Abstract
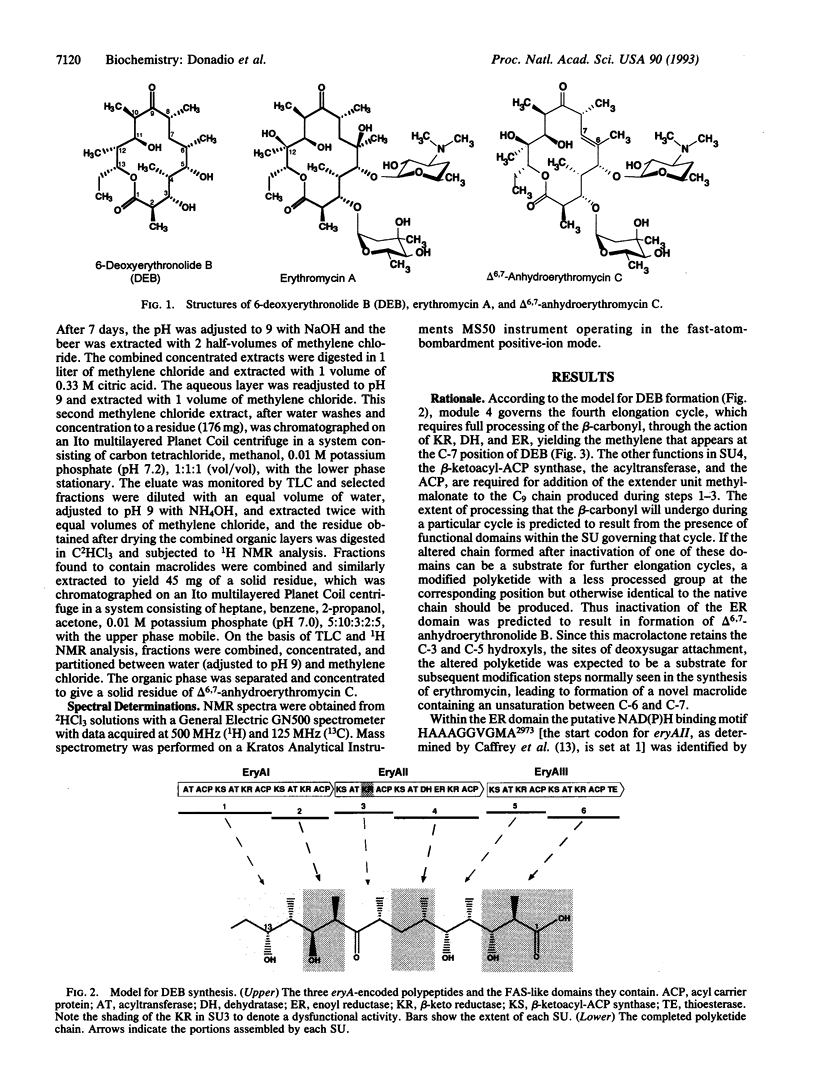
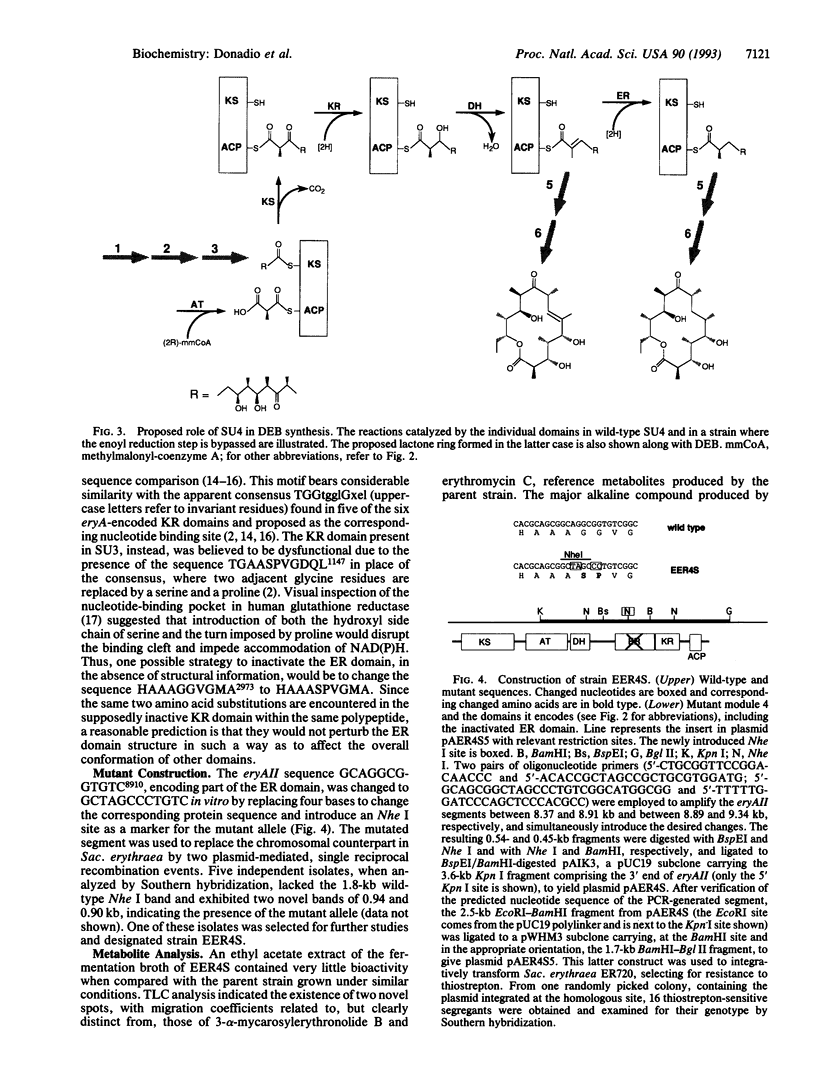
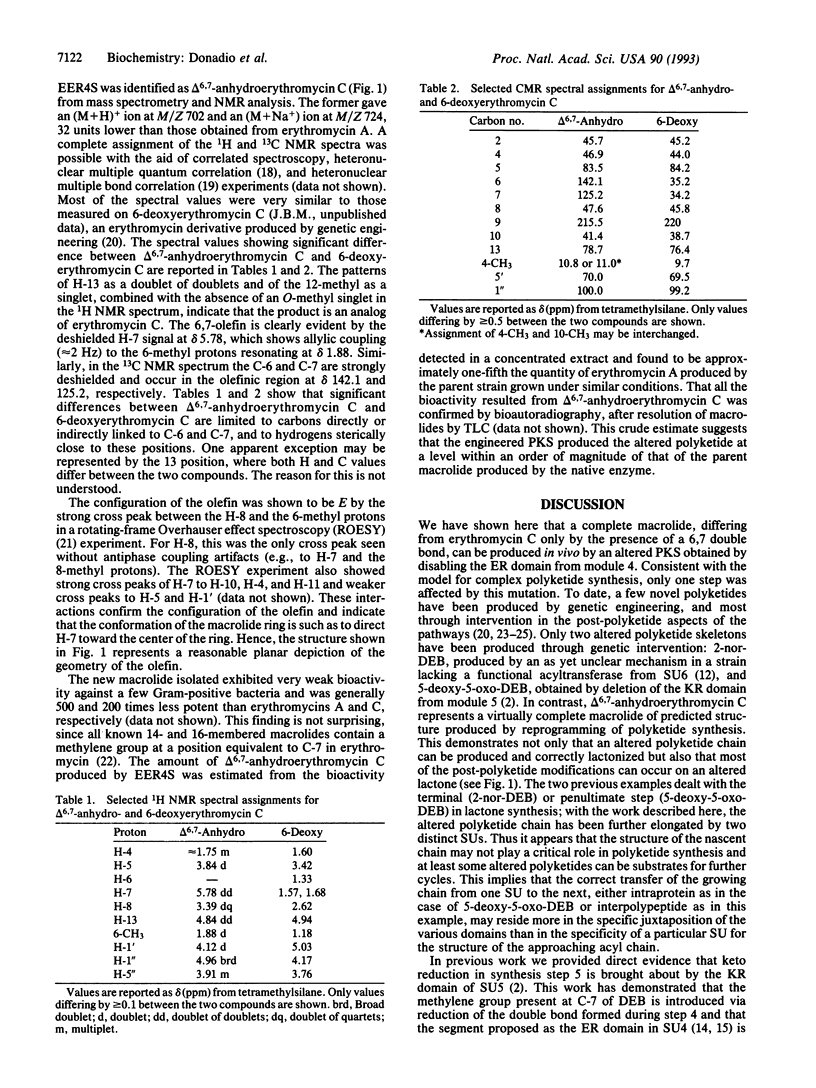
The polyketide-derived macrolactone of the antibiotic erythromycin is made through successive condensation and processing of seven three-carbon units. The fourth cycle involves complete processing of the newly formed beta-keto group (beta-keto reduction, dehydration, and enoyl reduction) to yield the methylene that will appear at C-7 of the lactone ring. Synthesis of this molecule in Saccharopolyspora erythraea is determined by the three large eryA genes, organized in six modules, each governing one condensation cycle. Two amino acid substitutions were introduced in the putative NAD(P)H binding motif in the proposed enoyl reductase domain encoded by eryAII. The metabolite produced by the resulting strain was identified as delta 6,7-anhydroerythromycin C resulting from failure of enoyl reduction during the fourth cycle of synthesis of the macrolactone. This result demonstrates the involvement of at least the enoyl reductase from the fourth module in the fourth cycle and indicates that a virtually complete macrolide can be produced through reprogramming of polyketide synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevitt D. J., Cortes J., Haydock S. F., Leadlay P. F. 6-Deoxyerythronolide-B synthase 2 from Saccharopolyspora erythraea. Cloning of the structural gene, sequence analysis and inferred domain structure of the multifunctional enzyme. Eur J Biochem. 1992 Feb 15;204(1):39–49. doi: 10.1111/j.1432-1033.1992.tb16603.x. [DOI] [PubMed] [Google Scholar]
