Abstract
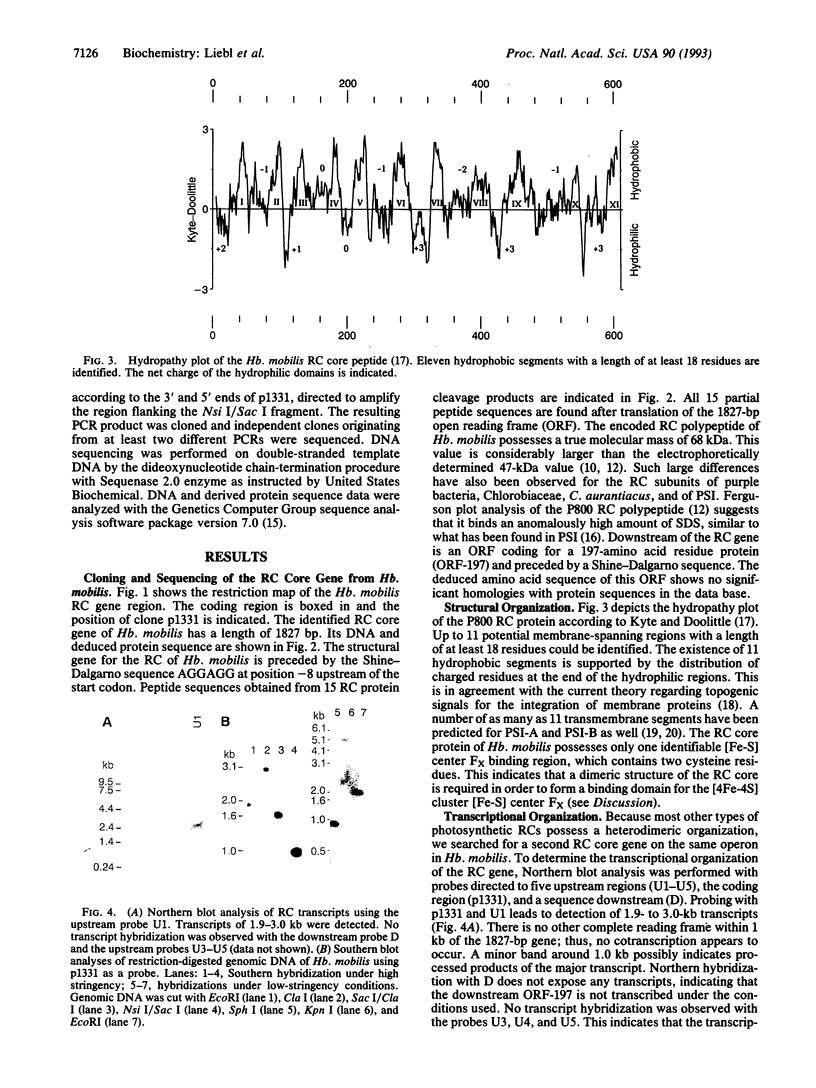
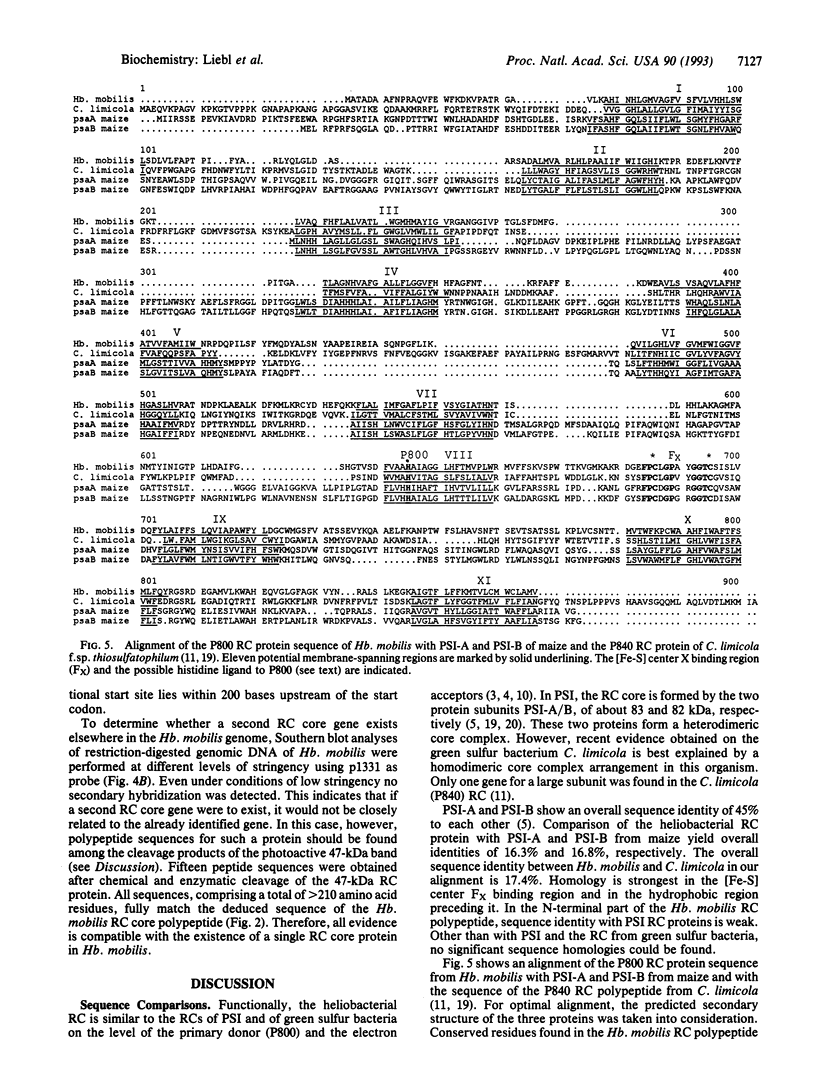
The gene for a reaction center core polypeptide from the anoxygenic photosynthetic bacterium Heliobacillus mobilis was cloned and sequenced. The deduced amino acid sequence consists of 609 residues with a molecular mass of 68 kDa. An adjacent open reading frame is not transcribed under our experimental conditions. No evidence for a second related reaction center core gene was found. The primary sequence of the reaction center protein (P800 protein) shows a high percentage of sequence identity to photosystem I in a cysteine-containing loop, which is the putative binding site of the iron-sulfur center FX and in the preceding hydrophobic region. Our data imply a homodimeric organization of the reaction center. This is fundamentally different from photosystem I and most other photosynthetic reaction centers, where the reaction center core is composed of two similar but nonidentical subunits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büttner M., Xie D. L., Nelson H., Pinther W., Hauska G., Nelson N. Photosynthetic reaction center genes in green sulfur bacteria and in photosystem 1 are related. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8135–8139. doi: 10.1073/pnas.89.17.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Komiya H., Yeates T. O., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: symmetry relations and sequence comparisons between different species. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9012–9016. doi: 10.1073/pnas.85.23.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Nitschke W., Feiler U., Rutherford A. W. Photosynthetic reaction center of green sulfur bacteria studied by EPR. Biochemistry. 1990 Apr 24;29(16):3834–3842. doi: 10.1021/bi00468a005. [DOI] [PubMed] [Google Scholar]
