Abstract
Male anterior urethral stricture disease is a commonly encountered condition that presents to many urologists. According to a National Practice Survey of Board Certified Urologist in the United States most urologists treat on average 6-20 urethral strictures yearly. Many of those same urologists surveyed treat with repeated dilation or internal urethrotomy, despite continual recurrence of the urethral stricture. In point of fact, the urethroplasty despite its high success rate, is underutilized by many practicing urologists. Roughly half of practicing urologist do not perform urethroplasty in the United States. Clearly, the reconstructive ladder for urethral stricture management that was previously described in the literature may no longer apply in the modern era. The following article reviews the etiology, diagnosis, management and comparisons of treatment options for anterior urethral strictures.
Keywords: Stricture, urethra, trauma, urethroplasty, urthrogram
Anatomy
An understanding of the urethral anatomy is paramount to the diagnosis and treatment of urethral stricture disease. The normal male urethra measures approximately 30 french, 20 cm long and is surrounded by the corpus spongiosum. The corpus spongiosum has a dual blood supply whereby both distal branches of the deep internal pudendal artery provide vascular supply. The deep internal pudendal artery then continues as the common penile artery.
The male urethra is divided into an anterior and posterior division. The anterior division consists of the fossa navicularis, pendulous and bulbar urethra. The posterior division consists of the membranous and prostatic urethra. The membranous urethra marks the dividing line between the anterior and posterior urethra. Medical nomenclature has commonly referred to narrowing of the urethra of the posterior division as contractures or stenosis. This has been in part due to the etiology of most posterior urethra injuries that are a consequence of a distraction or obliteration defect such as trauma or radical prostatectomy. Narrowing of the anterior division of the urethra are referred to as strictures (1).
The centricity of the male urethra differs along its course from the bladder neck to the meatus. The male urethra from its most distal end at the fossa navicularis is more centrally placed. This is in contrast, to the more eccentrically placed bulbar urethra. A good understanding of the anatomical location of the urethra along its course from the bladder neck to the meatus is important, as most straddle injuries to the urethra occur at the membranous urethra secondary to a loss of protection from surrounding tissues and structures. The urethra is also fixed along its course at the penoscrotal junction. This fixation point acts as a fulcrum when instrumentation of the urethra is being performed. A higher index of pressure occurs at this site, lending itself to the effects of pressure necrosis or irritation with long term catheters and during cystoscopy with rigid instruments (2). 80% of strictures that are secondary to instrumentation occur during the transurethreal resection of the prostate for benign prostatic hyperplasia occur at the membranous urethra. The other 20% generally occur at the penoscrotal junction. It has been documented that during a TURP the cystoscope can move back forth over the urethra at the penoscrotal junction 800 times during a single procedure. This can cause irritation to urethra with further development of stricturing (2).
Etiology
Before the 21st century the majority of anterior urethral strictures resulted from urethritis or urethral inflammation from sexually transmitted diseases (STD’s) (3). Approximately 40% of all strictures in 1960s-1980s were a direct result of urethritis (4). Today urethritis accounts for only <3% of all strictures in the developed world. Post inflammatory strictures are seldom seen today in the developed world secondary to increased awareness, prevention strategies, campaigns that have led to treating STD’s sooner in a more timely manner and with better antibiotics.
Instead, urethral strictures may be from prostatectomy, urethral catheterization, transurethral resection, cystoscopy, brachytherapy, hypospadius surgery, lichen sclerosis, urethritis, urethral tumor, penile fracture, perineal trauma, pelvic fracture and even idiopathic (4). The patient’s age and the site of the urethral stricture are important to clarify the etiology of the majority of most urethral strictures (4). (Refer to Table 1 & 2).
