Abstract
The aim of this study was to test whether extremely low frequency electromagnetic fields (ELF EMFs) affect health or not. Here, we constructed a 100-μT/50 Hz electromagnetic field atmosphere. A total of 128 rats were randomly assigned into two groups: the ELF EMF group and the sham group. The ELF EMF group was exposed to 100-μT/50-Hz ELF EMF for 20 h per day for three months; at the same time the other group was exposed to a sham device without ELF EMF. During the three months, the weight was recorded every 2 weeks, and the water intake and food intake of the animals were recorded weekly. The hematologic parameters were detected before and after the exposure, whereas blood chemistry analysis was performed every 4 weeks. The general condition of the exposed rats was not affected by ELF EMF. Compared with the sham group, the hematograms were not significantly altered in the ELF EMF group. Similarly, the blood chemistry (including lipid profile, blood glucose, liver function and renal function of rats) from the ELF EMF group showed no difference compared with rats from the control group during the three months exposure. The present study indicated that short-term exposure of 100-μT/50-Hz ELF EMF may not affect hematograms and blood chemistry in rats.
Keywords: extremely low frequency electromagnetic field, ELF EMF, hematogram, blood chemistry
INTRODUCTION
With the progress of science and technology, the use of electric power has become more and more popular. Meanwhile, the probability of exposure to extremely low frequency electromagnetic fields (ELF EMFs) in public or in the workplace has increased dramatically. ELF EMF is one of the electromagnetic fields, and it has an extremely low frequency, ranging from 1–300 Hz [1]. It should be noticed that ELF EMFs can be produced by powerlines, televisions, hair driers and other electrical appliances [2]. In response, a small current will be generated in the human or animal's body inside the ELF EMF [3]. This current might be a mechanism for ELF EMFs affecting human and animal health. In I979, Wertheimer and Leeper reported that children who lived near powerlines may be at risk of leukemia [4]. Since then, a great number of studies have focused on the effects of ELF EMFs on human health, mostly focused on cancer [5, 6], the endocrine system [2, 7], neurology and the psychiatric system [8, 9], and the cardiovascular system [10–12]. However, the results from various laboratories are controversial, and no definite conclusion can be drawn regarding the effects of ELF EMFs on human health.
Cancer is the most studied area, because ELF EMF is classified as ‘possibly carcinogenic’ [13]. A number of epidemiologic studies have suggested that prolonged exposure to ELF EMFs may be associated with increased risk of childhood cancer, particularly leukemia [14].
The relationship between ELF EMFs and the hematogram has drawn recent interest because the hematogram is one of the most important representations of leukemia. The hematologic effects of chronic exposure to ELF EMFs have been studied in animals. Cakir et al. reported that ELF EMF exposure induced a decrease in eosinophil, hemoglobin (Hb) and mean platelet volume (MPV) levels [15], while another study carried out by Margonato et al. showed that exposing rats to ELF EMFs did not affect their hematologic parameters [16].
It is well known that peripheral blood is composed of blood cells and plasma, which consists of lipids, proteins, electrolytes, etc. The homeostasis of these cells and molecules keeps the body healthy [17, 18]. Therefore, blood chemistry is another common examination that is used to evaluate human and animal health. As a consequence, the effect of ELF EMFs on blood chemistry is becoming an attractive field for study by scientists all around the world. Ragan et al. reported that exposure to ELF EMFs for 120 days affected the serum ion concentration and serum alkaline phosphatase concentrations [19]. However, conflicting results have been revealed by Zecca et al., who showed that exposure to ELF EMFs had no effect on serum chemistry [20].
Apparently, effects of ELF EMFs on the hematogram and blood chemistry still remain controversial, which may be due to the differing exposure conditions (e.g. field intensity, field's regularity), and this has led us to carry out the present study. Hence, we constructed an exposure device that produced a uniform 100-μT/50-Hz ELF EMF, and rats were used to evaluate the effects of ELF EMFs. The results showed that exposure to ELF EMFs had no effect on the hematogram, lipid profile or liver function and, more importantly, our study provided the first evidence that ELF EMFs did not affect renal function.
