Abstract
Both Foxp3+CD4+ regulatory T cells (Treg cells) and local immune responses within non-lymphoid tissues have long been recognized as important elements of a well-orchestrated immune system, but only recently have these two fields of study begun to intersect. There is growing evidence that Treg cells are present in various non-lymphoid tissues in health and disease, that they have a unique phenotype, and that their functions go beyond the classical modulation of immune responses. Thus, tissular Treg cells might add yet another level to classification of the Treg cell compartment into functional and/or phenotypic subtypes. This review summarizes recent findings in this new field, discussing knowns and unknowns about the origin, phenotype, function and memory of non-lymphoid tissue-resident Treg cells.
Introduction
Regulatory T cells that express the Foxp3 transcription factor, affectionately termed Treg cells, are one of the immune system’s main bastions against inappropriate or over-exuberant responses. They control autoimmunity, allergic and inflammatory reactions, and responses to infectious agents and tumors. Over the past decade, innumerable studies have addressed differentiation of the majority of Treg cells in the thymus, generation of a minority in the periphery, homeostasis of the Treg cell compartment, and cellular and molecular mechanisms of Treg-mediated suppression1. For the most part, these explorations have taken the average Treg cell residing in the spleen or lymph nodes to be paradigmatic.
However, it eventually became impossible to ignore the considerable heterogeneity of the Foxp3+CD4+ compartment, especially once transcriptome analysis had become a routine tool2. Initially, Treg cell subphenotypes were delineated based on expression of activation or memory markers; adhesion molecules, notably CD103; or homing receptors. But a major advance was the discovery of Treg cell functional diversity matched to the type of response being reined in. A subtype of Treg cells was discovered that depends on the transcription factor IRF4 to control T helper (TH)2 cells, which also critically rely on IRF4 (ref.3). In parallel, a discrete CXCR3+ Treg cell subtype was found, dependent on the T-bet transcription factor, that is specialized in regulating the activities of TH1 cells, which also require T-bet for their differentiation and functions4. Treg cells that optimally regulate interleukin 17 (IL-17)- or IL-27-dependent responses may be yet different subtypes5, 6. The relevance of these various subtypes was serendipitously confirmed in a recent study showing a mutation of Foxp3 to dampen arthritis in an IL-17, IL-4 dependent mouse model, while exacerbating type-1 diabetes in NOD mice, a TH1-type disorder7. Another striking match between the cells that regulate and those that are regulated is found in germinal centers: follicular regulatory (TFR) and helper (TFH) cells both depend on Blimp-1 and Bcl6 for their differentiation/homeostasis and CXCR5 for their localization8, 9. The advantage of such a matching is probably that it provokes co-localization to and/or co-survival within discrete locations. Arming regulatory and effector cells with the same capabilities could be dangerous, but safeguards are in place, for example TH1-type Treg cells poorly up-regulate IL-12Rβ2 upon interferon-γ induction of STAT1, meaning that their differentiation to TH1 effector cells is aborted10.
This review focuses on studies that go one step further, highlighting the phenotype and functions – sometimes exquisitely adapted – of Treg cells residing in non-lymphoid tissues. We will survey the populations of tissue-resident Treg cells, focusing on a few particularly interesting examples; consider their origin; discuss potential cellular targets; and weigh the concept of Treg cell memory. Lastly, we will highlight some general principles and knowledge gaps to fill.
Tissue-resident Treg cells: the landscape
The presence of a distinct population of Treg cells has been documented in several non-lymphoid tissues of both mice and humans: skin, intestinal mucosa, lung, liver, adipose tissue, autoimmune target tissues, infected tissues, grafts, placenta, tumors, atherosclerotic plaques and injured muscle are just some examples (11–21, D.B., C.B. and D.M., manuscript submitted). It is clear from this extensive list that Treg cells can localize in healthy tissues, in tissues with various types and grades of inflammation, and in immunoprivileged sites. In every case where the comparison has been made, tissue-resident Treg cells are distinguishable from classical lymphoid-organ Treg cells in phenotype and function. While they display some features of activated/effector cells22, certain properties make each tissue-resident Treg cell population unique, such as by the expression of specific transcription factors, chemokine receptors or effector molecules; or by a distinct T cell receptor (TCR) repertoire, migration pattern, mechanism of action or targets.