Table 1. Stricture etiology by patient age.
| No. Pts [%] | No. age [%] |
P value | ||
|---|---|---|---|---|
| 45 or greater | Less than 45 | |||
| Prostatectomy | 9 [3.36] | 9 [6] | 0 | 0.0024 |
| Perineal trauma | 6 [2.24] | 4 [2.67] | 2 [1.69] | Not significant |
| Urethral catheterization | 30 [11.19] | 20 [13.33] | 10 [8.47] | Not significant |
| Idiopathic/unknown | 80 [29.85] | 35 [23.33] | 45 [38.14] | 0.005 |
| TUR | 52 [19.4] | 48 [32] | 4 [3.39] | <0.0000001 |
| Hypospadias | 26 [9.7] | 3 [2] | 23 [19.49] | 0.0000005 |
| Pelvic fracture | 30 [11.19] | 8 [5.33] | 22 [18.64] | 0.0004 |
| Urethritis | 10 [3.73] | 5 [3.33] | 5 [4.24] | Not significant |
| Lichen sclerosus | 13 [14.85] | 10 [6.67] | 3 [2.54] | Not significant |
| Cystoscopy | 3 [1.12] | 3 [2] | 0 | Not significant |
| Tumor | 4 [1.49] | 2 [1.33] | 2 [1.69] | Not significant |
| Penile fracture | 3 [1.12] | 1 [0.67] | 2 [1.69] | Not significant |
| Brachytherapy | 2 [0.75] | 2 [1.33] | 0 | Not significant |
| Totals | 268 | 150 | 118 | |
The etiology of stricture disease as it corresponds to patient age. Data taken from Lumen et al.
Table 2. Etiology by stricture site.
| No. penile [%] | No. bulbar [%] | No. panurethral [%] | No. posterior [%] | |
|---|---|---|---|---|
| Prostatectomy | 0 | 3 [2.33] | 1 [2.78] | 5 [12.5] |
| Perineal trauma | 0 | 6 [4.65] | 0 | 0 |
| Urethral catheterization | 9 [14.29] | 13 [10.08] | 9 [25] | 0 |
| Idiopathic/unknown | 13 [20.63] | 62 [48.06] | 5 [13.89] | 0 |
| TUR | 7 [11.11] | 32 [24.81] | 9 [25] | 4 [10] |
| Hypospadias | 18 [28.57] | 5 [3.88] | 2 [5.56] | 0 |
| Pelvic fracture | 0 | 0 | 1 [2.78] | 29 [72.5] |
| Urethritis | 1 [1.59] | 6 [4.65] | 3 [8.33] | 0 |
| Lichen sclerosus | 10 [15.87] | 0 | 3 [8.33] | 0 |
| Cystoscopy | 0 | 1 [0.78] | 2 [5.56] | 0 |
| Tumor | 3 [4.76] | 0 | 1 [2.78] | 0 |
| Penile fracture | 2 [3.17] | 1 [0.78] | 0 | 0 |
| Brachytherapy | 0 | 0 | 0 | 2 [5] |
| Totals | 63 | 129 | 36 | 40 |
The site of urethral stricture as it relates to the etiology of stricture causality. Data taken from Lumen et al.
As stated earlier, strictures of unknown origin are extremely common in the developed world. They can arise anywhere in urethra, but most commonly arise at the bulbar urethra. They can arise earlier in life from unrecognized trauma, or traumatic catheterization, cystoscopy, TURP or hyspospadius repair. Kashefi et al. demonstrated in a 2008 publication in the Journal of Urology on Causality of Iatrogenic Urethral Strictures that an estimated 3.2 urethral injures per 1000 inpatients were secondary to traumatic catheterizations (5). In patients older than age 45, TURP and radical prostatectomy are the most common causes of urethral stricture/contracture. The urethral stricture event is a late complication of TURP in 2.2-9.8% of cases (6) and radical (8.4%) and simple (1.9%) prostatectomy (7). Causes of urethral stricture after TURP are secondarily trauma induced by the resectoscope at the bulbar urethra and the penoscrotal junction. In patients younger than age 45, hypospadius surgery, idiopathy, lichen sclerosis and pelvic fracture are among the most common causes of urethral strictures (4). When described by site anterior urethral strictures, regardless of age, are most commonly idiopathic (4).
