Abstract
OBJECTIVES: The purpose of this study was to describe the method of delivery, dosage regimens, and outcomes of sedatives administered by extravascular route for imaging procedures in children.
METHODS: Medline, Embase, International Pharmaceutical Abstracts, and Cochrane Database of Systematic Reviews were searched using keywords “child”, “midazolam”, “ketamine”, dexmedetomidine”, “fentanyl”, “nitrous oxide”, and “imaging.” Articles evaluating the use of extravascular sedation in children for imaging procedures published in English between 1946 and March 2015 were included. Two authors independently screened each article for inclusion. Reports were excluded if they did not contain sufficient details on dosage regimens and outcomes.
RESULTS: Twenty reports representing 1,412 patients ranging in age from 0.33 to 19 years of age were included for analysis. Due to discrepancies in doses and types of analyses, statistical analyses were not performed. Oral midazolam was the most common agent evaluated; other agents included intranasal (IN) ketamine, IN midazolam, IN fentanyl, IN and transmucosal dexmedetomidine, and N2O. Most agents were considered efficacious compared with placebo.
CONCLUSIONS: Most agents showed efficacy for sedation during imaging when delivered through an extra-vascular route. Selection of agents should be based on onset time, duration, patient acceptability, recovery time, and adverse events. More robust studies are necessary to determine the optimal agent and route to utilize for imaging procedures when sedation is needed.
INDEX TERMS: child, dexmetomidine, fentanyl, ketamine, midazolam, nitrous oxide
INTRODUCTION
Sedation is required for pediatric imaging studies that an adult could tolerate with minimal or no sedation. The American Academy of Pediatrics has published guidelines on the management and monitoring of pediatric patients undergoing sedation for diagnostic and therapeutic procedures.1 These guidelines mention that the goals of sedation during procedures should be to maximize patient safety, minimize discomfort and pain, control anxiety, control behavior/movement so that the procedure can be completed, and ensure the patient is safe enough to be discharged. It should be noted that most imaging studies are not painful, but it is imperative that the patient remains still throughout the procedure. The inability of the child to remain still is multifactorial: developmental immaturity, age-appropriate fearfulness, and limited cognitive ability to perceive these procedures as beneficial. However, several anatomic differences make children prone to respiratory depression and hypoxemia when undergoing sedation: an airway more susceptible to occlusion, higher metabolic activity, and incompletely developed lungs.2
Intravenous (IV) propofol and dexmedetomidine have been shown to be effective in providing sedation, but the placement of an IV line may be more noxious than the procedure. Hence, there is a great need for medications that can be delivered through the oral (PO), intranasal (IN), or transmucosal (TM) route. The agent of choice would be determined by the depth of sedation required and duration of the procedure. This review analyzed the current body of published reports that describe the method of delivery, onset time, effective dose, and recovery time needed for medications administered through an extravascular route, to provide sedation during a non-painful imaging procedure in pediatric patients.
METHODS
Review
Relevant articles were identified using MED-LINE (1946 to January 2015), EMBASE (1980 to January 2015), and the Cochrane Database of Systematic Reviews (2005 to January 2015), using the individual sedative agents (midazolam, ketamine, dexmedetomidine, fentanyl, nitrous oxide), imaging (computed tomography [CT], voiding cystourethrogram [VCUG], magnetic resonance imaging [MRI], echocardiography, and endoscopy) and child as key words. Results were limited to human studies published in English.
A two-step selection process was used for study inclusion. The initial reports were first screened by 2 authors (AT, JLM). The final selection process was determined by input from all authors. To be included for analysis, the published report had to include children 19 years of age and younger receiving extravascular sedation for an imaging study. Reports were excluded if they did not contain sufficient details regarding the dosage regimen, outcomes, and adverse events. Statistical analysis was not used, given the expected discrepancies in dosing and types of analyses (retrospective versus prospective design).
RESULTS
Twenty papers representing 1,412 patients ranging in age from 0.33 to 19 years old were included in the analysis. Characteristics of these 20 reports are reported in Tables 1 to 3.2–22 Most studies evaluated sedatives for VCUG. The most common agent evaluated was PO midazolam.7–14,19–20,22 Table 4 provides a summary of dosing information and adverse events reported in the included studies.
Table 1.
