A 40-year-old man first presented to medical attention 13 years before our evaluation with painful right-sided vision loss accompanied by neck pain and headache. There was mild proptosis of his right eye and disc edema on funduscopy. MRI of the brain with gadolinium demonstrated hypertrophic dural enhancement, orbital pseudotumor, and right optic nerve compression. CSF examination revealed an opening pressure of 35 cm, 18 × 106/L white blood cells (85% lymphocytes, 15% monocytes), 21 × 106/L red blood cells, a total protein of 168 mg/dL, and a glucose of 87 mg/dL (corresponding serum glucose 126 mg/dL). CSF bacterial, fungal, and acid-fast bacilli cultures were negative. There were no oligoclonal bands unique to the CSF. Cytology and flow cytometry were benign. Proteinase-3 and myeloperoxidase (anti-neutrophil cytoplasmic) antibodies were negative.
Right orbital decompression was performed at age 34 for progressive right optic neuropathy. Dural biopsy obtained during that procedure revealed dense, fibrous tissue with chronic, reactive inflammation. The patient was diagnosed with idiopathic hypertrophic pachymeningitis. A ventriculoperitoneal shunt was placed, and he was treated with glucocorticoids. Unfortunately, several steroid-related toxicities including diabetes mellitus and avascular necrosis of the femoral head necessitated steroid discontinuation. Methotrexate was self-discontinued by the patient because of lack of perceived benefit. A left compressive optic neuropathy with an inferior altitudinal defect and preserved visual acuity ultimately developed.
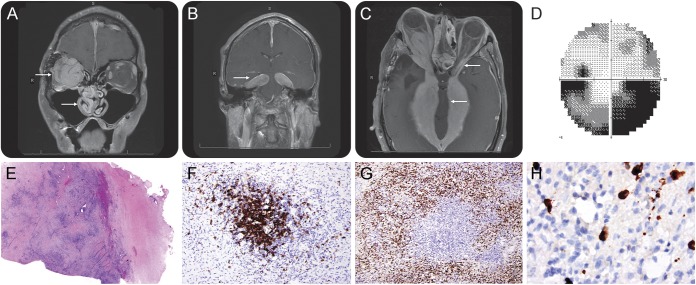
At the age of 40, the patient sought consultation with us in advance of plans for repeat hip arthroplasty. He had a new pseudobulbar affect and brisk deep tendon reflexes. The right optic nerve was atrophic and without light perception. MRI of the brain demonstrated significant disease progression (figure, A–C). Visual field loss in the left eye was stable (figure, D). Serum immunoglobulin G4 (IgG4) was elevated at 141 mg/dL (reference range 4–86 mg/dL). Peripheral lymphocyte subset analysis uncovered a low absolute number of CD8 T cells (203, reference range 270–918 × 106/L).
Figure. Imaging, neuro-ophthalmologic, and neuropathologic findings in aggressive IgG4-related hypertrophic pachymeningitis.
(A) Coronal T1 MRI following the administration of IV gadolinium reveals nasal and orbital invasion by IgG4 pseudotumor and (B) diffuse pachymeningitis within the tentorium cerebelli forming tumefactive masses. (C) There is severe midbrain compression at the level of the cerebral peduncles with compression of the left optic nerve as visualized on T1 fast spoiled gradient. (D) There is an inferior altitudinal field defect in the left eye on 30° Humphrey Visual Field testing (there is no light perception in the right eye). Dural biopsy shows (E) dense lymphocyte infiltration and storiform fibrosis on hematoxylin & eosin 40× with (F) collections of CD20-expressing cells surrounded by (G) infiltrating CD138+ plasma cells 200×. (H) A significant number of the plasma cells express IgG4 400×. IgG4 = immunoglobulin G4.
In light of emerging literature about IgG4-related hypertrophic pachymeningitis (IgG4-RHP) as a manifestation of IgG4-related disease (IgG4-RD),1–3 banked tissue from our patient's dural biopsy obtained 6 years prior was recut and reviewed (figure, E–H). The IgG4/IgG-positive plasma cell ratio was estimated at approximately 32.8%. On quantitative assessment, averages of between 20 and 60 IgG4-positive cells were seen per high-power field in different regions. Although there are no formally established diagnostic criteria for IgG4-RHP, these findings greatly exceed the cutoffs used to define this entity in a seminal description that set a threshold of 10 IgG4-positive plasma cells per high-power field when averaged over 5 consecutive fields.1 In that study, IgG4-RHP cases had an IgG4/IgG-positive plasma cell ratio ranging from 24% to 60% compared to 0% to 8% in controls. Taken together with the patient's clinical syndrome, the neuropathologic findings were consistent with IgG4-RHP. There was no evidence of IgG4-RD affecting other organ systems.