MATERIALS AND METHODS
Animal treatment
All experimental protocols complied with the Principles of Laboratory Animal Care (NIH publication No. 85-23, revised 1985), the OPRR Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 1986) and the US Animal Welfare Act. The Sprague–Dawley (SD) background rats were raised in the animal center of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Animals were housed under standard laboratory conditions (12 h light:12 h dark cycle, light cycle: 07 : 00–19:00 h with lights on at 07:00, 22–25°C room temperature, and 45–55% relative humidity). The food prepared for rats was bought from HFK Bioscience (Cat No. 1025, Beijing, China) and the composition was moisture (%) ≤ 8.0, crude protein (%) ≥ 18.0, crude fat (%) ≥ 4.0, crude fiber (%) ≤ 5.0, ash (%) ≤ 6.5, calcium (%) 1.2–1.4, phosphorus (%) 0.8–1.0. The food was sterilized with high pressure, and the water was supplied by a reverse osmosis water treatment system and sterilized by filtration and ozone. A total of 128 rats were randomly divided into two groups: the ELF EMF group and the sham group. Prior to the exposure, every animal's general conditions were recorded and their blood samples were collected. Then, rats were exposed to the 100-μT/50-Hz ELF EMF for 20 h per day. The food intake and water intake were recorded every week, and the weights of the rats were determined every two weeks.
Blood sample collection
The blood sample was collected every month via tail, as described previously [21, 22]. Briefly, puncture of the lateral tail vein was performed with the rat lying on its right side and the base of the tail placed between the index and middle fingers. Pressure was applied to distend the vein, and a 16-mm 25G hypodermic needle (Weigao, Shandong, China) was inserted into the vein in a cranial direction. Negative pressure was applied to the 1-ml syringe attached to the needle until venepuncture was achieved. The needle was removed, and digital pressure applied to the puncture site for 5–10 s.
Exposure device construction
The device was made of the ABS plastics (Yite Electric, Wuhan, China), and bronze wires (Yite Electric, Wuhan, China) were used to produce the ELF EMF. The whole device had five floors. The top and bottom floors were made with 400 round wires, and in the internal floors, there were 200 round bronze wires. A TDGC2 transformer (Mushidq, Shanghai, China) was connected and the voltage was measured by Fluke 17b voltmeter (Fluke, Everett, WA, USA). We adjusted the voltage to 34.9 V to make sure the intensity of the EMF was 100 μT. Inside the device, we placed a four-floor shelf, which was also made with ABS plastic in the center, and the height of every floor was the same as that of the device. We used plastic cages to raise the rats; the size of these cages was 45 × 20 × 35 cm (L × W × H). There were two rats moving freely in each cage. The distribution of the EMF was determined by Narda efa-300 (Narda, Pfullingen, Germany), and the distribution map was drawn before use.
Peripheral hematogram determined
Before and after the 3-month exposure, the peripheral hematogram (which included the total and differential white blood cell count, red blood cell count, hemoglobin and platelet count) was determined. The blood samples were anticoagulated using Ethylene Diamine Tetraacetic Acid (EDTA). After collection, hematograms of samples were determined using the Cell-Dyn 3700 hematology system (Abbott Laboratories, Abbott Park, Illinois, USA) over a period of 2 h.
Blood biochemical examination
The blood sample was centrifuged at 1600 g for 8 min after collection, and then stored at –20°C. Blood biochemical examinations, including liver function (alanine aminotransferase (ALT), aspartate aminotransferase (AST)), renal function (blood urea nitrogen (BUN) and creatinine (Cr)), blood glucose, blood lipid, calcium (Ca) and magnesium (Mg), were measured on an AEROSET Clinical Chemistry System (Abbott Laboratories, Abbott Park, Illinois, USA), as previously reported [23].
HE staining
After the animals were sacrificed, the liver and kidney were isolated and fixed with 4% neutral formalin, dehydrated, and then paraffin sections were prepared. Samples were stained with a hematoxylin-eosin (HE) kit (Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's protocol. Photographs were captured with an Olympus inverted microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to determine the statistical significance of differences between the two groups. In all cases, statistical significance was defined as P < 0.05.
RESULTS
The ELF EMF in the exposure device
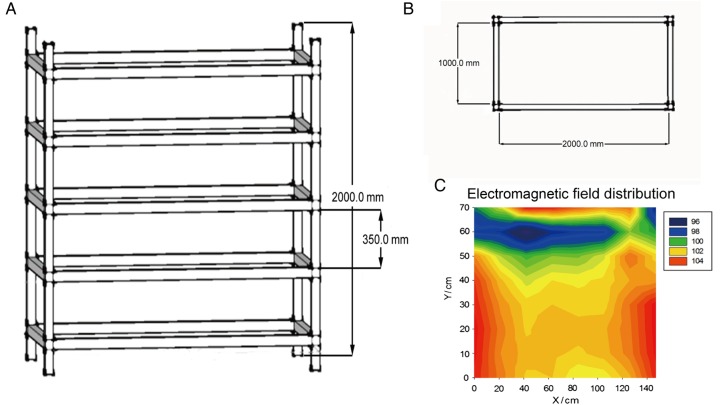
In order to evaluate the effects of ELF EMFs on animal health, we constructed an exposure system. This system consisted of an exposure device with five tiers and a transformer (Fig. 1A and B). A transformer was used to adjust the intensity of the EMF. We set the intensity to 100 μT, and the electromagnetic distribution was determined (Fig. 1C). As the figure showed, the distribution of the EMF in every floor was uniform. In addition, we built another exposure device without power to be used as the sham exposure system.