Currently, one of the best-characterized examples of tissue-resident Treg cells is the population found in visceral adipose tissue (VAT)12. Treg cells are enriched in VAT of lean, aged, male mice, constituting more than 50% of the CD4+ T cell compartment at that site, a significantly higher fraction than the 5–15% routinely observed in lymphoid organs, including in aged individuals. Microarray-based gene-expression profiling, confirmed by flow cytometry analyses, revealed VAT Treg cells to have a unique phenotype. While they are bona fide Treg cells, as shown by their recapitulation of most of the classical Treg signature and by their functionality in standard in vitro suppression assays, they differentially express a panoply of genes in comparison with lymphoid-organ Treg cells. The set of loci overexpressed in VAT Treg cells includes those encoding several chemokine receptors (for example, CC chemokine receptor (CCR)1, CCR2, CCR9), immunomodulatory cytokines (such as IL-10), and the transcription factor peroxisome proliferator-activated receptor (PPAR)-γ, all of which confer VAT Treg cells with unique functional properties12, 23. As flow cytometry studies showed that some of those markers are not expressed by 100% of the fat Treg cells12, 23, it is not clear how heterogeneous the population is and whether this has functional significance or more prosaically marks cells at different cell-cycle or differentiation stages. Their TCR repertoire is another feature contributing to a distinct phenotype: the TCR sequences they employ have little overlap with those used by lymphoid-organ Treg cells (12 and Kolodin et al., personal communication). In addition, there is a striking expansion of certain Treg clones in the adipose tissue, suggesting that recognition of particular antigens may have an important role in the accumulation of Treg cells in abdominal fat (further discussed below).
The major driver of VAT Treg cell accumulation, phenotype and function is PPAR-γ23. A member of the nuclear receptor superfamily characterized as the “master regulator” of adipocyte differentiation and function24, PPAR-γ interacts with Foxp3 to promote up-regulation of the VAT Treg signature in in vitro transduction experiments. Mice lacking PPAR-γ only in Treg cells show a substantial and specific reduction in the frequency and number of Treg cells in VAT vis-à-vis their wild-type littermates, without any changes in lymphoid-organ Treg populations. Moreover, the few VAT Treg cells remaining in PPAR-γ mutant mice show an under-representation of the VAT Treg signature. The fact that PPAR-γ expression in VAT Treg cells is much higher than in anyother Treg population, together with the observation that they can take up lipids by expressing CD36, the scavenger receptor23, suggests that tissue-resident Treg cells are attuned to local cues, which they can exploit for phenotypic and functional specialization, as well as for preferential survival within the tissue microenvironment (Fig.1).
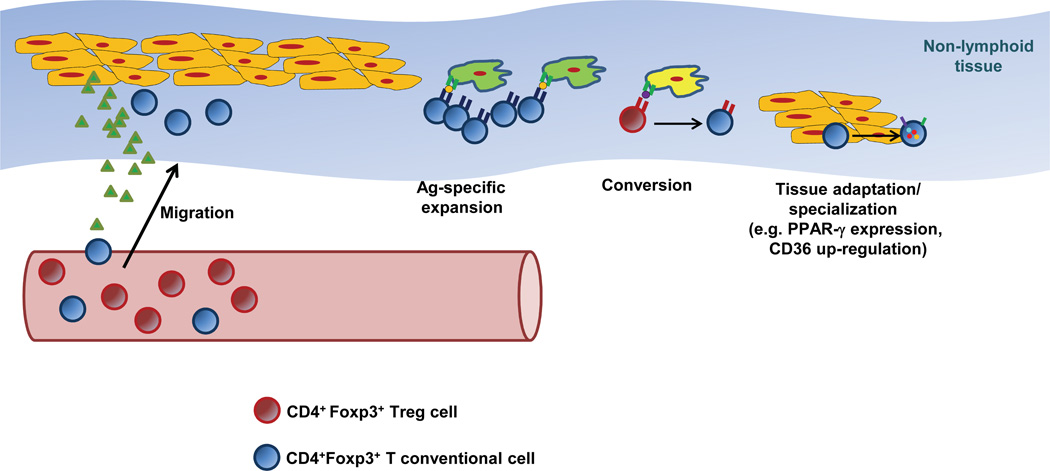
Figure 1. Mechanisms involved in the accumulation of Treg cells in non-lymphoid tissues.
Several mechanisms (alone or in combination) have been shown to increase the frequency and numbers of tissue-resident Treg cells: 1) Migration and retention of circulating Tregs, promoted by the expression of specific chemotactic molecules by parenchymal or stromal cells, and the expression of the specific receptors on Treg cells; 2) Expression of tissue-specific antigens that induce expansion of particular Treg clones; 3) Antigen-induced conversion of CD4+Foxp3− T conventional cells into pTregs; and 4) Acquisition of a tissue-specific phenotype that allows adaptation or specialization in the new environment, promoting survival and enhancing Treg functions.
Another tissue-resident population of great interest are Treg cells that infiltrate injured and regenerating skeletal muscle (D.B., C.B. and D.M., unpublished data). Within days after skeletal muscle injury (of various types), Treg cells start to accumulate locally, their frequency among CD4+ T cells increasing steadily to up to 50–60% and remaining at that frequency for weeks, long after local inflammation has resolved. Microarray-based gene-expression profiling revealed that muscle Treg cells have a unique transcriptome, more closely related to that of VAT Treg cells than to that of lymphoid-organ Treg cells. For example, genes encoding IL-10, amphiregulin (Areg) and T-cell immunoglobulin domain and mucin domain 3 (TIM-3) are highly expressed by Treg cells accumulated in muscle. Muscle Treg cells have a skewed TCR repertoire, which appears not to overlap with that of muscle conventional CD4+ T cells or of splenic Treg cells, and shows clear signs of clonal expansion. Interestingly, Treg cells also accumulate in skeletal muscle of dystrophic mice (such as dystrophin-deficient mdx mice and dysferlin-deficient mice), whose chronic muscle injury has a genetic origin.