- Caffrey P., Bevitt D. J., Staunton J., Leadlay P. F. Identification of DEBS 1, DEBS 2 and DEBS 3, the multienzyme polypeptides of the erythromycin-producing polyketide synthase from Saccharopolyspora erythraea. FEBS Lett. 1992 Jun 15;304(2-3):225–228. doi: 10.1016/0014-5793(92)80624-p. [DOI] [PubMed] [Google Scholar]
- DeWitt J. P. Evidence for a sex factor in Streptomyces erythreus. J Bacteriol. 1985 Nov;164(2):969–971. doi: 10.1128/jb.164.2.969-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio S., Hutchinson C. R. Cloning and characterization of the Saccharopolyspora erythraea fdxA gene encoding ferredoxin. Gene. 1991 Apr;100:231–235. doi: 10.1016/0378-1119(91)90372-i. [DOI] [PubMed] [Google Scholar]
- Donadio S., Katz L. Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin formation in Saccharopolyspora erythraea. Gene. 1992 Feb 1;111(1):51–60. doi: 10.1016/0378-1119(92)90602-l. [DOI] [PubMed] [Google Scholar]
- Donadio S., Shafiee A., Hutchinson C. R. Disruption of a rhodaneselike gene results in cysteine auxotrophy in Saccharopolyspora erythraea. J Bacteriol. 1990 Jan;172(1):350–360. doi: 10.1128/jb.172.1.350-360.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991 May 3;252(5006):675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- Epp J. K., Huber M. L., Turner J. R., Goodson T., Schoner B. E. Production of a hybrid macrolide antibiotic in Streptomyces ambofaciens and Streptomyces lividans by introduction of a cloned carbomycin biosynthetic gene from Streptomyces thermotolerans. Gene. 1989 Dec 28;85(2):293–301. doi: 10.1016/0378-1119(89)90421-6. [DOI] [PubMed] [Google Scholar]