Fig. 1.
The magnetic field distribution of the exposure device. (A) The scheme of the exposure device. (B) The sketch map of the device's cross-section. (C) The magnetic field distribution of the shelf settled in the middle of the device.
ELF EMFs had no effect on an animal's general condition
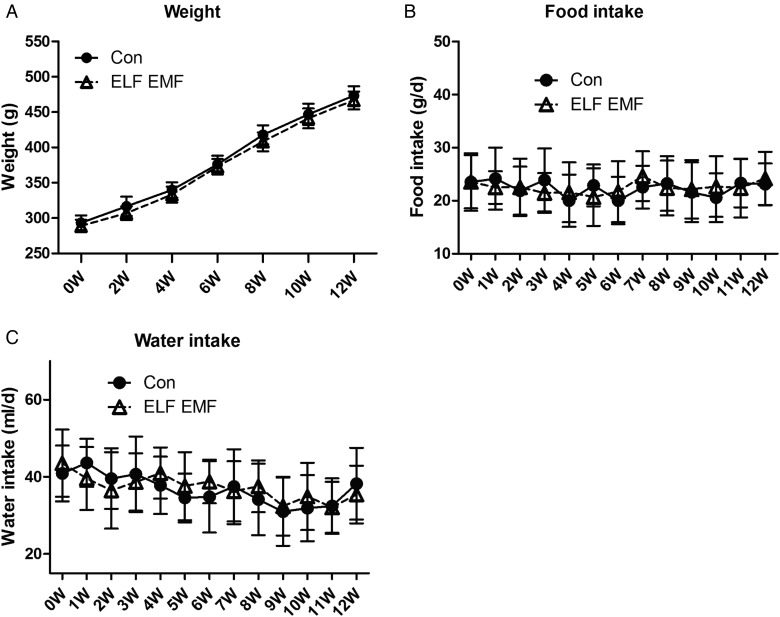
To evaluate the state of the rats, general condition, including weight, water intake and food intake were measured. Rats were weighed every 2 weeks, and it was found that after 3 months, the weight of the animals increased by ∼150 g, and there was no significant difference between the ELF EMF exposure group and the sham group (Fig. 2A). Meanwhile, we recorded the food intake and the water intake of rats every week. Results showed that the food (Fig. 2B) and water (Fig. 2C) intake of both groups were not changed during the whole 12 weeks. These data suggested that ELF EMFs had no effect on the general physiologic conditions of rats compared with the sham group.
Fig. 2.
General conditions of the rats during the 12-week exposure. (A) The weight of the rats in the control group and the ELF EMF–exposed group. (B) The food intake of the rats in the control group and the ELF EMF–exposed group. (C) The water intake of the rats in the control group and the ELF EMF–exposed group. Error bars indicate the standard deviation (SD) of the mean for n = 64 independent experiments.
