assess obliterative posterior urethral stricture: comparison to conventional retrograde urethrography with voiding cystourethrography. J Urol 2010;183:603-7. [DOI] [PubMed] [Google Scholar]
- 24.Koraitim MM, Reda IS. Role of magnetic resonance imaging in assessment of posterior urethral distraction defects. Urology 2007;70:403-6. [DOI] [PubMed] [Google Scholar]
- 25.Sung DJ, Kim YH, Cho SB, et al. Obliterative urethral stricture: MR urethrography versus conventional retrograde urethrography with voiding cystourethrography. Radiology 2006;240:842-8. [DOI] [PubMed] [Google Scholar]
- 26.Osman Y, El-Ghar MA, Mansour O, et al. Magnetic resonance urethrography in comparison to retrograde urethrography in diagnosis of male urethral strictures: is it clinically relevant? Eur Urol 2006;50:587-93; discussion 594. [DOI] [PubMed] [Google Scholar]
- 27.El-Kassaby AW, Osman T, Abdel-Aal A, et al. Dynamic three-dimensional spiral computed tomographic cysto-urethrography: a novel technique for evaluating post-traumatic posterior urethral defects. BJU Int 2003;92:993-6. [DOI] [PubMed] [Google Scholar]