- Hara O., Hutchinson C. R. A macrolide 3-O-acyltransferase gene from the midecamycin-producing species Streptomyces mycarofaciens. J Bacteriol. 1992 Aug;174(15):5141–5144. doi: 10.1128/jb.174.15.5141-5144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Malpartida F., Kieser H. M., Ikeda H., Duncan J., Fujii I., Rudd B. A., Floss H. G., Omura S. Production of 'hybrid' antibiotics by genetic engineering. Nature. 1985 Apr 18;314(6012):642–644. doi: 10.1038/314642a0. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A. Genetic manipulation of Streptomyces: integrating vectors and gene replacement. Methods Enzymol. 1991;204:430–458. doi: 10.1016/0076-6879(91)04023-h. [DOI] [PubMed] [Google Scholar]
- MacNeil D. J., Occi J. L., Gewain K. M., MacNeil T., Gibbons P. H., Ruby C. L., Danis S. J. Complex organization of the Streptomyces avermitilis genes encoding the avermectin polyketide synthase. Gene. 1992 Jun 15;115(1-2):119–125. doi: 10.1016/0378-1119(92)90549-5. [DOI] [PubMed] [Google Scholar]
- McAlpine J. B., Tuan J. S., Brown D. P., Grebner K. D., Whittern D. N., Buko A., Katz L. New antibiotics from genetically engineered actinomycetes. I. 2-Norerythromycins, isolation and structural determinations. J Antibiot (Tokyo) 1987 Aug;40(8):1115–1122. doi: 10.7164/antibiotics.40.1115. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Tuan J. S., Luebke V. E., Maine G. T., DeWitt J. P., Katz L. Mutation and cloning of eryG, the structural gene for erythromycin O-methyltransferase from Saccharopolyspora erythraea, and expression of eryG in Escherichia coli. J Bacteriol. 