Summary of Studies Evaluating Procedural Sedation for Computed Tomography and Magnetic Resonance Imaging Studies3–7
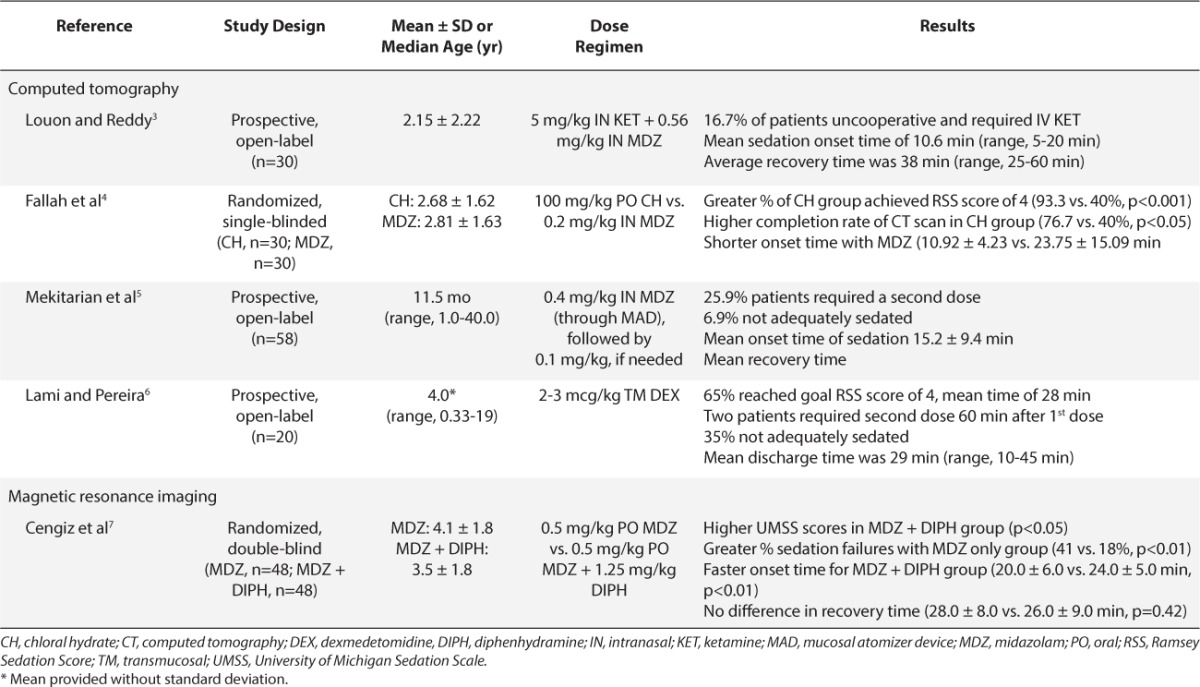
Table 3.
Summary of Studies Evaluating Procedural Sedation for Echocardiogram and Upper Gastrointestinal Endoscopy Imaging Studies19–22
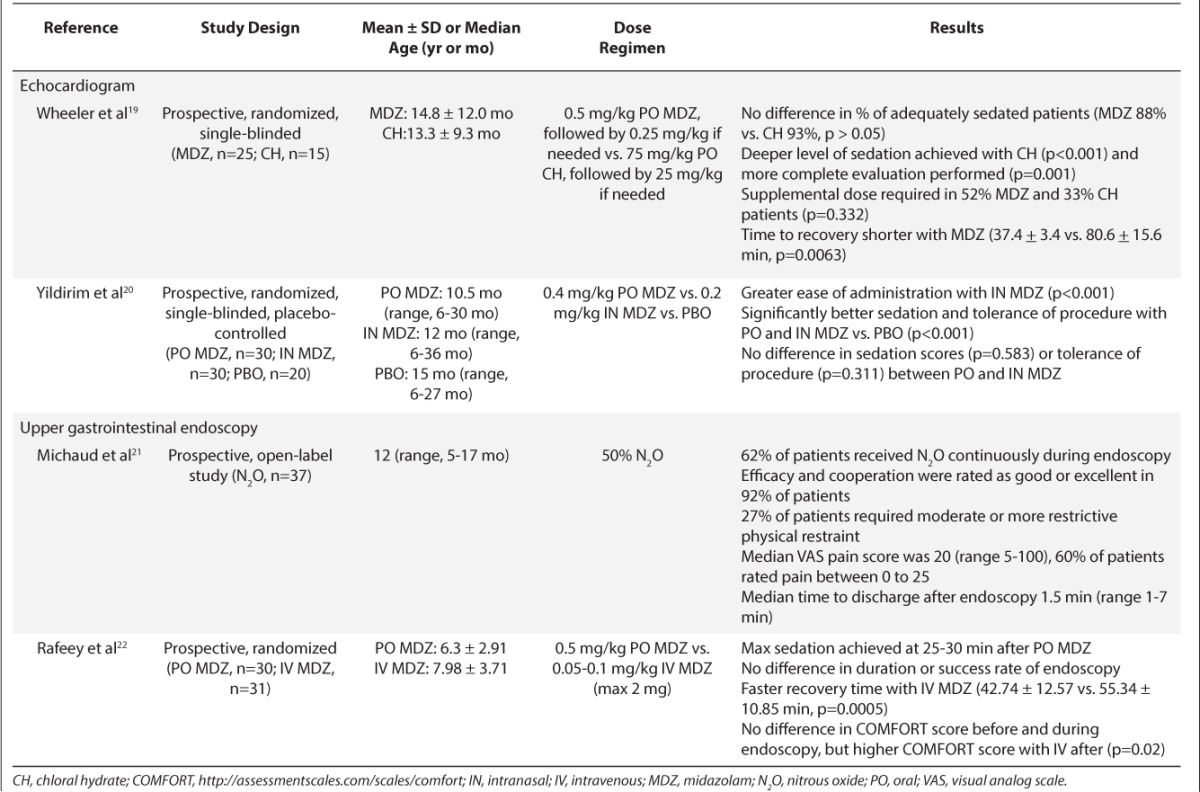
Table 4.