Given recent successes with immunomodulatory anti-tumor strategies (see Gajewski et al. reviewed in this issue), there is a growing interest in the more heterogeneous group of tumor-infiltrating Treg cells. In solid tumors of non-lymphoid origin, Treg cells can account for 30–50% of CD4+ T cells, depending on the tumor type19. Like other tissue-resident Treg cells, those found in tumors have a distinctive phenotype that differentiates them from Treg cells found in the circulation or in lymphoid organs. Microarray-based gene-expression profiling of tumor-associated Treg cells is still lacking25, but Foxp3+CD4+ T cells from several mouse and human tumors show increased expression of cell-surface markers such as Cytotoxic T-Lymphocyte-Antigen 4 (CTLA-4), Inducible T-cell Costimulator, (ICOS), TIM-3, glucocorticoid-induced TNFR family related gene (GITR); as well as of suppressive cytokines such as IL-10 and transforming growth factor (TGF)-β; and a variety of chemokine receptors19, 26–29.
Who gets there, when and how?
Treg cells are found in healthy tissues, although usually in very small numbers11. Upon challenges of different types (autoimmunity, infection, injury), Treg cell numbers, and often their frequencies, increase considerably. Diverse mechanisms, not mutually exclusive, have been proposed to explain the accumulation of Treg cells at different tissular sites: chemokine-based recruitment of circulating Treg cells, local TCR- or cytokine-driven expansion, conversion of local or circulating conventional CD4+ T cells, or prolonged survival (Fig. 1). In some cases, for example tumor Treg cells, all of these mechanisms have been championed by one investigator or another19, 25, 26, 30–34.
As for other types of immune-system cells, recruitment of Treg cells to non-lymphoid tissues is governed by specific combinations of chemotactic molecules and their receptors (reviewed in35). Treg cells residing in various tissues express distinct patterns of chemokine receptors and adhesion molecules in comparison with their lymphoid-organ counterparts, both at single cell level and in terms of population frequencies11, 23. This finding is true even for Treg cells residing in non-inflamed tissues like skin, lung and liver, wherein an enrichment for CCR4+CD103 (integrin αE)+ Treg cells is observed11. Ccr4−/− Treg cells show impaired accumulation in healthy non-lymphoid tissues, and this dearth has functional consequences as mice defective in CCR4+ Treg cells develop severe skin inflammation and a less severe lung infiltrate. It seems that antigen-specific activation of Treg cells in subcutaneous lymph nodes under inflammatory conditions induces up-regulation of CCR4, priming Treg cells to migrate to the affected tissue and to suppress immune responses locally. However, the origin of the small fraction of CCR4+CD103+ Treg cells found in lymphoid tissues in the absence of antigen challenge remains unclear. In this regard, a study showed that, in humans, only few Treg cells express CCR4 at birth but the majority expresses the gut-homing receptor α4β7, while the reverse pattern is true for adults36. The switch commences between 1.5 and 3 years of age and correlates with a change from a naïve to memory Treg phenotype, which has been suggested to depend on the recognition of microbial antigens in the gut, although this point has yet to be definitively demonstrated.
In addition to CCR4, an array of chemokine receptors can be involved, usually in a redundant fashion, in the recruitment of Treg cells to non-lymphoid tissues under various inflammatory conditions37. In some cases, the expression of a particular receptor by tissue-resident Treg cells is directly associated with the type of tissue, while in others it is related to the type of T cell response (TH1, TH2, TH17) occurring in the tissue and the accompanying Treg cell polarization, for example, the preferential recruitment of CCR6+ and CXCR3+ Treg cells to sites with an ongoing TH17- or TH1-driven inflammation, respectively4, 5.
The pattern and timing of trafficking between lymphoid and non-lymphoid tissues is not well-defined in most cases. Interestingly, in a model of pancreatic islet transplantation, Treg cells first migrate from blood to the inflamed allograft, in a process dependent on CCR2, CCR4, CCR5 and P- and E-selectin ligands38. Upon activation in the allograft, they subsequently move to the draining lymph nodes in a CCR2-, CCR5-, and CCR7-dependent fashion. This sequential migration is required for an efficient suppression of alloimmunity, since impairment of either of the steps by abolishing expression of the relevant receptor(s) in Treg cells results in decreased graft survival.