ELF EMFs had no effect on the peripheral hematogram
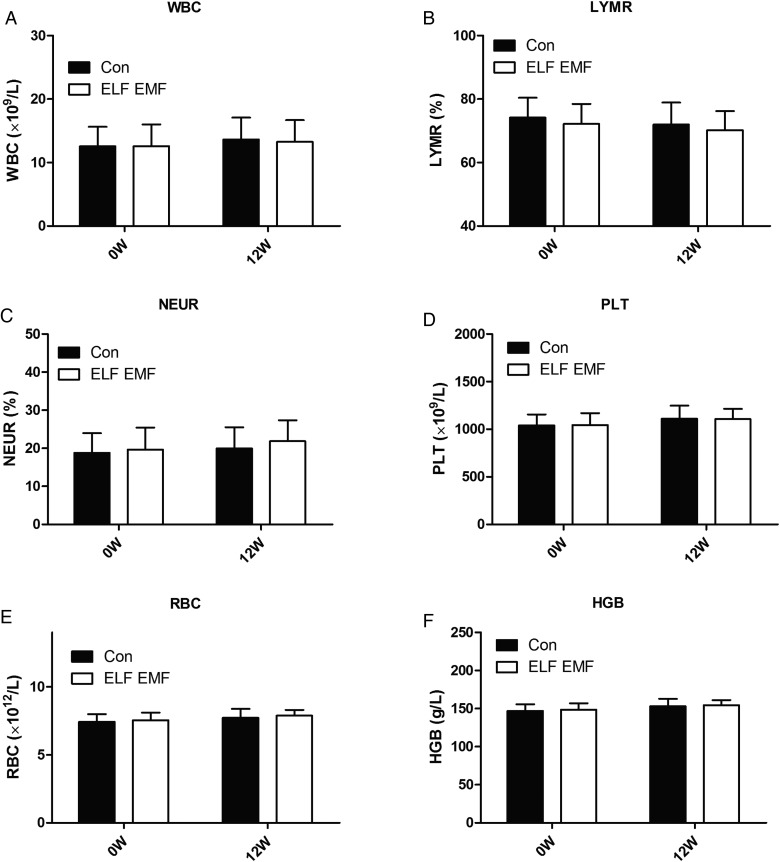
Before and after exposure, we collected the blood sample and determined the peripheral hematogram for every rat. The results showed that total and differential white blood cell (WBC) counts showed no significant difference between the two groups (Fig. 3A–C).
Fig. 3.
Peripheral hematogram of the two groups after the 12-week exposure. (A) The number of white blood cells (WBC). (B) The percentage of lymphocytes (LYMR). (C) The percentage of neutrophils (NEUR). (D) The number of platelets (PLT). (E) The number of red blood cells (RBC). (F) The amount of hemoglobin (HGB). Error bars indicate the standard deviation (SD) of the mean for n = 64 independent experiments.
At the same time, we analyzed the platelet and the red blood cell (RBC) counts. Consistently, ELF EMFs did not affect platelet count compared with the control (Fig. 3D). After a 3-month exposure, we compared the RBC count and the hemoglobin from ELF EMF–exposed rats with that of sham-exposed rats and found that there were no significant difference between the two groups (Fig. 3E and F). Together, the peripheral hematogram was not affected by the ELF EMF exposure.
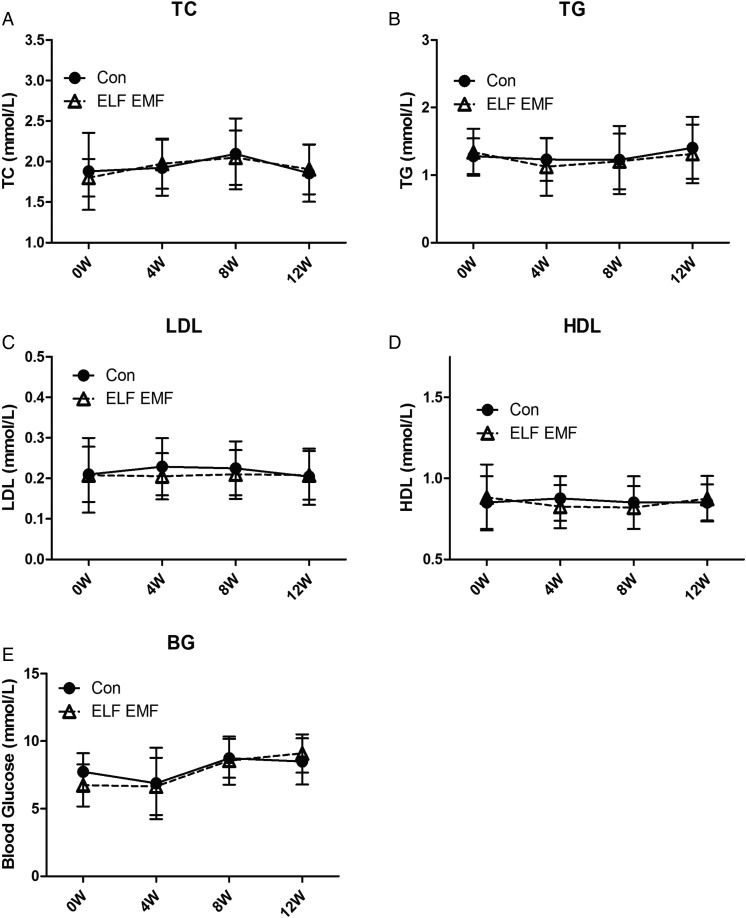
ELF EMFs had no effect on lipid profile or glucose
During the whole study, we collected blood every 4 weeks and determined the blood chemistry for both groups. The concentrations of lipid, including the total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL), were determined. As shown in Fig. 4A–D, the lipid profile of the ELF EMF–exposed rats showed no significant difference from that of control rats, which suggests that lipid metabolism was not affected by ELF EMF exposure. In addition, we determined the blood glucose (BG) as an indicator of glucose metabolism. As shown in Fig. 4E, there was no significant difference between ELF EMF–exposed animals and control animals.