Summary of Medications Used for Procedural Sedation3–22
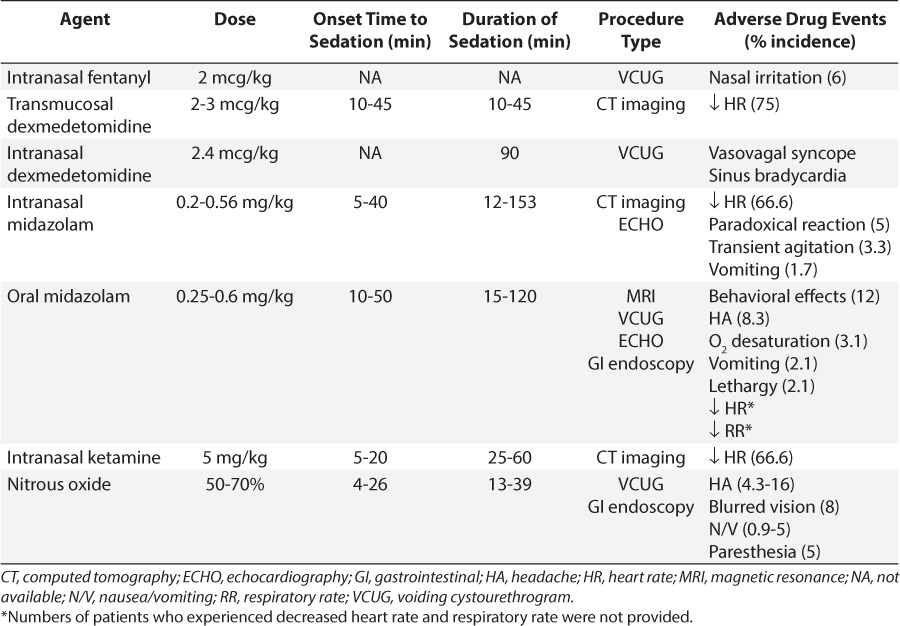
Table 2.
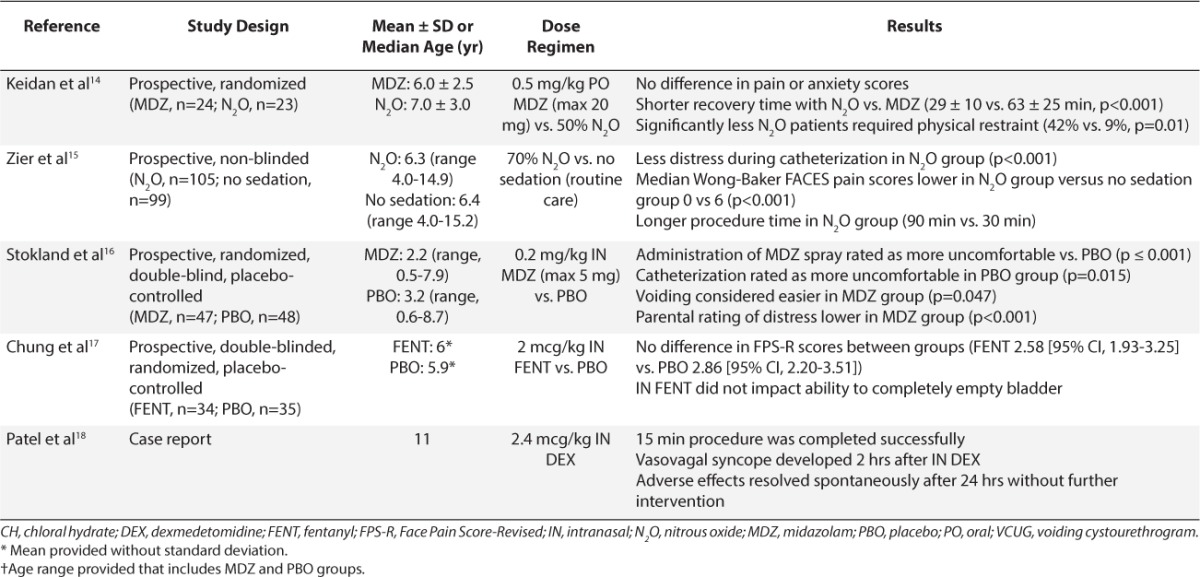
Summary of Studies Evaluating Procedural Sedation for Voiding Cystourethrogram Imaging Studies8–18
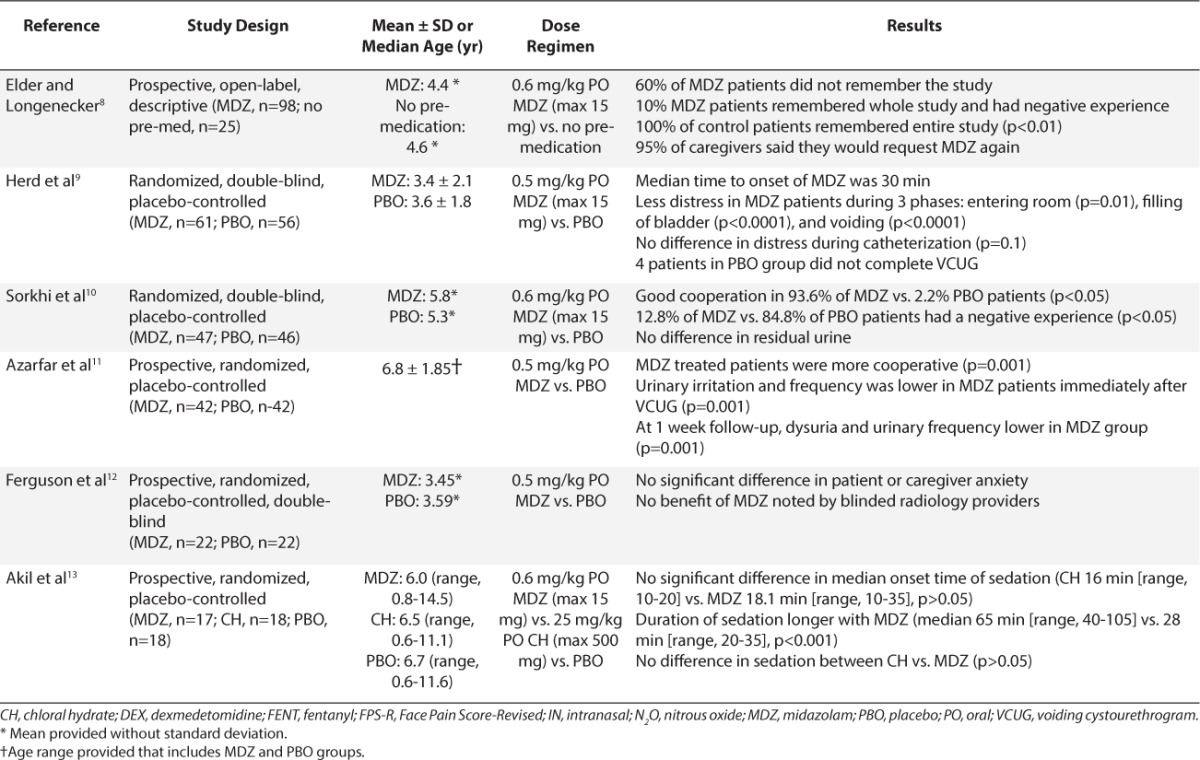
Table 2.
Summary of Studies Evaluating Procedural Sedation for Voiding Cystourethrogram Imaging Studies8–18 (cont.)
Computed Tomography Imaging
CT imaging requires the patient to be immobile to achieve a quality scan. The efficacy of a single agent or combination sedation regimen was evaluated in 4 studies of children undergoing a CT procedure (Table 1).3–6 Louon and colleagues3 were the only investigators to evaluate combination IN midazolam, 0.56 mg/kg and ketamine 5 mg/kg. Only 13.3% of patients were agitated during instillation. Adequate sedation was achieved in 83.3% of patients, with a mean onset time of 10.6 minutes; the remaining 16.7% of children received a rescue IV ketamine dose. Bradycardia occurred in 66.7% of the patients, but this was not considered clinically significant.
Two studies evaluated the use of IN midazolam as monotherapy for CT imaging (2–3). Fallah and colleagues4 compared IN midazolam, 0.2 mg/kg, to PO chloral hydrate. The investigators targeted a Ramsay Sedation Scale (RSS) score of 4 (i.e., response to light glabellar tap). Adequate sedation was achieved in 40% of patients in the IN midazolam group compared to 93.3% in the chloral hydrate group (p<0.001). Lack of treatment success was attributed to a low IN midazolam dose. Overall, IN midazolam was well tolerated, and no serious adverse effects were reported. Mekitarian-Filho and colleagues5 evaluated IN midazolam, 0.4 mg/kg, administered using a nasal mucosal atomizer device (MAD). A minimum RSS score of 3 (i.e., awake and responsive to commands) was considered adequate for sedation. Adequate sedation was achieved in 75% of the patients; with 93.3% of the CT images considered good quality. Adverse events were minor, including paradoxical reactions in 3 patients (5%) and vomiting in 1 patient (1.7%). The authors noted that 28.3% of patients cried during IN midazolam administration, but the cause of crying was not elucidated further.
