Abstract
Background
Conventional pharmaco-cavernosography provides little information on penile venous anatomy, although it is indispensible in documenting veno-occlusive erectile dysfunction (ED). We propose an innovative method, which may provide additional insight into the penile venous structure.
Methods
From July 2010 to November 2012, 96 impotent men, aged 20 to 75 years, underwent this method of pharmaco-cavernosography in which two sets of 60 mL of 50% omnipaque solution administered intracavernously by themselves. The first set of pilot cavernosograms was taken at intervals of five, ten, twenty and thirty seconds after the commencement of the injection. The second set of cavernosograms was taken in the same intervals within 30 minutes following the pilot set, preceded by the injection of 20 µg prostaglandin E1 (PGE1). Analysis was conducted on the drainage veins including deep dorsal vein (DDV), cavernosal veins (CVs) and para-arterial veins (PAVs) accordingly. The veins demonstrated in the pilot cavernosograms, and the second set, were compared in terms of venous numbers and presentation percentage.
Results
There was a statistically significant difference (P<0.001) between the total number of independent venous drainage channels and the presentation percentage of DDV, CVs and PAVs observed in the pilot cavernosograms, and those in second set (4.5 vs. 2.1; 97.47%, 60.33%, and 38.91% vs. 57.06%, 29.34%, and 19.08%, respectively).
Conclusions
Compared with conventional pharmaco-cavernosography methods, pilot cavernosograms are readily able to show detailed penile venous anatomy. It is therefore may be concluded that pilot cavernosograms is a valuable addition to conventional protocols of pharmaco-cavernosography.
Keywords: Pilot cavernosograms in pharmaco-cavernosography, cavernosography in the human penis, deep dorsal vein (DDV), cavernosal vein (CV), para-arterial veins (PAVs)
Introduction
In clinical medicine, imaging may be the most optimal tool for investigating patient’s anatomical and physiological status, and to inform decisions regarding therapeutic intervention (1-4). Knowledge of anatomy can be gained from dissection, however in most cases this applies to non-living tissue. In contrast, imaging is primarily applicable to live subjects. Imaging may drive the advancement of anatomy and vice versa, although the methods employed need to adequately capture the anatomical detail, otherwise a discrepancy may exist between the two. The drainage venous system of the corpora cavernosa may be one of the rare cases in medical history (5) of such a discrepancy.
The human penis has long been the subject of many exhaustive studies and its vascular anatomy is commonly well established (6,7). Since 1970s the imaging cavernosography has been in use and consequently the penile venous anatomy is recognized (8-12). The penile veins are currently regarded as superficial, intermediate and deep according to their respective position in each anatomical layer (13). Among them there is a single deep dorsal vein (DDV) flanked on either side by a pair of dorsal artery between the Buck’s fascia and the tunica albuginea (14). However, a recent anatomical exploration associated with an imaging study has reported that in addition to the DDV, there also exists a pair of cavernosal veins (CVs) and two pairs of para-arterial veins (PAVs), each of which has its own respective set of emissary veins drained from the sinusoids of the corpora cavernosa (15).
Conventional methods of pharmaco-cavernosography are not able to show the penile venous distribution in detail due to the fact that most venous vasculature are masked in it. Thus, conventional pharmaco-cavernosography is unable to provide proper information regarding penile venous anatomy to researchers, clinicians and patients, and may prevent its further study. In this study we aim to discover whether the use of pilot films may provide more anatomical information than conventional cavernosograms.
Patients and methods
From July 2010 to November 2012, erectile dysfunction (ED) prompted 125 men, aged 20 to 77 years, to consult us. These patients received multidisciplinary diagnostic approaches. Patients were excluded from undergoing cavernosography if they had an untreated chronic systemic disease (e.g., diabetes mellitus, chronic liver disease, renal failure, hormonal insufficiency, psychoneurotic disorders, etc.) or other obvious aetiologies such as prostate surgery, major pelvic surgery and trauma. Subjects are included only if they experienced ED for at least one year and excluded if they had a medical history of either a congenital vascular disorder or Peyronie’s Disease. Among these 96 males underwent this dual pharmaco-cavernosography. A solution of 25 mL Iohexol (Omnipaque, GE Healthcare, Cork, Ireland) and 35 mL normal saline in a 60 mL syringe was connected to a 19-gauge scalp needle. The needle was firmly affixed to the lateral aspect of the penile shaft, which was stretched in the meantime. A scout film was first undertaken after 7-8 mL solution was injected in all patients via an anterior-posterior (AP) view (Figure 1), then patients were placed in a 30-degree right oblique position while lying on a reclined board. The set (Figure 2A,B,C) of pilot cavernosograms was first taken serially at 5.0, 10.0, 20.0 and 30.0 seconds while the 60 mL solution was being injected by the patient himself. The second set was made within 30 minutes following the injection of 20 µg prostaglandin E1 (PGE1) via the same intracavernous route to document veno-occlusive dysfunction (VOD) if venous channels (Figure 2D) were shown during a rigid erection following an infusion of iohexol solution and, if necessary, 60-120 mL additional normal saline was used to induce erection if PGE1 failed to achieve so.