- Nitschke W., Sétif P., Liebl U., Feiler U., Rutherford A. W. Reaction center photochemistry of Heliobacterium chlorum. Biochemistry. 1990 Dec 18;29(50):11079–11088. doi: 10.1021/bi00502a010. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Koike H., Katoh S. Multiple forms of chlorophyll-protein complexes from a thermophilic cyanobacterium Synechococcus sp. Arch Biochem Biophys. 1982 Nov;219(1):209–218. doi: 10.1016/0003-9861(82)90151-5. [DOI] [PubMed] [Google Scholar]
- Trost J. T., Blankenship R. E. Isolation of a photoactive photosynthetic reaction center-core antenna complex from Heliobacillus mobilis. Biochemistry. 1989 Dec 26;28(26):9898–9904. doi: 10.1021/bi00452a003. [DOI] [PubMed] [Google Scholar]
- Trost J. T., Brune D. C., Blankenship R. E. Protein sequences and redox titrations indicate that the electron acceptors in reaction centers from heliobacteria are similar to Photosystem I. Photosynth Res. 1992;32:11–22. [PubMed] [Google Scholar]
- Webber A. N., Malkin R. Photosystem I reaction-centre proteins contain leucine zipper motifs. A proposed role in dimer formation. FEBS Lett. 1990 May 7;264(1):1–4. doi: 10.1016/0014-5793(90)80749-9. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vermaas WFJ. Transcript Levels and Synthesis of Photosystem II Components in Cyanobacterial Mutants with Inactivated Photosystem II Genes. Plant Cell. 1990 Apr;2(4):315–322. doi: 10.1105/tpc.2.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]