Overall, IN midazolam monotherapy or combination therapy with IN ketamine appears to be effective for CT imaging. Louon and colleagues3 noted a higher percentage of patients successfully completed the CT scan with combination therapy, but there were no direct comparisons of combination versus monotherapy. IN midazolam doses ranged from 0.2 to 0.56 mg/kg.3–5 Based on the results of these studies, higher IN midazolam doses of 0.4 to 0.5 mg/kg would be appropriate for monotherapy.
Lami and colleagues6 evaluated TM dexmedetomidine in 20 children scheduled for CT imaging. The 100 mcg/mL IV dexmedetomidine formulation was administered buccally at a dose of 2 to 3 mcg/kg in order to achieve an RSS score of 4. Sixty percent of patients achieved adequate sedation with a single dose. Additional sedatives and anesthetics were required for 7 patients, but these measures were not further defined. Seventeen children (85%) developed a 20% to 40% reduction in heart rate from baseline, but no pharmacologic treatment was administered. A recommendation for the use of TM dexmedetomidine cannot be made due to the small sample size of this study.
Magnetic Resonance Imaging
Sedation is essential for MRI in children because of the time and immobility needed to complete the scan. Only 1 study was identified that used a sedative through an extravascular route for MRI. Cengiz and colleagues7 compared PO midazolam, 0.5 mg/kg, to a combination of PO midazolam, 0.5 mg/kg, and diphenhydramine, 1.25 mg/kg. Midazolam monotherapy failed to achieve adequate sedation in significantly more patients than in the group receiving combination therapy (41% vs. 18%, respectively, p=0.01). It is important to note there were no significant differences in duration of MRI between the group that received combination therapy and the monotherapy group (31 ± 9 minutes vs. 29 ± 7 minutes, respectively, p=0.17). The authors noted that sedation failure was greater in older children (5 ± 1 years of age). In addition, use of combination therapy significantly shortened the time to procedure readiness (20 ± 6 vs. 24 ± 5 minutes, respectively, p=0.01), but this difference was not clinically significant. There were no statistical differences in the time to recovery between the groups (28 ± 8 vs. 26 ± 9 minutes, respectively, p=0.42). Combination therapy resulted in a greater reduction in respiratory rate and oxygen saturation than the midazolam group, but no patient required intervention.
Oral midazolam appears to be an option for MRI sedation, although it was associated with a high failure rate. The addition of diphenhydramine was found to increase the rate of MRI completion, although sedation was more likely to fail in children ≥5 years of age.7 It is not clear why older children had less response. The study evaluated only children 1 to 7 years old, so this finding could be a result of a small sample size. Combination therapy shortened time to procedure readiness by 5 minutes, but this benefit came with an increase in adverse events.
Voiding Cystourethrogram
VCUG can be unpleasant due to urinary catheter insertion and anxiety associated with examination of the genitalia. Sedatives may be needed to minimize anxiety and allow the child to remain cooperative without affecting voiding. Ten studies and 1 case report included in this report describe sedation during VCUG.8–18 Midazolam was the medication most widely studied for VCUG. Elder and colleagues8 were the first to evaluate the safety and efficacy of PO midazolam. Ninety-eight children received a PO dose of 0.6 mg/kg of an IV midazolam solution, which was artificially sweetened with a Kool-Aid mixture (Kraft Foods, Dover, DE), approximately 20 to 30 minutes prior to VCUG. Another 25 children were recruited as control patients, with no medication. A telephone follow-up was completed in 94 of the midazolam group and all 25 controls. Nineteen patients (20%) in the midazolam group versus all control patients remembered the entire study. Of those in the midazolam group who remembered, only 9 patients (9.6%) reported the experience as “negative.” Behavioral side effects were noted in 12% of children receiving midazolam (e.g., aggression and inconsolability). However, there was no effect on post-voiding residual urine volume.
Four more recent studies also compared PO midazolam, 0.5 to 0.6 mg/kg, versus placebo for sedation during VCUG.9–12 Three of these studies reported that patients treated with midazolam were more cooperative and tolerant versus those treated with placebo.9–12 Herd and colleagues7 divided the procedure into 5 phases: entering the room, catheterization, filling, voiding, and leaving. Patients who received midazolam were significantly less distressed than those given placebo during room entrance (p=0.01), filling (p<0.0001), and voiding phases (p<0.0001). No differences in distress were noted during catheterization (p=0.1). Sorkhi and colleagues10 reported that 12.8% of patients remembered the entire procedure as a “negative” experience, similar to the percentage reported by Elder and colleagues.8 Likewise, no differences in residual volume at completion of procedure were noted between groups. Azarfar and colleagues11 also followed up 1 week after the VCUG procedure; midazolam-treated patients had less urinary irritation, resulting in less dysuria and decreased urinary frequency (p=0.001). The authors suggested that if patients are more cooperative during catheterization, then insertion of the catheter would be less traumatic physically.