Diagnosis
Initial diagnosis of urethral stricture begins with a thorough history and physical examination. Patient history should focus on previous urethral instrumentation, genital injury, history of sexual transmitted disease, urethral discharge, hematuria, and dysuria. Lower urinary tract symptoms should be assessed with an AUA system index (2). Weak stream, incomplete emptying and frequency were noted to be the most prevalent symptoms for patients undergoing urethroplasy for anterior urethral strictures (8) (Refer to Table 3). Physical examination should be tailored with emphasis on palpation of the penile shaft for thick urethral scarring, examination of the glans penis, meatus and perineum.
Table 3. Urethral stricture disease signs and symptoms.
| No. Pts [%] | |
|---|---|
| Weak stream | 104 [49] |
| Incomplete emptying | 57 [27] |
| Frequency | 42 [20] |
| Urinary retention | 35 [16] |
| Dysuria | 34 [16] |
| Urgency | 31 [14] |
| Nocturia | 25 [12] |
| Staining | 21 [10] |
| Spraying stream | 19 [9] |
| Hesitancy | 17 [8] |
| Post-void dribbling | 11 [5] |
| Intermittency | 10 [5] |
| Hematuria | 11 [5] |
| Incontinence | 9 [4] |
Stricture related symptoms. Data adapted from Mundy et al.
The diagnostic study of choice for urethral stricture should confirm the length, location, depth and density. The diagnosis of a urethral stricture is evaluated using direct visualization, sonography and contrast imaging (9). To identify a stricture direct visualization with a cystoscopy is most commonly undertaken. Cystoscopy can help gauge the caliber of the stricture and its location along the course of the urethra. Cystoscopy is limited however in its inability to determine the length and depth of the stricture. To determine stricture length, radiographic techniques such as a dynamic retrograde urethrogram and voiding cystourethrogram provide such information. Retrograde urethrogram has been the gold standard for urethral stricture diagnosis (10) (Refer to Figure 1).
Figure 1.
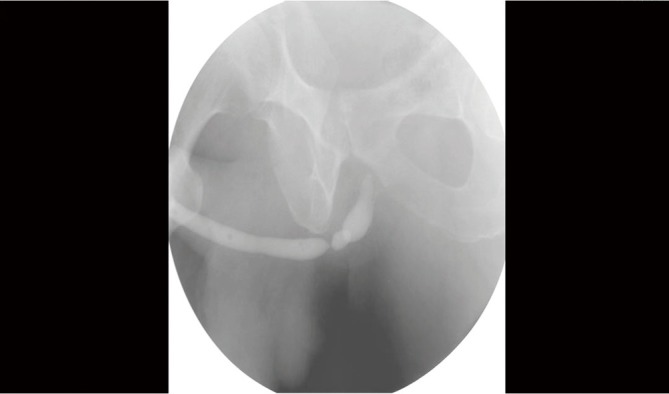
Retrograde urethrogram revealing a bulbar urethral stricture.
There are two types or retrograde urethrograms; static vs. dynamic. The static urethrogram consists of obtaining films after retrograde injection of contrast in the urethra. The static urethrogram gives limited information because it only images the anterior urethra. After a male voids, the urine that is retained in the posterior urethra is “milked back” into the bladder by the intrinsic sphincter. Taking films after injection of contrast in the urethra rather than during injection (dynamic RUG) fails to view the posterior the urethra secondary to the “milk-back” phenomenon. A dynamic retrograde urethrogram is performed by placing the patient in the 45 degree lateral oblique position and taking films during injection of contrast. Taking films during injection of contrast prevents the posterior urethra from emptying prior to taking films and thus a complete anatomical depiction of the urethra can be imaged. Retrograde Urethrogram has a specificity and sensitivity of 90% (11).
In addition to a dynamic retrograde urethrogram an accompanying voiding cystogram provides information on the dynamics of voiding and can demonstrate occult processes proximal to a narrow caliber urethral segment. Occasionally pathology is seen on the voiding study which is not demonstrated on the retrograde study. For example high pressure VUR, reflux into ejaculatory ducts and seminal vesicles, old abscess cavities around the posterior urethra may only be visualized on a voiding study. The VCUG provides no addition morbidity and can be peformed at the same time as the RUG.