Although not universally accepted, it has been suggested that the Treg-cell TCR repertoire is biased towards the recognition of self-antigens1, which could facilitate the localization of Treg cells in non-lymphoid tissues to prevent harmful autoimmune attacks, and collateral damage to the tissue resulting from inflammation or injury. Sequencing of the TCR repertoire of VAT Treg cells in either wild-type mice or a mouse line engineered to have only limited TCR diversity yielded several interesting observations (12, Kolodin et al, personal communication). First, the sequences expressed by VAT Treg cells have little overlap with those expressed by conventional CD4+ T cells, either in adipose tissue or in lymphoid organs, arguing against Treg cell conversion in this context. Second, there are striking clonal expansions of Treg cells in VAT even in standard mice. Lastly, in the “limited” line, it is possible to find examples, in the same or different individuals, of a repeated complementary-determining region (CDR)-3 protein sequence specified by different nucleotide sequences. These findings argue that VAT Treg cells are responding to one or more local antigens, whether they be derived from lipids or proteins. By combining an ovalbumin (OVA)-specific TCR transgenic model and the expression of OVA in the skin, a recent study showed that engineered expression of a self-antigen in a peripheral tissue can drive the activation and proliferation of TCR-transgenic Treg cells, which mediate resolution of organ-specific autoimmunity39. These antigen-specific Treg cells remain in the target tissue and are primed to attenuate subsequent autoimmune reactions when the antigen is re-expressed (discussed in the next section), suggesting that antigen recognition is important for tissue Treg cell function and memory.
A recent report provided the first clear identification of the antigen specificity of a natural population of tissue-resident Treg cells40. By sequencing the TCR repertoire of prostate-tumor-infiltrating Treg cells, the authors identified a TCR αβ pair recurrently enriched in independent mice, whose sequences they used to generate a TCR transgenic line. In tumor-free TCR-transgenic mice, Treg cells are found selectively in the prostate and its draining lymph nodes, suggesting that the cloned TCR is specific for an antigen expressed in the normal prostate in addition to the malignant tissue. Interestingly, selection of the TCR-transgenic Treg cells is dependent on expression of Aire in the thymus, thereby linking two major mechanisms of immunological tolerance - one central, one peripheral. Although similar demonstrations for other tissue-resident Treg specificities will be necessary before one can definitively generalize, this work supports the hypothesis that tissue-resident Treg cells are generated in the thymus in response to self-antigens, and that this TCR specificity facilitates their localization and amplification in non-lymphoid tissues, especially in pathological situations such as inflammation or cancer in which an enhanced self-antigen presentation can occur in the periphery.
While the majority of the Treg cells differentiates in the thymus, a small fraction of them can be generated in the periphery, by conversion of Foxp3−CD4+ T cells into Foxp3+CD4+ Tregs, termed “peripheral Treg cells” (pTreg cells) to distinguish them from their thymic-derived counterparts. There has been a lot of interest in conversion as a mechanism of Treg cell accumulation in non-lymphoid tissues, although few studies have clearly demonstrated that, in certain sites and/or conditions, pTreg cells constitute a substantial proportion of tissular Treg cells. The intestinal lamina propria Treg pool is one of the main populations of non-lymphoid-tissue Treg cells impacted by conversion, and pTreg cells have been shown to contribute substantially to oral tolerance (reviewed in41). Exposure to agonist administered orally and specific microbial products strongly induce conversion in the lamina propria, which is reflected in the increased frequency of Treg cells, as well as in the increased proportion of Helios− Treg cells in that tissue42–45. The analysis of a mutant mouse bearing a deletion in the CNS1 enhancer region of the Foxp3 locus necessary for peripheral induction confirmed previous observations on lamina propria Treg cells and suggested that the accumulation and function of pTreg cells might be important at mucosal and feto-maternal interfaces, and perhaps little else17, 46, 47. Different populations of tolerogenic antigen-presenting cells promote Foxp3 induction at mucosal surfaces, such as CD103+ dendritic cells that produce retinoic acid in the intestinal lamina propria42, 48, and tissue-resident macrophages in the lung mucosa49. Interestingly, a report showed that neurons can elicit conversion of encephalitogenic Foxp3−CD4+ T cells into Treg cells in vitro, although evidence for in vivo conversion was weak50. The generation of pTreg cells has also been proposed to play a role in the accumulation of Treg cells in tumors34, 51, although the exact contribution of this mechanism versus recruitment/expansion of thymic-derived Treg cells remains controversial25, 41. In fact, a study using a mouse tumor model showed that both mechanisms can coexist and that the induction of tumoral pTreg cells is intrinsically influenced by the tumor microenvironment and the presence of a tumor-specific antigen51.
Lastly, the question remains as to how and when the few Treg cells found in healthy, unchallenged tissues are recruited to these sites. It is possible that a combination of specific expression of chemokine receptors and antigen specificity constantly brings a small number of Treg cells to the tissues throughout life. Alternatively, early seeding with Treg cells precursors during fetal life might occur, paralleling the phenomenon observed in tissue macrophages52, followed by self-renewal of the tissue-resident Treg cells. In theory, seeding during embryonic life could occur after or before thymic development, although extrathymic differentation of TCRαβ T cells remains controversial.