Ferguson et al12 found no significant differences in patient or caregiver anxiety scores when PO midazolam was given for VCUG sedation. They evaluated the efficacy of 0.5 mg/kg midazolam oral syrup versus placebo in children 2 to 6 years old 15 minutes prior to the procedure. Anxiety was assessed using the modified Yale Preoperative Anxiety Scale, and caregiver anxiety was assessed using the State-Trait Anxiety Inventory. Only half of the desired patients were enrolled based on the a priori power calculation, which might have allowed the introduction of a type II error. They also gave midazolam 15 minutes prior to VCUG versus administration 20 to 30 minutes prior to VCUG by Elder and colleagues,8 which also might have influenced the results.
Two studies compared PO midazolam to other sedatives for VCUG sedation.13–14 Akil and colleagues13 compared sedative effects of PO midazolam, 0.6 mg/kg, to those of PO chloral hydrate, 25 mg/kg, administered 15 to 30 minutes prior to VCUG; they also included a placebo group. Sedation was assessed using the Brietkopf-Buttner Classification of Emotional Status score. They also evaluated the physician's perception of patient cooperation during the procedure. Patients receiving midazolam were more sedated versus those receiving placebo (p=0.01), but this was not statistically different from patients receiving chloral hydrate. The median onset time to sedation was also not significantly different between midazolam (18.1 minutes) and chloral hydrate (16 minutes; p>0.05). However, the duration of effect was twice as long with midazolam (65 minutes vs. 28 minutes, p=0.018). There were no adverse events reported.
Keidan and colleagues14 compared the safety and efficacy of PO midazolam versus N2O in children undergoing VCUG. The investigators compared 0.5 mg/kg midazolam, administered 30 minutes prior to VCUG versus 50% N2O initiated just before the patient undressed. Similar ratings for pain and anxiety were found; however, fewer patients in the N2O group required physical restraint (9% vs. 42%, respectively, p=0.01). In addition, mean recovery time was 34 minutes shorter in patients receiving N2O (p<0.001).
One additional study evaluated N2O in children undergoing VCUG. Zier and colleagues15 compared 70% N2O versus no sedation. The investigators assessed pain through the Wong-Baker FACES pain scale (Wong-Baker FACES Foundation, Oklahoma City, OK), where they were assigned a pain score ranging from 0 (“no hurt”) to 10 (“hurts worse”). They noted that distress and pain scores were higher in non-sedated patients (p<0.001).
The investigators of these 7 studies used different primary outcomes (e.g., anxiety versus level of sedation) and different assessment tools. The midazolam dose ranged from 0.5 to 0.6 mg/kg, and administration time ranged from 15 to 30 minutes prior to VCUG, which might have affected the onset and efficacy of PO midazolam. Differences in efficacy also might have been related to the PO midazolam dose form. Commercially available midazolam oral solution was not on the market prior to 2005, so 3 of the earlier studies compounded an oral solution from the IV solution.8–10,13 Elder and colleagues8 noted that compounded midazolam solutions using artificially sweetened liquids should be used because sugar-sweetened drinks were associated with decreased efficacy.
N2O appears to be a promising agent for VCUG because of its fast onset and offset times. In studies that evaluated N2O, concentrations ranged from 50% to 70%. However, to our knowledge, there are no studies comparing higher versus lower N2O doses.
IN midazolam has also been evaluated as a pre-VCUG sedative. Stokland and colleagues16 compared the use of 0.2 mg/kg midazolam IN using 5 mg/mL IV solution versus placebo administered 3 to 5 minutes prior to catheterization. No statistical differences were noted in completion rate or duration of procedure. IN midazolam administration was rated more uncomfortable (p<0.001). Patients in the placebo group were more uncomfortable during catheterization (p=0.015).
IN midazolam offers another route of administration prior to VCUG. It was administered 2 to 3 minutes before the VCUG, whereas PO midazolam required administration 15 to 30 minutes prior because of time for enteral absorption. The administration time favors IN midazolam and could decrease the preparation time for the procedure. One negative point to highlight is that some discomfort can occur with IN midazolam. More studies are needed to determine the optimal dosing in a larger sample.
IN fentanyl versus placebo was assessed by Chung and colleagues17 in children 4 to 8 years old undergoing VCUG. The investigators were assessing pain with catheter insertion versus procedural sedation. The investigators found no statistical differences in pain. The authors believed that the lack of significant analgesic effect was a result of how the fentanyl was administered. They administered the IN fentanyl dose in one nostril over 30 to 60 seconds and believed that it might have been more effective if the total dose was divided into aliquots and administered in both nostrils. Incomplete bladder emptying at VCUG completion was noted in 1 fentanyl (2.9%) and 2 placebo patients (5.7%). Although this finding was not significant, urinary retention is a common adverse event with opioids, and thus, IN fentanyl may not be the best choice for sedation in VCUG.