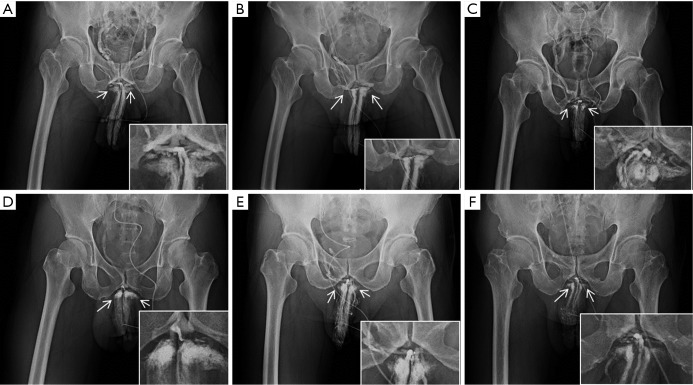
Figure 1.
Representative scout films of anterior-posterior (AP) view. (A) On a 64-year-old male, a scout film was taken when 7-8 mL solution was injected. There are seven independent drainage channels tangentially leaving the corpora cavernosa at the penile hilum. The penile crura (arrows) were important reference points in all films; (B) the reduction of the number of drainage channels to one after surgery is shown; (C) an AP film of a 57-year-old man showing eight drainage channels; (D) an AP film of a 45-year-old man showing four drainage channels; (E) an AP film of a 61-year-old man showing five drainage channels; (F) an AP film of a 31-year-old man showing 6 drainage channels.
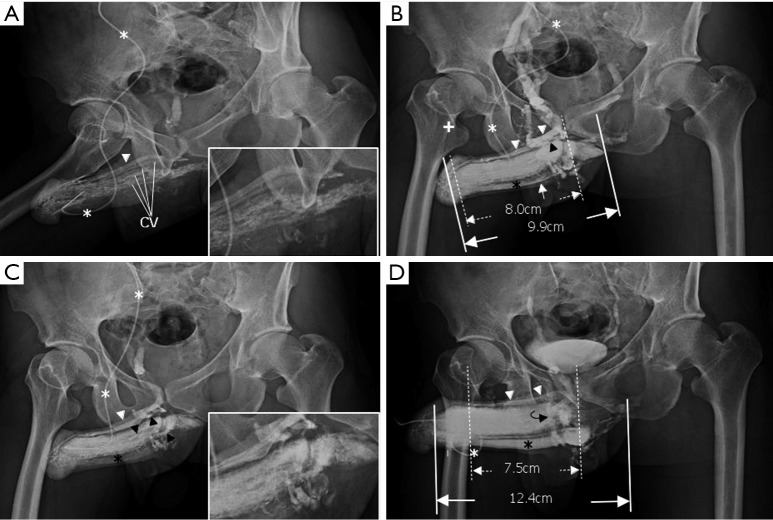
Figure 2.
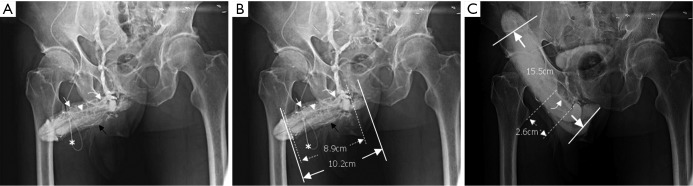
Representative cavernosography of a 32-year-old male in the cavernosal vein group. (A) While the tip of a 19-gauge scalp needle (white asterisk) was positioned at the lateral aspect of the right corpus cavernosum, four independent channels of the cavernosal veins and the deep dorsal vein (white arrowhead) were visible; (B) four cavernosal veins (black arrowheads) and the deep dorsal vein (white arrowhead) were enhanced. The corpus spongiosum (black asterisk) was identifiable. Even two penile crura could be differentiated in addition to the septum; (C) those tissues were enhanced and the bulbo-urethral vein (white arrow) was visible; (D) the assembly of the deep dorsal vein (white arrowhead), a circumflex vein (black curved arrow) and the corpus spongiosum (black asterisk) were presented when an artificial erection was made.