Fig. 4.
The effect of ELF EMF exposure on blood lipid profile and blood glucose level. The blood lipid and glucose were determined every 4 weeks during the 12 weeks' observation. (A) The level of total cholesterol (TC). (B) The level of triglyceride (TG). (C) The level of low-density lipoprotein (LDL). (D) The level of high-density lipoprotein (HDL). (E) The level of blood glucose (BG). Error bars indicate the standard deviation (SD) of the mean for n = 64 independent experiments.
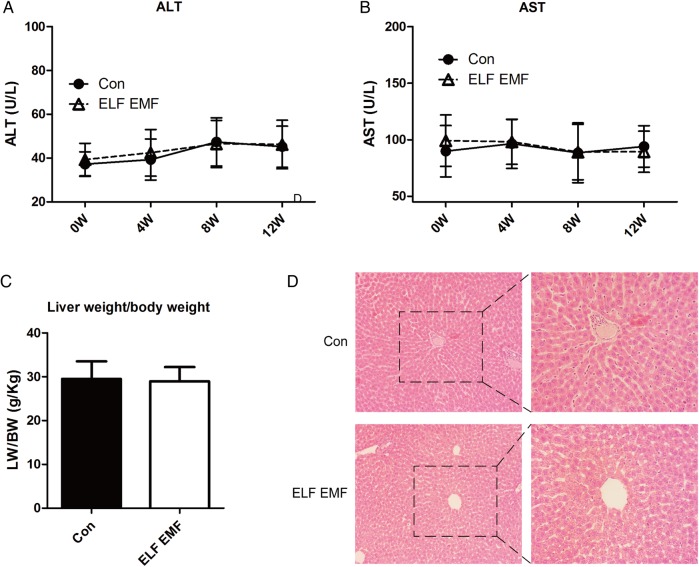
ELF EMFs had no effect on liver function
Moreover, we estimated the liver function by measuring AST and ALT. The results showed that both AST and ALT showed no significant difference after 100-μT ELF EMF exposure and they did not differ from the sham group (Fig. 5A and B). These results suggested that ELF EMFs might not influence liver function. In addition, as shown in Fig. 5C, the ratio of liver weight to body weight was similar between the two groups. Moreover, compared with the control group, the results of HE staining showed that exposure to ELF EMFs did not alter the structure of the liver (Fig. 5D)
Fig. 5.
ELF EMF exposure did not affect the liver function during the 12 weeks' observation. (A) The level of alanine transaminase (ALT). (B) The level of aspartate aminotransferase (AST). (C) The ratio of the liver weight to the body weight. (D) The HE staining of the liver section: the left images were taken at a magnification of × 100, while the right images were taken at a magnification of × 200. Error bars indicate the standard deviation (SD) of the mean for n = 64 independent experiments.
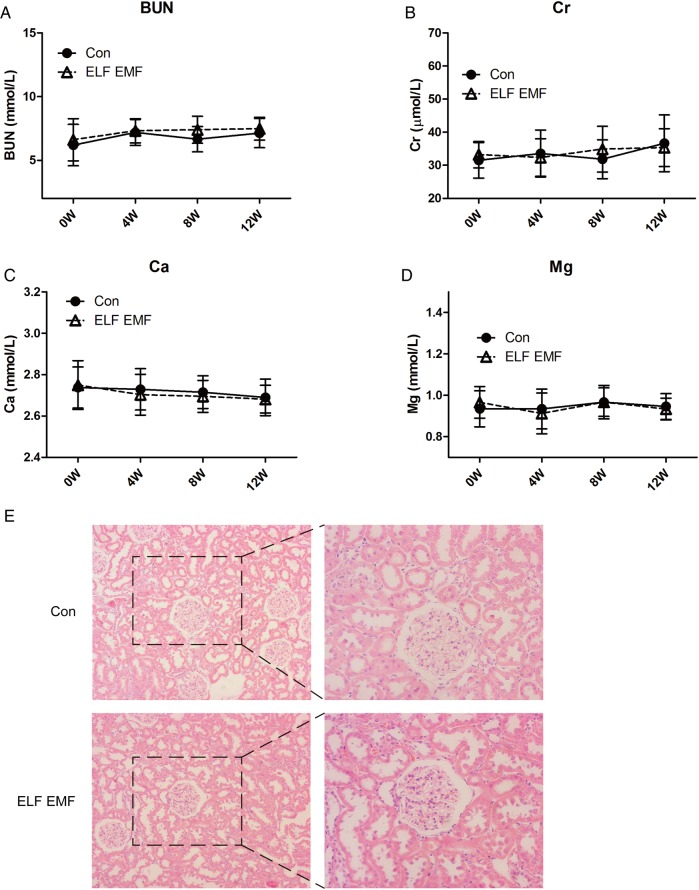
Exposure to ELF EMFs did not affect renal function
BUN and Cr are important and direct indicators for the evaluation of the renal function. Thus, we measured the concentrations of BUN and Cr every 4 weeks. As shown in Fig. 6A and B, the concentrations of BUN and Cr were not influenced by ELF EMF exposure compared with the control group. Further, we evaluated the level of blood Ca and Mg, and the results showed that exposure to ELF EMFs had no effect on the concentrations of Ca and Mg (Fig. 6C and D). To further study the structure of the renal function, HE staining was conducted. The results showed that the structure of the renal glomerulus did not differ between the two groups (Fig. 6E).