Immunological and non-immunological targets
One of the major functions of Treg cells residing in non-lymphoid tissues is to control local inflammation. Since their initial discovery, Foxp3+CD4+ cells were recognized to be potent suppressors of T cell responses, and this activity has been extended to tissular Foxp3+ cells. In addition, tissue-resident Treg cells can strongly impact myeloid populations in the vicinity, inhibiting neutrophils and pro-inflammatory macrophages, and promoting the activities of anti-inflammatory macrophages and monocyte subsets (Fig. 2).
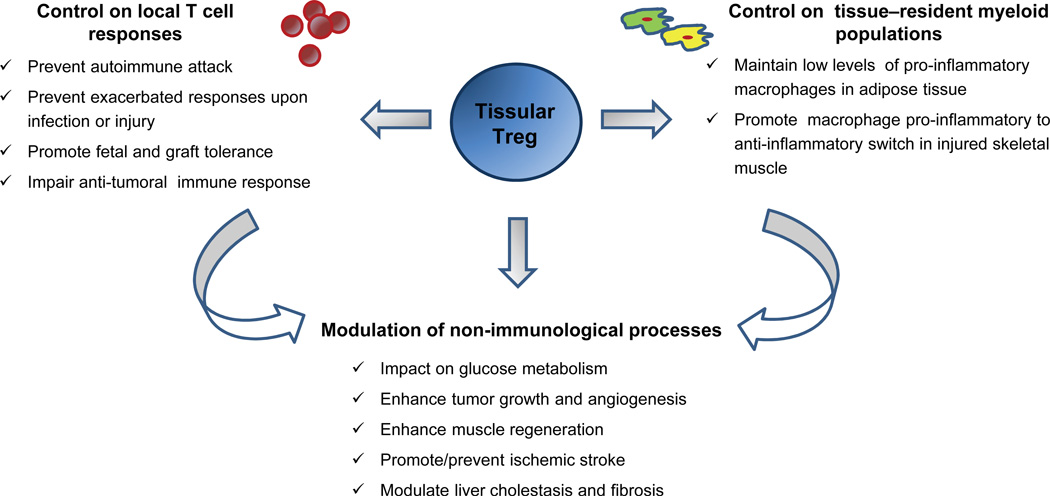
Figure 2. Functions of tissue-resident Treg cells.
The functions of tissue resident Treg cells can be divided in three main groups (representative examples of each group are listed in the figure): control on local T-cell responses, control on tissue-resident myeloid populations and modulation of non-immunological processes. The latter may occur by direct interaction between Treg cells and their non-immune targets, or indirectly through the regulation of other tissue-resident leukocytes which in turn could affect those targets.
As a particular example, VAT Treg cells may influence a broad spectrum of targets. Their elevated expression of factors like IL-10 and CTLA-4, and their effectiveness in the typical in vitro suppression assay12 suggest that VAT Treg cells are capable of controlling conventional CD4+ and CD8+ T cell populations in the adipose tissue, although in vivo experiments to substantiate this point are still lacking. They may also control co-resident myeloid cells, as suggested by an inverse correlation between the frequency of Treg cells and that of pro-inflammatory myeloid populations (CD11b+CD11c+F4/80+ macrophages and pro-inflammatory CD11b+Ly6chi monocytes) observed in VAT depots23, 53, 54. Similarly, muscle Treg cells are important in controlling the pro-inflammatory to anti-inflammatory switch that occurs in the myeloid infiltrate of injured muscle; punctual ablation of Treg cells at the time of muscle injury results in prolonged accumulation of pro-inflammatory Ly6chi monocytes in the injured tissue (D.B., C.B. and D.M., unpublished data).
Because of the ability of Treg cells to dampen immune responses that can eradicate malignancies, their accumulation in tumors is considered to be a tumor ‘escape’ mechanism. Tumor-infiltrating Treg cells can be as effective as peripheral-blood Treg cells in T cell-suppression assays30 or even significantly better29; and, in many cases, a high frequency of intra-tumoral Treg cells is correlated with poor prognosis30, 55, 56. On the other hand, Treg cells can also have anti-tumoral effects by inhibiting inflammation in the tumor environment. A strong link between inflammation and cancer exists in many tissues: inflammation can promote proliferation and survival of malignant cells, angiogenesis and metastasis57, 58. In line with these observations, in certain tumors, like colorectal cancers, a high density of Treg cells correlates with a favorable prognosis59, 60. It has recently been proposed that Treg-mediated suppression of pro-inflammatory TH17 responses to the dense microbiome of the large intestine is key to preventing tumor growth in the intestinal epithelium60.
There is growing evidence that Treg cells (in particular those residing in tissues) not only control T lymphocytes and other immune-system cells, but also regulate certain non-immunological processes, including systemic metabolic indices12, 23, 54, 61, ischemic stroke62, 63, formation of atherosclerotic plaques64, 65, cardiac remodeling after myocardial infarction66, liver cholestasis and fibrosis67 and skeletal muscle regeneration (D.B., C.B. and D.M., manuscript submitted). It is still unclear to what extent such influences reflect direct interaction between Treg cells and their non-immunological target cells or an indirect effect of Treg suppression of local inflammation (Fig. 2). In any case, these non-traditional roles can have profound impact on both homeostatic and pathophysiological processes and should be considered an important facet of Treg cell function.