IgG4-RHP is the manifestation of a chronic inflammatory response. One hypothesis is that T-helper type 2 cells stimulate oligoclonal lymphoplasmacytosis and storiform fibrosis through the production of the interleukins 4, 5, 10, and 13 and transforming growth factor β.2 Meningeal antigens are suspected immune targets, but none have yet been identified. High-dose glucocorticoids are a first-line treatment, although toxicity can be dose-limiting.3 Methotrexate may be beneficial in steroid-refractory cases.3 B cell–depleting monoclonal antibodies that target CD20, such as rituximab, may also be beneficial for IgG4-RD and IgG4-RHP.4,5 Our patient declined additional glucocorticoids and elected to proceed with rituximab. Three months later, his MRI was stable, and there was near normalization of his IgG4 serum concentration to 87 mg/dL.
Two large epidemiologic studies have found that between 8% and 29% of patients with hypertrophic pachymeningitis in fact have IgG4-RHP.6,7 Published imaging and histopathology in IgG4-RHP primarily describe relatively mild pachymeningeal findings characterized by linear dural thickening with sparse, focal inflammatory infiltrates.3 The case presented here illustrates a more severe side of the IgG4-RHP spectrum with disease progression presumably from lack of sustainable immune suppression. These imaging findings are also a testament to the tremendous capability of the cerebral peduncles to withstand slow mechanical compression. This case emphasizes the importance of early recognition and treatment of IgG4-RHP and underscores the value of re-reviewing biopsies labeled as idiopathic hypertrophic pachymeningitis, since many cases were read out in the era before IgG4-RHP was recognized.
Footnotes
Author contributions: R.D.S.: study concept and design, acquisition of data, wrote the manuscript. M.W.: acquisition of data, critical revision of the manuscript. M.H.L.: acquisition of data, critical revision of the manuscript. A.P.: acquisition of data, critical revision of the manuscript. J.M.G.: study concept and design, acquisition of data, critical revision of the manuscript, study supervision.
Study funding: NIH National Center for Advancing Translational Research KL2TR000143 (J.M.G.).
Disclosure: R.D. Schubert reports no disclosures. M. Wood received research support from NIH. M.H. Levin received research support from NIH, University of California, Clinical and Translational Science Institute, Research to Prevent Blindness Career Development award, That Man May See, does private medical-legal consulting. A. Perry was chief editor for and is now a senior editor of Brain Pathology. J.M. Gelfand served on a scientific advisory board for MedImmune, Roche, received research support from Quest Diagnostics, NIH, received compensation for expert witness medical-legal consulting related to inflammatory neurologic disease, his spouse served on a scientific advisory board for Eli Lilly, received research support from Allergan and EMKinetics, received compensation for expert witness medical-legal consulting related to migraine and headache disorders. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Lindstrom KM, Cousar JB, Lopes MB. IgG4-related meningeal disease: clinico-pathological features and proposal for diagnostic criteria. Acta Neuropathol 2010;120:765–776. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012;366:539–551. [DOI] [PubMed] [Google Scholar]
- 3.Lu LX, Della-Torre E, Stone JH, Clark SW. IgG4-related hypertrophic pachymeningitis: clinical features, diagnostic criteria, and treatment. JAMA Neurol 2014;71:785–793. [DOI] [PubMed] [Google Scholar]
- 4.Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 2010;62:1755–1762. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis 2015;74:1171–1177. [DOI] [PubMed] [Google Scholar]
- 6.Wallace ZS, Carruthers MN, Khosroshahi A, et al. IgG4-related disease and hypertrophic pachymeningitis. Medicine 2013;92:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonekawa T, Murai H, Utsuki S, et al. A nationwide survey of hypertrophic pachymeningitis in Japan. J Neurol Neurosurg Psychiatry 2014;85:732–739. [DOI] [PubMed] [Google Scholar]