Urethral sonography provides an adjunct to the retrograde urethrogram in defining the degree of stricture spongiofibrosis and for surgical planning. Sonography can more accurately describe and detect anterior urethral strictures (11). Sonography is able to visualize spongiofibrosis which appears as an anechoic irregular denistity next to and/or invading the urethral lumen (10). Ultrasound can further help delinate periurethral pathology that includes calculi, abscesses, fistula or diverticulae. A study by Nash et al. in 1995 compared RUG and urethral sonography in 101 patients. During intraoperative measurement of the stricture length, sonography correlated more closely with urethral stricture length vs. RUG. RUG has a propensity to underestimate urethral stricture length in the bulbar urethra (10).
Management
The treatment for urethral strictures dates back in the literature to >600 years BC (12). Urethral dilation was first described by Susruta in India. He described using an iron, wood or shellac rod lubricated with butter and introduced into the urethra every 3 days with rods of increasing size. The principles of management have changed little since the beginning of urethral dilation; however, our imaging and operative techniques have improved to provide patients with better curative outcomes. It has been the mantra of urologists to treat urethral strictures according to the “reconstructive ladder”; use simple methods first before progressing to more complex treatment options. This ideology has become “passé”. Improved imaging and surgical interventions have led some urologist to treat initially with urethroplasty if the urethral strictures characteristic and patient understanding allows. Literature has shown that repeated futile attempts at less invasive procedures can make future surgical repair more difficult.
The current options for urethral stricture management and cure include urethral dilation,direct visual internal urethrotomy (DVIU), urethral stent insertion and urethroplasty. Prior to any stricture intervention the physician patient consultation should include discussion about curative versus management options. Intervention should be tailored to each individual case, stricture characteristic and patient expectations.
Urethral dilation has been practiced for centuries and has been a means of management rather curative surgical intervention. The assumption of the procedures effectiveness is its ability to create small tears within the scar that heal by epithelialization with healthy mucosa. However the tear can also heal by further scar formation thereby contracting the stricture more and worsening symptoms and creating a more difficult future surgical repair. The ideal dilation stretches the scar overtime rather than creating large tears that may or may not heal with further scarring. Urethral dilation is not the choice of management for patients with balanitis xerotica obliterans, because dilation can worsen the stricture and extend it more proximally (2). Urethral dilation has been found to be most effective, and sometimes curative, in patients with superficial scars or folds in the mucosa. Repeated dilation does not treat the underlying spongiofibrosis in patients with more complex strictures. Spongiofibrosis found on ultrasound should direct intervention to include DVIU or urethroplasty.
Direct Visual Internal Urethrotomy (DVIU) treats the stricture directly by incision. Patients who have superficial spongiofibrosis may benefit from DVIU when the incision is carried out through all depths of the scar. Predictors of success include stricture length and degree of spongiofibrosis (13). DVIU is best utilized for short strictures less than 1.5 cm that involve the bulbar or pendulous urethra (9). DVIU has a long term success rate of 74% if the above criteria are met. DVIU success can be improved if urethral dilation is performed greater than 1 year (80%) (1). Repeated urethrotomies without improvement in stricture caliber portend a worse prognosis for urethral stricture cure (1). Some physicians advocate only performing DVIU two to three times before proceeding to a more invasive surgical intervention. Literature comparison of DVIU vs. repeated urethral dilation revealed no significant difference in stricture recurrence or reduction in urethral caliber up to 4 years post procedure (14).