An unusual property of VAT Treg cells is their control of metabolic indices, inhibiting local and systemic insulin resistance and glucose intolerance. VAT of obese individuals exhibits low grade, chronic inflammation which has been directly linked to the appearance of metabolic abnormalities, ultimately type 2 diabetes and the metabolic syndrome68, 69. That the frequency of VAT, but not lymphoid-organ Treg cells drops severely upon feeding mice with a high-fat diet (HFD) or in genetic models of obesity first suggested that this Treg subset might influence local and/or systemic metabolism12, 53. Loss-of-function experiments, relying on punctual and specific Treg ablation in a Foxp3-DTR mouse model, demonstrated increases in inflammatory mediators in the visceral fat depot, a decrease in insulin-stimulated insulin receptor tyrosine phosphorylation in VAT and the liver, and insulin resistance upon loss of Treg cells12. Conversely, gain-of-function experiments, which entailed treatment of HFD-fed mice with IL-2–anti-IL-2 complexes, resulted in enhanced IL-10 expression in VAT and improved insulin sensitivity and glucose tolerance. These results were mirrored in a more recent report on experiments manipulating PPAR-γ expression or signaling: mice devoid of PPAR-γ only in Treg cells show a strong reduction in VAT Treg cells and degradation of metabolic indices, while HFD-fed wild-type mice injected with a PPAR-γ agonist, pioglitazone, have a substantially more robust Treg cell population and metabolic improvements. Strikingly, and unexpectedly, the restoration of metabolic indices typically induced by treatment of HFD-fed mice with pioglitazone is only very partial in mice lacking PPAR-γ specifically in Treg cells23, demonstrating not only that the beneficial effects of this drug occur in part by targeting VAT Treg cells, but also that VAT (and not lymphoid) Tregs are important players in the control of the metabolic indices downstream of obesity and inflammation of the adipose tissue. At least part of this Treg cell influence may be directly on adipocytes, as one of their major mediators, IL-10, can engage receptors on adipocytes to down-regulate pro-inflammatory cytokines and increase glucose uptake.
Treg cells that accumulate in injured skeletal muscle also seem to impact non-immunological processes, in this case tissue regeneration (D.B., C.B. and D.M., unpublished data). Mice punctually depleted of Treg cells mice show impaired muscle repair according to multiple criteria, notably the functionality of satellite cells, key players in the regeneration of skeletal muscle. Muscle Treg cells overexpress Areg, a growth factor that induces in vitro differentiation of satellite cells, which express Areg receptors (D.B., C.B. and D.M., manuscript in preparation), suggesting that local Treg cells could have a direct effect on skeletal muscle precursor cells.
The influence of Treg cells on the pathophysiology of ischemic stroke provides yet another example of non-immune processes regulated by Tregs. However, in this case, opposite effects have been reported by different investigators, so whether Treg cells are beneficial or detrimental to the outcome of ischemic stroke remains controversial. One study showed that Treg cells can be found in the post-ischemic brain; and that their depletion profoundly enhances brain damage and exacerbates the functional outcome62. IL-10 production by Treg cells may be their major mechanism of immunomodulation in the brain and of Treg -mediated cebroprotection. Unfortunately, these results conflict with another study published subsequently that indicated that indicating that Treg cells promote acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature63. Notably, this negative impact of Treg cells on recovery from stroke is unrelated to their classical immunoregulatory functions; in fact, the CD4+ T cell and macrophage populations infiltrating the ischemic tissue are not affected by the depletion of Treg cells. Rather, the negative influence seems to result from an interaction between Treg cells and activated cerebral endothelial cells and platelets. These contradictory findings on the role of Treg cells in stroke issue from different models of Treg depletion (anti-CD25-mediated depletion vs a Foxp3-DTR line), by the different stroke models used (permanent vs temporal; different ischemia times), or the timing of read-out (late vs acute stages).
A liaison between Treg cells and endothelial cells has also been observed within the tumor microenvironment, in this case reflecting a pro-angiogenic effect by tumor-infiltrating Foxp3+CD4+ cells70. Tumor hypoxia promotes the recruitment of Treg cells through induction of expression of the chemokine, CC-chemokine ligand 28 (CCL28), in tumor cells. Elevated numbers of Treg cells expressing CCR10 accumulate in CCL28-positive tumors, which exhibit increased growth and angiogenesis in comparison with CCL28-negative tumors. Angiogenesis is dependent on the presence of tumor-infiltrating Treg cells, which not only enhance expression of vascular endothelial growth factor A (VEGFA) in tumor cells, but also directly contribute to the tumor VEGFA pool by producing the pro-angiogenic factor themselves70. Tumor-infiltrating Treg cells also promote mammary carcinoma metastasis in the lung by producing receptor activator of nuclear factor kappa-B ligand (RANKL)71. Thus, the impact of tumor-resident Treg cells on tumor growth and patient prognosis reflects both traditional (on immune targets) and non-traditional (on non-immune targets) Treg functions. Dissection of the individual pathways regulated by tissue Treg cells will be important for the design of effective and specific therapies aimed at modulating Treg cells and their products in cancer and other diseases.