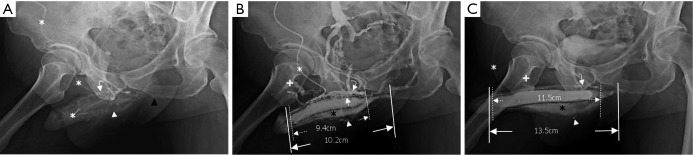
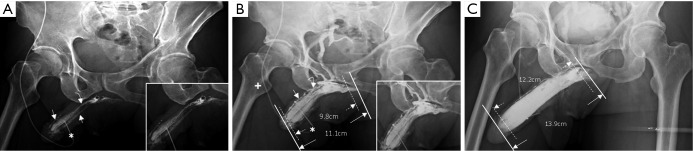
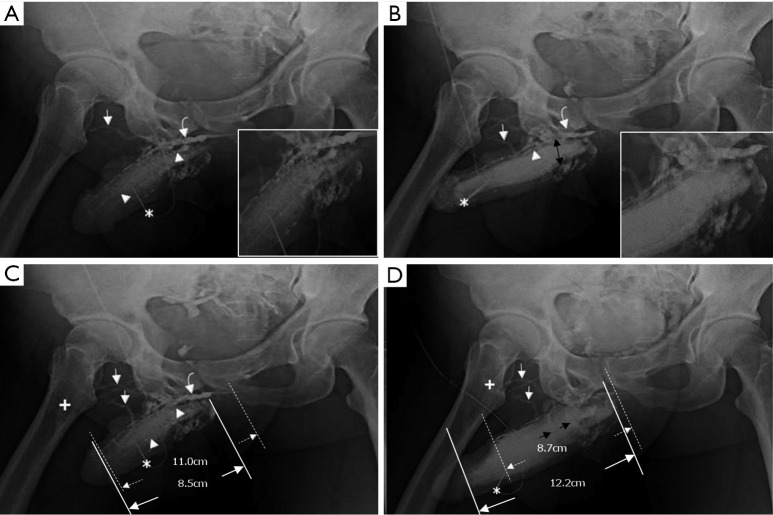
The total number of venous drainage channels consists of those channels leaving tangentially the corpora cavernosa independently by the AP view at the penile hilum demarcated with penile crura (Figure 1). In both the first (Pilot cavernosograms) and second (Pharmaco-cavernosograms) (Figure 2C vs. 2D; Figure 3A,B vs. 3C; Figure 4A,B vs. 4C; Figure 5A,B vs. 5C; Figure 6A,B vs. 6C; Figure 7A,B,C vs. 7D respectively) sets of cavernosograms the maximal length of the opacified corpus cavernosum (X) and the DDV (Y) was measured in centimeters. Subsequently the presentation percentage was derived from Y dividing X in %. The same calculations were conducted for the CVs and PAVs when these were present. When an explanation of these films was made to patients, it was intentionally initiated from the second set documenting the VOD if it existed (N=85). Then the first set of cavernosograms was subsequently disclosed, and patients were asked whether the films benefited their understanding.
Figure 3.
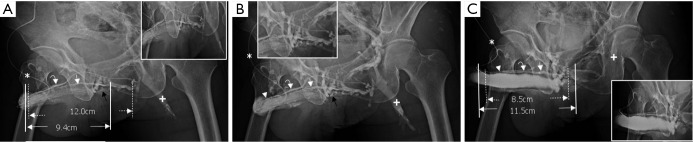
Cavernosograms of a 56-year-old man in the deep dorsal vein group with conspicuous corpus spongiosum. (A) While the tip of a 19-gauge scalp needle (white asterisk) was positioned in the corpora cavernosa, the deep dorsal vein (white curved arrow) and the bulbo-urethral vein (white arrowhead) were shown after injection of the contrast medium; (B) those veins were enhanced while the corpus spongiosum (black asterisk) was conspicuously demarcated. In addition the cavernosal vein (white arrow) was presented beneath the deep dorsal vein (DDV) (between dotted lines) and the left corpus cavernosum (between lines). Note that the right femoral vein (cross) was well-opacified; (C) the deep dorsal vein (white curved arrow and also between dotted lines), part of the bulbo-urethral vein (white arrowhead) were still presented and the corpus spongiosum (black asterisk) was filled when the introduction of an artificial erection. Again the corpus cavernosum (between lines) was measured.