Fig. 6.
The effect of ELF EMF exposure on renal function. (A) The concentration of the blood urea nitrogen (BUN). (B) The level of creatine (Cr). (C) The concentration of total calcium (Ca). (D) The level of total magnesium (Mg). (E) The HE staining of the renal section: the left images were taken at a magnification of ×100, while the right images were taken at a magnification of ×200. Error bars indicate the standard deviation (SD) of the mean for n = 64 independent experiments.
DISCUSSION
In the present study, we constructed an exposure device that could produce a 100-μT ELF EMF, and rats were used as the subject to evaluate the effects of 100-μT ELF EMFs on the hematogram and blood chemistry. Our results suggested that the general condition of the rats were not affected by the ELF EMF. In addition, the results from blood hematogram and blood chemistry showed that the WBC, RBC, platelets, blood lipid profile, blood glucose, liver function and renal function of rats from the ELF EMF group were not significant different from those of the sham group. Moreover, we also demonstrated that exposure to a 100-μT ELF EMF did not influence the histological structure of the liver and kidney compared with the control group.
Our investigation showed that exposure to ELF EMFs had no effect on hematogram, which is consistent with the previous epidemiological studies [24] and animal studies [20]. However, Seto et al. reported that total WBC count, lymphocyte count, and eosinophil count were significantly lower in 60-Hz high-intensity field (80-KV/m)–exposed subjects [25], which was supported by Cakir et al. using a 0.98-mT ELF EMF as the exposure source [15]. These studies suggest that the intensity of the ELF EMF is an important factor influencing the hematologic response.
As for the hematogram examination, blood chemistry is usually used to evaluate the health of humans and animals. The results of the lipid profile and blood glucose level showed that exposure to ELF EMFs had no effect on them, which is similar to the results of the study conducted by Ragan et al. [19]. Recently, Torres-Duran et al. found that 60-Hz/2.4-mT ELF EMF exposure did not influence the triglyceride or total cholesterol, whereas the HDL and free fatty acid levels were increased [26].
Liver function detection showed that the liver enzyme, liver weight and liver structure were not affected by exposure to ELF EMFs. Coincidentally, the liver weight was not influenced in rats exposed to an EMF for a longer period or at a higher intensity [27, 28]. However, there remains a controversy about the effects of ELF EMFs on liver function. Hashish et al. reported that exposure to a 50-Hz ELF EMF for 30 days caused a significant increase in AST level [29], which may due to oxidative stress or apoptosis caused by the ELF EMF [30].
Importantly, we are the first to report the effects of ELF EMFs on renal function. Both the direct indicators (BUN, Cr) and indirect indicators (blood Ca and Mg) were used to assess the renal function. No significant change was found after exposure to ELF EMFs. Some studies have reported that high-frequency EMF exposure has had some harmful effects on renal morphology and function [31, 32]. In addition, it has been reported that exposure to ELF EMFs could cause oxidative stress in kidneys [33, 34]. However, ELF EMFs may not be strong enough to damage the renal function.
In summary, in our present study, we found that exposure to ELF EMFs had no effect on the hematogram and blood chemistry, indicating that 50-Hz/100-μT ELF EMFs might be safe for human and animal health. This is similar to the conclusion reached by the World Health Organization (WHO) in 2007 after ten years of follow-up, which declared that ELF EMFs might have no effect on human health [35].