Patel and colleagues18 also described the use of IN 2.4 mcg/kg dexmedetomidine for an 11-year-old female with recurrent urinary tract infections who underwent a VCUG procedure. This report did not focus on the efficacy of the sedation for the procedure but rather focused on a description of a vasovagal syncope episode that the patient experienced following IN dexemdetomidine sedation. This being said, the patient did successfully complete the 15-minute procedure. Approximately 12 hours after receiving IN dexmedetomidine, the patient collapsed and had heart rate of 36 beats per minute and blood pressure of 78/51 mm Hg. Electrocardiography was conducted and showed sinus bradycardia. The patient was admitted for observation and telemetry monitoring; no further interventions were needed, and she was discharged home the next day.
Echocardiography
Sedation during transthoracic echocardiography is required to decrease excessive movement and anxiety during the procedure. Two studies were identified that evaluated procedural sedation during echocardiography.19–20 Wheeler and colleagues19 compared the use of PO midazolam, 0.5 mg/kg, versus PO chloral hydrate, 75 mg/kg. There were no differences in the number of patients who were adequately sedated to complete a partial echocardiographic evaluation (p>0.05). However, a more comprehensive echocardiogram evaluation was performed in the chloral hydrate group because deeper sedation was achieved (p<0.001). There were no statistical differences in need for additional sedation (p=0.332). There also were no difference in onset time of sedation, but the mean recovery time in midazolam patients was approximately 40 minutes faster (p=0.0063). The shorter recovery time could be financially advantageous because the patient would require shorter post-procedural monitoring. However, this advantage appears to come with a trade-off. Children receiving midazolam were not able to achieve a deeper level of sedation, and almost 40% of evaluations were terminated before a full comprehensive evaluation was completed.
Yildirim and colleagues20 compared IN midazolam, 0.2 mg/kg, versus PO midazolam, 0.4 mg/kg, in children undergoing echocardiography. In addition, a third group received placebo. There were no statistical differences between IN and PO midazolam in regard to level of sedation (p=0.583) or tolerability of the procedure (p=0.311). However, children receiving IN and PO midazolam were better sedated (p<0.001) and more comfortable than those receiving placebo (p<0.001). The IN route had greater patient acceptability than PO midazolam (93.3% vs. 36.7%, respectively, p<0.001). One patient (3.3%) in the PO group, 3 patients (10%) in the IN group, and 7 patients (35%) in the placebo group did not complete the procedure. No adverse effects were noted.
Chloral hydrate and midazolam may be effective for sedation during echocardiograms in children. In the present studies, chloral hydrate allowed for a full comprehensive evaluation but had a prolonged duration of activity when compared with PO midazolam.19 Current data suggest that IN midazolam may be more effective than PO midazolam. Wheeler and colleagues19 noted a significant number of echocardiograms were terminated early due to inadequate sedation. In addition, Yildrim and colleagues20 noted that IN midazolam had greater acceptability. More studies should evaluate the optimal dose, route, and agent for children undergoing echo-cardiograms without IV access.
Upper Gastrointestinal Endoscopy
Procedural sedation is necessary during upper gastrointestinal (GI) endoscopy procedures because the gag reflex needs to be diminished for successful completion. Two studies evaluated procedural sedation in pediatric upper GI procedures.21–22 Michaud and colleagues21 studied the safety and efficacy of 50% N2O for sedation during endoscopy. N2O was administered for 3 to 8 minutes prior to endoscopy in all patients and was continuously given during the procedure in 62% of patients. Efficacy was rated on a 3-point scale (i.e., ranging from excellent to poor) by the pediatric endoscopist and nurse. They noted excellent or good efficacy in 89% to 92% of children, and the procedure was completed in all children. Despite the perceived efficacy, moderate restraint and restrictive physical restraint were required in 16% and 5% of children, respectively. Patients assessed pain during the procedure using a Visual Analog Scale (VAS). The investigators noted a wide range of VAS scores with a median score of 20 (range 5 to 100), but the majority (60%) had a pain score ranging from 0 to 25. Minor adverse events reported included headache (16%), blurred vision (8%), nausea/vomiting (5%), and paresthesia (5%).
Rafeey and colleagues22 compared PO midazolam, 0.5 mg/kg, to IV midazolam, 0.05 to 0.1 mg/kg, in 61 children undergoing esophagogastroduodenoscopy. The IV midazolam solution was used to make a 2.5 mg/mL oral solution, which was administered 30 minutes prior to the procedure. Level of sedation was assessed by the COMFORT scale (http://assessmentscales.com/scales/comfort). Overall, there were no differences in level of sedation; the procedure was successfully completed in all patients. Patients in the PO midazolam group took 12.5 minutes longer to recover than those receiving IV therapy. During endoscopy, both groups had a decrease in peripheral oxygen saturation below 95%, and no patient required intervention. No major adverse effects occurred. The most common procedure-related symptoms included dizziness (32%), somnolence (29%), and headache (16%), which were not different between groups.