Figure 4.
Cavernosograms of a 57-year-old man in the para-arterial vein group. (A) While the tip of a 19-gauge scalp needle (white asterisk) was positioned in the corpus cavernosum, the para-arterial veins (white arrow), the right cavernosal vein (white arrowhead) and the deep dorsal vein (white curved arrow) were shown following injection of the contrast medium (white asterisk); (B) the para-arterial veins (white arrow) and the deep dorsal vein (white curved arrow) were shown more prominently while the deep dorsal vein and the cavernosal vein were seen beneath the symphysis pubis, and the right femoral vein (white cross) was seen as well; (C) the deep dorsal vein (white curved arrow) and the para-arterial veins (white arrow) with few circumflex veins were presented while the right femoral vein (white cross) was well-demonstrated.
Figure 5.
Cavernosograms of a 66-year-old man in the bulbo-urethral vein group. (A) The bulbo-urethral vein (black arrow), the deep dorsal vein (white curved arrow), the cavernosal vein (white arrowhead) and the para-arterial veins (white arrow) were shown while the 19-gauge scalp needle (asterisk) was used to inject the contrast medium. A parallel venous complex of the deep dorsal vein and the cavernosal vein system was noted beneath the symphysis pubis; (B) those veins were enhanced. The glans penis was significantly opacified as shown in panels A and C; (C) after waiting 2 minutes to drain the contrast medium, the para-arterial veins (white arrow), the cavernosal vein (white arrowhead), and the bulbourethral vein (black arrow) were still shown although the parallel venous complex beneath the symphysis pubis faded away.
Figure 6.
Cavernosograms of a 67-year-old man in the superficial dorsal vein group. (A) While the tip of a 19-gauge scalp needle (white asterisk) was positioned in the right corpus cavernosum, the superficial dorsal vein (white curved arrow), the deep dorsal vein (white arrow) and the cavernosal vein (black arrow) were shown after injection of the contrast medium. The left femoral vein (white cross) was well-opacified; (B) those veins were enhanced while the para-arterial vein (arrowhead) was clearly shown; (C) the superficial dorsal vein (white curved arrow), the deep dorsal vein (white arrow), the para-arterial vein (white arrowhead) and the left femoral vein (cross) were still demonstrated despite the introduction of an artificial erection.
Figure 7.
Cavernosograms of a 45-year-old man in the deep dorsal vein group. (A) While the tip of a 19-gauge scalp needle (white asterisk) was positioned in the right corpus cavernosum, the deep dorsal vein (white curved arrow), the superficial dorsal vein (white arrows), and the cavernosal vein (white arrowhead) were shown after injection of the contrast medium. Several huge circumflex veins presented proximally; (B) those veins were enhanced while the right femoral vein (cross) was poorly opacified; (C) the venous complex of deep dorsal vein (white curved arrow), cavernosal veins (white arrowheads) and several superficial dorsal veins (white arrows) were clearly demonstrated. There was an extraordinarily complex venous plexus (black double arrows) composed of circumflex veins beneath the penile base; (D) the circumflex veins (black arrows) seemingly drained the cavernosal sinusoidal bloods to the right femoral vein (white cross) via several superficial dorsal veins (white arrows) when an artificial erection was induced.
Statistically, the paired t-test was used for parameters with continuous values and Fisher’s exact test with discontinuous parameters.
Results
Table 1 summarizes the demographic data of the 96 males. In all males the number of independent drainage channels varied from 3-8 (average 4.5), and 1-3 (average 2.1), in the pilot set and second set of cavernosograms respectively. The number of patients who presented the specific vessels on DDV, CVs and PAVs were 96, 87, and 75 vs. 75, 27, and 7 men corresponding to the first (pilot) set and second set of films, respectively. There was statistically significant difference (P<0.001) between the presentation percentage of DDV, CVs and PAVs in the first set and second set (97.46%, 60.28%, 38.96% vs. 57.05%, 29.29%, 19.04%, respectively). In all males, the presentation of the aforementioned veins is consistently of overwhelming superiority in the first set. In addition, a direct shunt to the superficial dorsal vein (SDV) and bulbo-urethral vein (BV) was shown in 19 (28.8%) and 25 (37.9%) men, respectively.