However, there are some studies that suggest that ELF EMFs could affect human health [1, 36] and even cause cancer [5, 37–39]. These conflicting results for the effects of ELF EMFs on hematograms and blood chemistry may be due to variation in the duration of exposure or to secondary electric field effects (e.g. noise), especially field strengths. Most of the positive effect studies employed higher exposure intensity (>1 mT, which is much higher than humans encounter in daily life). Generally, the maximum allowable of ELF EMFs for the public is 100 μT [3], which is the strength that was used in the present study. Thus, the results from our present study may be more suitable to use as a guide for public exposure.
However, there are some limitations in our study. First, a longer exposure time may result in more comprehensive observation. Second, the precise safety limits of ELF EMFs remain unknown and need further investigation. In addition, the effects on diseased rats remain unknown. Therefore, additional work is needed to make the conclusion more rigorous.
In conclusion, our study suggested that 100-μT ELF EMFs may not affect an animal's hematogram or blood chemistry after a 12-week exposure.
FUNDING
This work was supported by a Science and Technology Project of State Grid Corporation of China [GY71-13-057]. Funding to pay the Open Access publication charges for this article was provided by a Science and Technology Project of State Grid Corporation of China [GY71-13-057].
REFERENCES
- 1.Feychting M, Ahlbom A, Kheifets L. EMF and health. Annu Rev Public Health 2005;26:165–89. [DOI] [PubMed] [Google Scholar]
- 2.Karasek M, Woldanska-Okonska M. Electromagnetic fields and human endocrine system. ScientificWorld Journal 2004;4 Suppl 2:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 Khz). Health Phys 2010;99:818–36. [DOI] [PubMed] [Google Scholar]
- 4.Wertheimer N, Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol 1979;109:273–84. [DOI] [PubMed] [Google Scholar]
- 5.Repacholi M. Concern that “EMF” magnetic fields from power lines cause cancer. Sci Total Environ 2012;426:454–8. [DOI] [PubMed] [Google Scholar]
- 6.Roosli M. [Health effects of electromagnetic fields]. Ther Umsch 2013;70:733–8. [DOI] [PubMed] [Google Scholar]
- 7.Aydin M, Cevik A, Kandemir FM, et al. Evaluation of hormonal change, biochemical parameters, and histopathological status of uterus in rats exposed to 50-Hz electromagnetic field. Toxicol Ind Health 2009;25:153–8. [DOI] [PubMed] [Google Scholar]
- 8.Sobel E, Davanipour Z, Sulkava R, et al. Occupations with exposure to electromagnetic fields: a possible risk factor for Alzheimer's disease. Am J Epidemiol 1995;142:515–24. [DOI] [PubMed] [Google Scholar]
- 9.Ahlbom IC, Cardis E, Green A, et al. Review of the epidemiologic literature on EMF and Health. Environ Health Perspect 2001;109 Suppl 6:911–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamee DA, Legros AG, Krewski DR, et al. A literature review: the cardiovascular effects of exposure to extremely low frequency electromagnetic fields. Int Arch Occup Environ Health 2009;82:919–33. [DOI] [PubMed] [Google Scholar]
- 11.Sorahan T, Nichols L. Mortality from cardiovascular disease in relation to magnetic field exposure: findings from a study of UK electricity generation and transmission workers, 1973–1997. Am J Ind Med 2004;45:93–102. [DOI] [PubMed] [Google Scholar]
- 12.Savitz DA, Liao D, Sastre A, et al. Magnetic field exposure and cardiovascular disease mortality among electric utility workers. Am J Epidemiol 1999;149:135–42. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. Nonionizing radiation. Part I: static and extremely low frequency (ELF) electric and magnetic fields. Monographs 2002;80:429. [PMC free article] [PubMed] [Google Scholar]
- 14.Repacholi MH, Greenebaum B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics 1999;20:133–60. [DOI] [PubMed] [Google Scholar]
- 15.Cakir DU, Yokus B, Akdag MZ, et al. Alterations of hematological variations in rats exposed to extremely low frequency magnetic fields (50 Hz). Arch Med Res 2009;40:352–6. [DOI] [PubMed] [Google Scholar]
- 16.Margonato V, Nicolini P, Conti R, et al. Biologic effects of prolonged exposure to ELF electromagnetic fields in rats: II. 50 Hz magnetic fields. Bioelectromagnetics 1995;16:343–55. [DOI] [PubMed] [Google Scholar]