Treg cell memory in tissues
The generation of memory is one of the hallmarks of adaptive immunity, and it is becoming clear that Treg cells are no exception to the rule. During viral infections, the expansion of antigen-specific Treg cells is followed by a contraction phase and the formation of a memory pool72, 73. Upon re-infection, these memory Treg cells rapidly expand and effectively control specific anti-viral responses by CD4+ and CD8+ T cells. An accelerated accumulation of memory Treg cells is observed in the non-lymphoid tissues targeted by the infection; furthermore, memory Treg cells are capable of suppressing the collateral tissue damage and inflammation elicited by recall expansion of non-Treg memory CD4+ T cells. A link between Treg memory and tissue-resident Treg cells has also been demonstrated in the context of autoimmunity39. Using a TCR–neo-self-antigen (OVA) double-transgenic model, a recent study showed that upon local induction of ovalbumin expression, ovalbumin-specific Treg cells accumulate in the skin. After resolution of the inflammatory response, activated Treg cells remain in the target tissue in high frequencies and are primed to attenuate subsequent autoimmune responses when the antigen is re-expressed. These memory Treg cells can survive in the non-lymphoid tissues in the absence of overt expression of antigen, and show enhanced functional activity.
In human skin, resident Treg cells comprise 5–10% of total skin T cells74. These Tregs express the memory T cell marker CD45RO, and are thus typically referred to as ‘effector-memory Treg cells’, although their true memory nature has not been formally demonstrated. They reside preferentially in the epidermis, where Langerhans cells (LCs) induce their proliferation in an MHC-class-II- and IL-15-dependent manner75. It has been proposed that self-peptides or the normal skin microbiome might be the source of antigens presented by LCs. Human skin-resident Treg cells also proliferate in response to pathogens75 and during the recall response induced by injection of tuberculin purified-protein derivative (PPD) in individuals immunized with bacille Calmette-Guérin (BCG)76.
Memory Treg cells seem also to have an important function in preventing fetal rejection in successive pregnancies77. Fetal-antigen-specific Treg cells, generated by conversion of Foxp3−CD4+ T cells, expand >100 fold through parturition, remain at enriched numbers after delivery, and re-accumulate with accelerated kinetics during subsequent pregnancies. The precise localization of memory Treg cells in feto-maternal non–lymphoid tissues remains unknown. Although in the case of pregnancy the target tissue is present only transiently, and memory Treg cells seem to be maintained in lymphoid organs, their fetal-antigen specificity and existing evidence of the presence of Treg cells in placenta and utero78–80 suggest that memory Treg cells might migrate to these tissues in recurrent pregnancies to exert their protective role.
Thus, evidence suggests that there is a close association between tissue-resident Treg cells and memory Treg cells. The generation of regulatory memory is emerging as an important factor to control the accelerated and enhanced recall responses to secondary exposure to antigens. The localization of memory Treg cells in the target non-lymphoid tissues seems to be key for their function and, in some cases, for the maintenance of the memory Treg cell pool during the remission period.
Perspectives
Tissue-resident Treg cells is a relatively new area of Treg cell biology. It is not surprising, then, that some key questions remain unanswered, or even unaddressed. We would like to highlight three questions that particularly intrigue us:
First, how widespread is the phenomenon? Given the distinct Treg cell compartments already found to be associated with adipose tissue, muscle, the colon, etc, it is theoretically possible that there is a specialized type of Treg cell dedicated to maintaining homeostasis within each tissue. Or it might be that evolution has provided dedicated Treg cell types only for those tissues particularly prone to insult (for example, the skin and muscle) or in especially close communication with the environment (such as the skin and the gut). An alternative possibility is that there are a limited number of Treg cell types and each tissue Treg compartment represents a different optimum mix of the various subsets – that is what we are seeing are really only unique “averages.” A broader survey of tissue Treg cells, especially in single-cell-analysis mode, should yield critical information bearing on this question. It may also prove informative to compare Treg compartments from tumors and their matched normal tissues.
Second, where do tissue-resident Treg cells come from? So far, it seems clear that, except for the case of the gut and placental compartment, tissue Treg cells do not emanate from conversion of conventional Foxp3−CD4+ T cells. It is perhaps simplest to envisage that a precursor cell, already Foxp3+ but not committed to a particular Treg cell sub-phenotype, gets retained within a particular tissue, likely because its TCR reacts to an antigen therein and, in response to tissue-specific cues, takes on a tissue-specific sub-phenotype. But it is difficult to rule out the possibility that small numbers of precursors precommitted to a particular Treg cell sub-phenotype are generated in the thymus and monitor their corresponding peripheral tissues, either as residents or as members of the circulating T cell pool. Such a scenario is reminiscent of the recent finding that populations of tissue-resident macrophages are generated before birth and are important elements in enforcing tissue homeostasis52. Perhaps these macrophages need Treg cell monitors to keep them in line. Identifying the origin of tissue-resident Treg cells would be aided by generating appropriate inducible lineage-tracer mice, for example in PPAR-γ reporters for VAT Treg cells.