Table 1. Summary of 96 patients whom underwent pharmaco-cavernosography.
| Grouping | No. of patients | No. of independent drainage channels b average/range | Corpus cavernosum (X) |
Deep dorsal vein (Y) |
Cavernous vein (Y) |
Para-arterial vein (Y) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length in cm | Length in cm | No./% patient shown | Length in cm | No./% patient shown | Length in cm | No./% patient shown | ||||||
| First set | 96 | 4.5/3-8 | 7.88 | 7.68 | 96/97.46 | 4.75 | 87/60.28 | 3.07 | 57/38.96 | |||
| Second set | 96 | 2.1/1-3 | 11.71 | 6.68 | 75/57.05 | 3.43 | 27/29.29 | 2.23 | 07/19.04 | |||
| P valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
a, Univariate comparisons were performed using the paired t-test as necessary for parameters with continuous values and Fisher’s exact test with discontinuous parameters; b, number of independent drainage channels account for the channels leaving the corpora cavernosa detected via anterior-posterior view.
In the first set of films, usually the CVs were shown first, followed by the DDV, which is the predominant one without exception. A rail-like parallel venous complex of the DDV and the CV system was noted between the symphysis pubis and penile hilum, whereas the PAVs were visualized as flare-like eruptions in 59.38% (n=57) cases—although only 45.62% (26/57) of them are extraordinarily conspicuous. The PAVs were confluent to the DDV distally and not visible proximally (Figure 3B). In addition, a direct shunt to the SDV was shown in 28.13% (n=27) of men and 55.56% (15/27) of them were found to drain directly to the femoral vein of which the ratio of right (Figures 2,3,4,7) to left (Figure 6) side was 30:2. Therefore, the SDV, perhaps, forms part of the erectile apparatus. Furthermore BV demonstrated in 36.46% (35/96) of the subjects while the corpus spongiosum was well demarcated in 18.75% (18/96) of them. N.B., these important pieces of information cannot be obtained from the second set of films.
Unsurprisingly, all patients (n=96/96) categorically feel more informed, particularly those patients who are diagnosed with VOD (n=85) after the physician shows them the first set of images in this investigation.
Discussion
The definition of VOD in cavernosography may not be beyond controversy despite a high detection rate of venous leakage being reported in the literature (16-18). Conventional imaging techniques only show a small portion of the leaky DDV and completely fail to display either the CVs or the PAVs. The failure of conventional surgery to repair venous leaks should therefore be ascribed to conventional understanding of the venous anatomy and the conventional imaging techniques based on that understanding; it follows that a comprehensive blueprint of the penile venous anatomy is a prerequisite for satisfactory venous surgery. Absent this blueprint, the surgeon will fail to remove all residual veins, thereby leading to either an insufficient or disappointing post-surgical outcome (19,20).
In our experience of dynamic infusion cavernosography, an occurrence of penile edema or hematoma resulting in intractable pain ensues if the injection needle is displaced outside the corpora cavernosa. This situation is worsened by using an infusion pump, which provides pressure during injection in order to enhance the venous visibility. To resolve this dilemma, we decided upon patient self-administration of a contrast solution. A clearer imaging (Figure 3) and dynamic films are essential to guarantee success of the study—it even enables differentiation of the septum and separation of CVs (black arrowheads in Figure 3B,C). Other advanced diagnostic tools have been recommended for diagnosis of patients with ED; they may indeed be helpful (21,22). It appears, however, that there is no rendering as clear as the images produced in this study which so closely reveals the detailed structure and venous anatomy.
The hemodynamic relationship between the penile vasculature and the tunica albuginea is unique. The tunica albuginea of the corpora cavernosa is a bi-layered structure with multiple sub-layers through which emissary veins pass obliquely (23). Two circulatory routes within the human corpus cavernosum penis have been described (24), one system for metabolism and the other for erectile function. For the latter case, there is no corresponding venous vasculature to the cavernosal artery, which is the major arterial supply to the corpora cavernosa. Thus, we could regard the sinusoids of the corpora cavernosa as an end-arterial system just like the well-documented retina and kidney. This study has confirmed the long-understood function of the DDV and has also shown the importance of the CV and PAVs as demonstrated in our serial films. These veins (the CV and PAVs) have their own specific emissary drainage system from the subtunical sinusoids. Even the SDV has been shown to directly drain the cavernosal blood and at times shunt directly to the femoral veins instead of the internal pudendal veins as common thought. Understanding and renderings of the corporal drainage veins must be refined to acknowledge the importance of the CV, PAV, BV and SDV. This paramount information is unable to be presented in conventional pharmaco-cavernosography unless pilot films are introduced as in this study.