There has much research in the field of direct visual internal urethrotomy. Adjunctive procedures have been studied and have shown improvements in outcomes. Mitomycin C submucosal injection at incision sites has shown to reduce scar formation. Mitomycin C has both antifibroblast and anticollagen properties. Mazdak and associates randomized 40 male patients with anterior urethral strictures to receive urethrotomy vs. urethrotomy with Mitomycin C injection at incision sites. At 6 month follow up, only 2 patients who received Mitomycin C restructured versus 10 patient who had only urethrotomy. The study revealed a 10% risk of restricture with Mitomycin C vs. 50% for DVIU alone during a 6 month follow up (15). Triamcinolone has also been studied when injected at incision sites. A double blinded placebo controlled study performed by Tabassi and associates of 70 patients who received either triamcinolone injection vs. sterile water at urethromotomy incision sites. Results revealed a decrease time to recurrence in the experimental group versus control. Stricture recurrence was prolonged to 8 months versus DVIU alone.
A randomized prospective trial performed by Atak and associates randomized 51 patients with iatrogenic annular urethral strictures to DVIU with a cold knife vs. Holmium: YAG laser. Postoperative follow up at 3, 6, 9, and 12 months were analyzed via uroflowmetry and retrograde urethrogram. Result revealed performing DVIU with the holmium: YAG laser reduced operative time and higher recurrence free rate of stricture recurrence at 6, 9, and 12 months compared to DVIU using cold knife (16).
Urethral stent placement has been another supplement to DVIU. There are two types of urethral stents. The Wallstent/Urolume and the Memokath stent. Only the Urolume stent is FDA approved to be used in the United States. The Urolume stent becomes incorporated into the urethral wall while Memokath stent is temporary and later removed. Removable urethral stents are designed to prevent the process of epithelialization from incorporating the stent into the urethral wall and are left in place for as long as 6 months to 1 year before they are removed. Studies in United Kingdom have revealed long term success rates of 85% at 4-4.5 years (17). Long terms studies of urethral stents revealed a high rate of complications (47%) that consisted of stent migration and reoperation secondary to stent encrusting, stenosis and urethral obstruction. Urethral stents should be used for patients with bulbar urethral strictures less than 3 cm and prostatic obstruction secondary to benign prostatic hyperplasia and those who are unfit to undergo bulbar urethroplasty.
Urethoplasty provides the optimal surgical repair of strictures that consist of excision with primary anastomosis. (Refer to Figure 2) In some instances, stricture excision produces a gap that is too long for an end-end anastomosis and placement of a buccal mucosal graft or penile shaft flap to bridge the gap is used. There are many different types of urethroplasty, each applicable in their own circumstance. The type of stricture, length and nature of underlying problem and history of previous surgery dictates urethroplasty technique (2). Although urethroplasty has a 95 percent success rate many urologist have little experience performing the procedure. This has lent itself to many urologist performing repeated endoscopic procedures despite futile results. According to a study of Medicare Beneficiaries undergoing treatment of urethral strictures from 1992-2001, urethroplasty rates of 0.6-0.8% compared to other treatment modalities revealed its underuse despite its efficacious treatment outcomes (18). A study in England from 2006 revealed 500 urethroplasties being performed vs. 7,000 urethrotomies. 57.8% of Urologist do not perform urethroplasties and 33% would refer to another physician for urethrotomy (19).
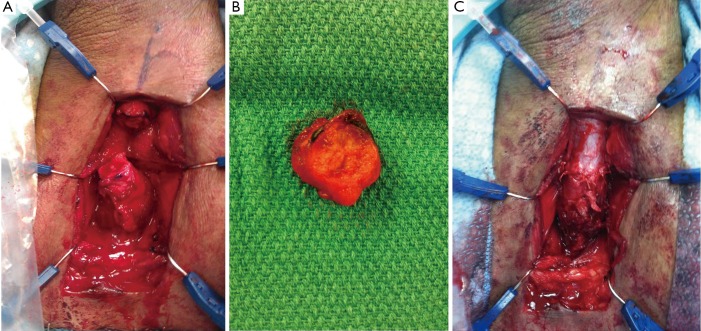
Figure 2.
Intraoperative photos taken during an urethroplasty for a bulbar urethral stricture. A. Urethral defect and length after excision of urethral stricture; B. Excised urethra with contained stricture; C. Urethral anastomosis.