1990 May;172(5):2541–2546. doi: 10.1128/jb.172.5.2541-2546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton N. S., Berry A., Perham R. N. Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. Nature. 1990 Jan 4;343(6253):38–43. doi: 10.1038/343038a0. [DOI] [PubMed] [Google Scholar]
- Tuan J. S., Weber J. M., Staver M. J., Leung J. O., Donadio S., Katz L. Cloning of genes involved in erythromycin biosynthesis from Saccharopolyspora erythraea using a novel actinomycete-Escherichia coli cosmid. Gene. 1990 May 31;90(1):21–29. doi: 10.1016/0378-1119(90)90435-t. [DOI] [PubMed] [Google Scholar]
- Vara J., Lewandowska-Skarbek M., Wang Y. G., Donadio S., Hutchinson C. R. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J Bacteriol. 1989 Nov;171(11):5872–5881. doi: 10.1128/jb.171.11.5872-5881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Leung J. O., Swanson S. J., Idler K. B., McAlpine J. B. An erythromycin derivative produced by targeted gene disruption in Saccharopolyspora erythraea. Science. 1991 Apr 5;252(5002):114–117. doi: 10.1126/science.2011746. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Losick R. The use of a chromosome integration vector to map erythromycin resistance and production genes in Saccharopolyspora erythraea (Streptomyces erythraeus). Gene. 1988 Sep 7;68(2):173–180. doi: 10.1016/0378-1119(88)90019-4. [DOI] [PubMed] [Google Scholar]
- Witkowski A., Rangan V. S., Randhawa Z. I., Amy C. M., Smith S. Structural organization of the multifunctional animal fatty-acid synthase. Eur J Biochem. 1991 Jun 15;198(3):571–579. doi: 10.1111/j.1432-1033.1991.tb16052.x. [DOI] [PubMed] [Google Scholar]