A CV is specific to each corpus cavernosum. This vein is consistently shown to be part of the deep venous system of the penis and is described in the literature as being short, running nearby the penile hilum. Our study shows that this venous system course throughout almost the entire length of the corresponding corpus cavernosum, although it does become smaller distally. This vein, as a rule, sends a communicating branch to the DDV regardless of its size. Thus, it is not surprising to find an immediate demonstration of it before the presence of the DDV (Figure 3). The PAVs are always masked by the DDV and CVs for the caliber of the latter are larger. We note their presence by a “volcano-like” eruption which is always seen in this study. This finding is in accordance with anatomical studies that show the venous diameters are less than the DDV and CV proximally.
The corpora cavernosa could be regarded as an ideal vessel to apply Pascal’s law, which states that pressure applied to any part of an enclosed fluid at rest is transmitted equally throughout the fluid and to the walls of the containing vessel—provided that there is no venous leakage (25). Thus, the entire venous system between Buck’s fascia and the tunica albuginea should be regarded as a single enclosed unit (15). In addition, in this study 15.63% (15/96) of men exhibit return of the cavernosal blood to femoral veins, where blood return (Figure 7D) will be commensurate with arterial perfusion—in other words, an accelerated venous return from this route will ensue in case of lower body exercise. It is commonly believed that pelvic steal syndrome explains why some men experience a reduction in erection rigidity when they sustain an exercise of gluteal muscles, such as an attempt to stand up or change position. Does venous return contribute to this issue?
In conventional pharmaco-cavernosography, it appears that some of the drainage channels are prone to be masked once the injected contrast medium is filled in the corpora cavernosa. With the first set of cavernosograms in this study, the entire corporal drainage veins can be clearly shown, which is informative for both surgeons and patients because it provides a detailed blueprint of the delicate venous anatomy that is necessary both for effective surgery (26) and for properly illustrating to the patient his condition. It is likely that this information is exclusively available via this method of cavernosography.
Conclusions
We can conclude that the venous drainage of the corpora cavernosa is initially via corresponding CVs, PAVs, DDV, BV and to SDV in one-third of patients. The DDV, CVs, PAVs, BV and even the SDV appear to be able to drain the sinusoidal blood of the corpora cavernosa independently. A rail-like parallel venous complex of the DDV and CVs system forms categorically between the symphysis pubis and penile hilum, rather than a single venous route. Therefore the entire penile venous system—including DDV, CVs, PAVs, BV and SDV—should be regarded as one intrinsic unit, which can only be disclosed in the first pilot set of cavernosograms. We therefore believe that pilot films should be required in pharmaco-cavernosography.