Conclusions
This article serves to provide a review of the current literature and data surrounding etiology, diagnosis and management of anterior urethral strictures. It is clear that urethroplasty, the gold standard, has been underutilized as a first line treatment. The “minimally invasive” treatments tend to be less successful and potentially more costly because it would require more visits to the doctor and more surgeries. In the age of glooming medical costs, perhaps we should be more prudent as urologists and look again to the gold standard treatment as a viable and preferred option for first line treatment of urethral stricture disease.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wein AJ, Kavoussi LR, Novick AC, et al. Campbell-Walsh Urology Tenth Edition. Saunders, 10th revised edition, hardback. 2011. [Google Scholar]
- 2.Mundy AR, Andrich DE. Urethral Stricture Review Article Institute of Urology, London, UK. Br J Urol 2012. [Epub ahead of print]. [Google Scholar]
- 3.Beard DE, Goodyear WE. Urethral stricture; a pathological study. J Urol 1948;59:619-26. [DOI] [PubMed] [Google Scholar]
- 4.Lumen N, Hoebeke P, Willemsen P, et al. Etiology of urethral stricture disease in the 21st century. J Urol 2009;182:983-7. [DOI] [PubMed] [Google Scholar]
- 5.Kashefi C, Messer K, Barden R, et al. Incidence and prevention of iatrogenic urethral injuries. J Urol 2008;179:2254-7; discussion 2257-8. [DOI] [PubMed] [Google Scholar]
- 6.Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol 2006;50:969-79; discussion 980. [DOI] [PubMed] [Google Scholar]
- 7.Varkarakis I, Kyriakakis Z, Delis A, et al. Long-term results of open transvesical prostatectomy from a contemporary series of patients. Urology 2004;64:306-10. [DOI] [PubMed] [Google Scholar]
- 8.Nuss GR, Granieri MA, Zhao LC, et al. Presenting symptoms of anterior urethral stricture disease: a disease specific, patient reported questionnaire to measure outcomes. J Urol 2012;187:559-62. [DOI] [PubMed] [Google Scholar]
- 9.Jezior J. Management of Bulbous Urethral Stricture. AUA update series. Eastern Viginia Medical School.
- 10.Nash PA, McAninch JW, Bruce JE, et al. Sonourethrography in the evaluation of anterior urethral strictures. J Urol 1995;154:72-6. [PubMed] [Google Scholar]
- 11.El-Ghar MA, Osman Y, Elbaz E, et al. MR urethrogram versus combined retrograde urethrogram and sonourethrography in diagnosis of urethral stricture. Eur J Radiol 2010;74:e193-8. [DOI] [PubMed] [Google Scholar]
- 12.Das S. Shusruta of India, the pioneer in the treatment of urethral stricture. Surg Gynecol Obstet 1983;157:581-2. [PubMed] [Google Scholar]
- 13.Dubey D. The current role of direct vision internal urethrotomy and self-catheterization for anterior urethral strictures. Indian J Urol 2011;27:392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyns CF, Steenkamp JW, De Kock ML, et al. Treatment of male urethral strictures: is repeated dilation or internal urethrotomy useful? J Urol 1998;160:356-8. [DOI] [PubMed] [Google Scholar]
- 15.Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol 2007;51:1089-92; discussion 1092. [DOI] [PubMed] [Google Scholar]
- 16.Atak M, Tokgöz H, Akduman B, et al. Low-power holmium:YAG laser urethrotomy for urethral stricture disease: comparison of outcomes with the cold-knife technique. Kaohsiung J Med Sci 2011;27:503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain M, Greenwell TJ, Shah J, et al. Long-term results of a self-expanding wallstent in the treatment of urethral stricture. BJU Int 2004;94:1037-9. [DOI] [PubMed] [Google Scholar]
- 18.Anger JT, Buckley JC, Santucci RA, et al. Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology 2011;77:481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bullock TL, Brandes SB. Adult anterior urethral strictures: a national practice patterns survey of board certified urologists in the United States. J Urol 2007;177:685-90. [DOI] [PubMed] [Google Scholar]