Third, what is the advantage of having unique populations of tissue-resident Treg cells? It makes perfect evolutionary sense to optimize Treg compartments for both effective survival and appropriate effector activities. This is especially true for Treg cells that will reside in a given tissue long-term, either as tissue-resident sentries or in response to chronic insult. For example, adipose tissue is an environment not generally hospitable for lymphocytes – VAT Treg cells co-opting of PPAR-γ provides these cells with properties (such as expression of the lipid transport, CD36) that promote survival in adipose tissue. Similarly, high-level expression of Areg by Treg cells in regenerating tissues (for example muscle or intestine) arms them with the capacity to favorably impact their local environment. We wonder how common it is for tissue-resident Treg cells to co-opt transcriptional programs characteristic of tissular cells (like VAT Treg use of PPAR-γ, the “master-regulator” of adipocytes), and whether there might be something special about the chromatin organization or epigenetic make-up of Treg cells that favors such adaptability.
Interesting realms to explore!
Acknowledgments
The lab’s work on tissue Treg cells is supported by NIH Grants R01DK092541 and R37AI051530 (to C.B. and D.M.). DB was supported by a Kaneb Fellowship.
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall AO, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darce J, et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36:731–741. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch MA, et al. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LT, Jacobs J, Mathis D, Benoist C. Where FoxP3-dependent regulatory T cells impinge on the development of inflammatory arthritis. Arthritis Rheum. 2007;56:509–520. doi: 10.1002/art.22272. [DOI] [PubMed] [Google Scholar]
- 15.Suffia IJ, et al. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee I, et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samstein RM, et al. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilburgs T, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 19.Tanchot C, et al. Tumor-Infiltrating Regulatory T Cells: Phenotype, Role, Mechanism of Expansion In Situ and Clinical Significance. Cancer Microenviron. 2012 doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Boer OJ, et al. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS. ONE. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng X, et al. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol. Med. 2012;18:598–605. doi: 10.2119/molmed.2011.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol. 2013;34:74–80. doi: 10.1016/j.it.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Cipolletta D, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 25.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gobert M, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 27.Gao X, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS. ONE. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park HJ, et al. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012;278:76–83. doi: 10.1016/j.cellimm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Strauss L, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 30.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 31.Tan MC, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quezada SA, et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp. Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuczma M, et al. Intratumoral convergence of the TCR repertoires of effector and Foxp3+ CD4+ T cells. PLoS. ONE. 2010;5:e13623. doi: 10.1371/journal.pone.0013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu VC, et al. Tumor evasion of the immune system by converting CD4+ J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Xu J, Bromberg JS. Regulatory T cell migration during an immune response. Trends Immunol. 2012;33:174–180. doi: 10.1016/j.it.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grindebacke H, et al. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J Immunol. 2009;183:4360–4370. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenblum MD, et al. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–542. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 42.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haribhai D, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J. Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soroosh P, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp. Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 51.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 52.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deiuliis J, et al. Visceral adipose inflammation in obesity is associated with critical alterations in T regulatory cell numbers. PLoS. ONE. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilan Y, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bates GJ, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 56.Perrone G, et al. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur. J Cancer. 2008;44:1875–1882. doi: 10.1016/j.ejca.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Colotta F, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 58.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 60.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol. Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eller K, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liesz A, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 63.Kleinschnitz C, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2012 doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ait-Oufella H, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 65.Klingenberg R, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang TT, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 67.Katz SC, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187:1150–1156. doi: 10.4049/jimmunol.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 69.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 70.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 71.Tan W, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez AM, Zhu J, Huang X, Yang Y. The development and function of memory regulatory T cells after acute viral infections. J Immunol. 2012;189:2805–2814. doi: 10.4049/jimmunol.1200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brincks EL, et al. Antigen-Specific Memory Regulatory CD4+Foxp3+ T Cells Control Memory Responses to Influenza Virus Infection. J Immunol. 2013;190:3438–3446. doi: 10.4049/jimmunol.1203140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seneschal J, et al. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vukmanovic-Stejic M, et al. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev. Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 79.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ 'effector' regulatory T cells in the gravid uterus. Proc Natl Acad Sci U S A. 2007;104:594–599. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perez Leiros C, Ramhorst R. Tolerance induction at the early maternal-placental interface through selective cell recruitment and targeting by immune polypeptides. Am. J Reprod. Immunol. 2013;69:359–368. doi: 10.1111/aji.12087. [DOI] [PubMed] [Google Scholar]