Acknowledgements
We would like to thank Benedict S. A. Murrell for his English editing, along with Ms Hsiu-Chen Lu, Nicola Chen and Luan-Hua Ho for their preparations of photos for this manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Velcek D, Evans JA. Cavernosography. Radiology 1982;144:781-5. [DOI] [PubMed] [Google Scholar]
- 2.Benoit G, Delmas V, Gillot C, et al. The anatomy of erection. Surg Radiol Anat 1987;9:263-72. [DOI] [PubMed] [Google Scholar]
- 3.Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. In: Wein AJ, Kavoussi LR, Novick AC, et al. eds. Campbell-Walsh Urology. 10th ed. Philadelphia, Pa: Saunders, 2011;688-720. [Google Scholar]
- 4.Dzik-Jurasz AS. Pelvic malignancy: integrating form and function. Br J Radiol 2005;78:S86-93. [DOI] [PubMed] [Google Scholar]
- 5.Bookstein JJ, Lurie AL. Selective penile venography: anatomical and hemodynamic observations. J Urol 1988;140:55-60. [DOI] [PubMed] [Google Scholar]
- 6.Gray RR, Keresteci AG, St Louis EL, et al. Investigation of impotence by internal pudendal angiography: experience with 73 cases. Radiology 1982;144:773-80. [DOI] [PubMed] [Google Scholar]
- 7.Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res 2008;20:17-29. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick T. The corpus cavernosum intercommunicating venous drainage system. J Urol 1975;113:494-6. [DOI] [PubMed] [Google Scholar]
- 9.Delcour C, Wespes E, Vandenbosch G, et al. Impotence: evaluation with cavernosography. Radiology 1986;161:803-6. [DOI] [PubMed] [Google Scholar]
- 10.Moscovici J, Galinier P, Hammoudi S, et al. Contribution to the study of the venous vasculature of the penis. Surg Radiol Anat 1999;21:193-9. [DOI] [PubMed] [Google Scholar]
- 11.Newman HF, Northup JD, Devlin J. Mechanism of human penile erection. Invest Urol 1964;1:350-3. [PubMed] [Google Scholar]
- 12.Eardley I, Sethia K. Anatomy and physiology of erection. In: Eardley I, Sethia K. eds. Erectile dysfunction, current investigation and management. 2nd ed. London, UK: Elsevier Limited, 2003;7-23. [Google Scholar]
- 13.Tanagho EA. Anatomy of the genitourinary tract. In: Tanagho EA, McAninch JW. eds. Smith’s General Urology. 17th ed. New York, NY: Mc-Graw Hill, 2008;1-16. [Google Scholar]
- 14.Benson GS, Boileau MA. The penis: sexual function and dysfunction. In: Gillenwater JY, Grayhack JT, Howards SS, et al. eds. Adult and pediatric urology. Philadelphia, Pa: Lippincott Williams & Wilkins: 2002;1935-74. [Google Scholar]
- 15.Hsu GL. The hypothesis of human penile anatomy, erection hemodynamic and their clinical applications. Asian J Androl 2006; 8:225-34. [DOI] [PubMed] [Google Scholar]
- 16.Rajfer J, Rosciszewski A, Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol 1988;140:69-71. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs AM, Mehringer CM, Rajfer J. Anatomy of penile venous drainage in potent and impotent men during cavernosography. J Urol 1989;141:1353-6. [DOI] [PubMed] [Google Scholar]
- 18.Elhanbly S, Abdel-Gaber S, Fathy H, et al. Erectile dysfunction in smokers: a penile dynamic and vascular study. J Androl 2004;25:991-5. [DOI] [PubMed] [Google Scholar]
- 19.Cakan M, Yalçinkaya F, Demirel F, et al. Is dorsale penile vein ligation (dpvl) still a treatment option in veno-occlusive dysfunction? Int Urol Nephrol 2004;36:381-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen SC, Hsieh CH, Hsu GL, et al. The progression of the penile vein: could it be recurrent? J Androl 2005;26:53-60. [PubMed] [Google Scholar]
- 21.Kurbatov DG, Kuznetsky YY, Kitaev SV, et al. Magnetic resonance imaging as a potential tool for objective visualization of venous leakage in patients with veno-occlusive erectile dysfunction. Int J Impot Res 2008;20:192-8. [DOI] [PubMed] [Google Scholar]
- 22.Kawanishi Y, Izumi K, Muguruma H, et al. Three-dimensional CT cavernosography: reconsidering venous ligation surgery on the basis of the modern technology. BJU Int 2011;107:1442-6. [DOI] [PubMed] [Google Scholar]
- 23.Hsu GL, Brock GB, Martinez-Pineiro L, et al. The three-dimensional structure of the tunica albuginea: anatomical and ultrastructural levels. Int J Impot Res 1992;4:117-29. [Google Scholar]
- 24.Banya Y, Ushiki T, Takagane H, et al. Two circulatory routes within the human corpus cavernosum penis: a scanning electron microscopic study of corrosion casts. J Urol 1989;142:879-83. [DOI] [PubMed] [Google Scholar]
- 25.Halliday D. Pascal’s principle, fluids. In: Halliday D, Resnick R, Walker J. eds. Fundamentals of Physics. 5th ed. New York, NY: John Wiley & Sons: 1997;355-6. [Google Scholar]
- 26.Hsieh CH, Liu SP, Hsu GL, et al. Advances in understanding of mammalian penile evolution, human penile anatomy and human erection physiology: clinical implications for physicians and surgeons. Med Sci Monit 2012;18:RA118-25. [DOI] [PMC free article] [PubMed] [Google Scholar]