- 17.Scheiermann C, Frenette PS, Hidalgo A. Regulation of leucocyte homeostasis in the circulation. Cardiovasc Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agus ZS, Wasserstein A, Goldfarb S. Disorders of calcium and magnesium homeostasis. Am J Med 1982;72:473–88. [DOI] [PubMed] [Google Scholar]
- 19.Ragan HA, Buschbom RL, Pipes MJ, et al. Hematologic and serum chemistry studies in rats exposed to 60-Hz electric fields. Bioelectromagnetics 1983;4:79–90. [DOI] [PubMed] [Google Scholar]
- 20.Zecca L, Mantegazza C, Margonato V, et al. Biological effects of prolonged exposure to ELF electromagnetic fields in rats: III. 50 Hz electromagnetic fields. Bioelectromagnetics 1998;19:57–66. [DOI] [PubMed] [Google Scholar]
- 21.Fitzner Toft M, Petersen MH, Dragsted N, et al. The impact of different blood sampling methods on laboratory rats under different types of anaesthesia. Lab Anim 2006;40:261–74. [DOI] [PubMed] [Google Scholar]
- 22.Lee G, Goosens KA. Sampling blood from the lateral tail vein of the rat. J Vis Exp 2015:e52766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Chen C, Wang H, et al. Protective effects of Acyl-coA thioesterase 1 on diabetic heart via PPARalpha/PGC1alpha signaling. PLoS One 2012;7:e50376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selmaoui B, Bogdan A, Auzeby A, et al. Acute exposure to 50 Hz magnetic field does not affect hematologic or immunologic functions in healthy young men: a circadian study. Bioelectromagnetics 1996;17:364–72. [DOI] [PubMed] [Google Scholar]
- 25.Seto YJ, Majeau-Chargois D, Lymangrover JR, et al. Chronic 60-Hz electric field exposure-induced subtle bioeffects on hematology. Environ Res 1986;39:143–52. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Duran PV, Ferreira-Hermosillo A, Juarez-Oropeza MA, et al. Effects of whole body exposure to extremely low frequency electromagnetic fields (ELF-EMF) on serum and liver lipid levels, in the rat. Lipids Health Dis 2007;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Lee HJ, Choi SY, et al. Toxicity bioassay in Sprague–Dawley rats exposed to 20 kHz triangular magnetic field for 90 days. Bioelectromagnetics 2006;27:105–11. [DOI] [PubMed] [Google Scholar]
- 28.High WB, Sikora J, Ugurbil K, et al. Subchronic in vivo effects of a high static magnetic field (9.4 T) in rats. J Magn Reson Imaging 2000;12:122–39. [DOI] [PubMed] [Google Scholar]
- 29.Hashish AH, El-Missiry MA, Abdelkader HI, et al. Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf 2008;71:895–902. [DOI] [PubMed] [Google Scholar]
- 30.Emre M, Cetiner S, Zencir S, et al. Oxidative stress and apoptosis in relation to exposure to magnetic field. Cell Biochem Biophys 2011;59:71–7. [DOI] [PubMed] [Google Scholar]
- 31.Ulubay M, Yahyazadeh A, Deniz OG, et al. Effects of prenatal 900 MHz electromagnetic field exposures on the histology of rat kidney. Int J Radiat Biol 2015;91:35–41. [DOI] [PubMed] [Google Scholar]
- 32.Odaci E, Unal D, Mercantepe T, et al. Pathological effects of prenatal exposure to a 900 MHz electromagnetic field on the 21-day-old male rat kidney. Biotech Histochem 2015;90:93–101. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Samano J, Torres-Duran PV, Juarez-Oropeza MA, et al. Effects of acute electromagnetic field exposure and movement restraint on antioxidant system in liver, heart, kidney and plasma of Wistar rats: a preliminary report. Int J Rad Biol 2010;86:1088–94. [DOI] [PubMed] [Google Scholar]
- 34.Duan Y, Wang Z, Zhang H, et al. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct 2013;4:1252–62. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Environmental Health Criteria 238. Extremely Low Frequency (ELF) Fields. Geneva: World Health Organization, 2007. [Google Scholar]
- 36.Anderson LE. ELF: exposure levels, bioeffects, and epidemiology. Health Phys 1991;61:41–6. [DOI] [PubMed] [Google Scholar]
- 37.Kheifets L, Shimkhada R. Childhood leukemia and EMF: review of the epidemiologic evidence. Bioelectromagnetics 2005;Suppl 7:S51–9. [DOI] [PubMed] [Google Scholar]
- 38.Teepen JC, van Dijck JA. Impact of high electromagnetic field levels on childhood leukemia incidence. Int J Cancer 2012;131:769–78. [DOI] [PubMed] [Google Scholar]