Based on the results of these two studies, both midazolam and inhaled N2O appear to be safe and effective for procedural sedation in children undergoing an upper GI endoscopy. Midazolam has many qualities that make it an ideal agent for this procedure, including its amnestic and anxiolytic effects. However, N2O may be an acceptable alternative because it has a rapid onset and recovery from sedation, along with minimal side effects. To our knowledge, no studies have directly compared N2O and midazolam for procedural sedation in children undergoing endoscopy.
DISCUSSION
Practical Considerations
Overall, most of the medications included were considered efficacious for sedation compared to placebo. It is difficult to compare the findings of these 19 studies considering the different procedure types, primary outcomes, and sedation and/or anxiety tools used. Seven studies included a comparator group; all with a relatively small sample size, making it difficult to draw conclusions.4,7,13–14,19–20,22 Three studies compared PO or IN midazolam to PO chloral hydrate.4,13,19 It should be noted that chloral hydrate is no longer commercially available, although some institutions may compound a solution.
The type of procedure should drive the selection of a medication. Longer procedures (e.g., MRI) require medications with longer activity whereas in shorter procedures (e.g., endoscopy) a more rapid offset time would be desired. A faster offset time would require less time monitoring the patient post-procedure. Based on the results of these studies, N2O appears to be ideal for shorter procedures because of the rapid onset and shorter duration of activity compared to midazolam. This being said, N2O may not be available at many institutions due to cost and other logistical issues (e.g., training requirements). Table 4 includes a summary of the onset and duration of sedation for the included studies. There was a wide range reported between the studies. It is difficult to compare recovery time between these studies because different definitions for recovery time were used.
Additional factors should be considered when selecting an agent for procedural sedation, including patient acceptability. Oral midazolam has a bitter taste, which affects tolerance. Eight studies provided details for preparation of PO midazolam; all 8 studies used the IV midazolam solution in a sweetened drink.7–14,19–20,22 Oral midazolam syrup is now commercially available, which is considered more tolerable. TM dexmedetomidine with the IV formulation was said to be well tolerated because of bland taste and minimal volume required, and no children refused it in the study by Lami et al.6 IN midazolam was administered using the IV product delivered by dropper or MAD. None of these studies compared the 2 delivery devices, but administration using a MAD may be more effective because of greater dispersion of the particles and increased surface area for absorption. It was noted by several authors that IN midazolam caused a burning sensation which limited tolerability. The use of 10 mg of lidocaine spray prior to IN midazolam administered through a MAD has been shown to prevent discomfort.23 N2O must be delivered through facemask, which is not well tolerated by children <5 years old, and mild restraint may be needed with initiation.24
Adverse events associated with a specific agent must also be taken into consideration. Table 4 includes a summary of the major adverse events noted. Decreased blood pressure was noted with PO midazolam, whereas decreased heart rate was noted with IN and PO midazolam. Both of these adverse events were not considered clinically significant. In addition, behavioral changes and paradoxical reactions were noted with use of midazolam, and this impacted successful completion of the procedure. The most commonly reported adverse effects for N2O were headache and nausea/vomiting. The nausea/vomiting with N2O is dose- and duration-dependent (>10–15 minutes after administration).24–26 Some authors have reported delayed vomiting with N2O, and caregivers should be informed that this can occur post-discharge.25 Vasovagal syncope was described in 1 case report included in this review with IN dexmedetomidine.18 The clinical significance of vasovagal syncope and other adverse events associated with dexmedetomidine administered through this route is unknown. Some of the studies used combination therapy for procedural sedation. This practice may be considered because lower doses of each agent may be used; however, combination therapy could result in increased adverse effects because of overlapping effects between the agents (e.g., over-sedation).
There remains a need for future research in this area. As noted, several studies included a comparison of sedatives with placebo. One may argue that using a placebo group may be unreasonable or unethical considering that many children may exhibit pain or fear as a result of imaging procedures. However, at the time these studies were conducted, the standard of care for some of these imaging procedures was no sedation. Future research should involve comparison of agents for specific imaging procedures.
CONCLUSIONS
Sedation is necessary during imaging procedures because a cooperative, still child is needed to complete the study. Administration of sedatives for non-painful, imaging procedures through the extravascular route has been evaluated for IN ketamine, IN/PO midazolam, TM dexmedetomidine, IN fentanyl, and N2O. Midazolam is the most widely studied agent across all procedure types. Selection of agents should be based on the onset time, duration, patient acceptability, and adverse events. Further research comparing these agents for specific procedures is needed.
ABBREVIATIONS
- CH
chloral hydrate
- CT
computed tomography
- DEX
dexmedetomidine
- DIPH
diphenhydramine
- FENT
fentanyl
- FPS-R
Face Pain Score-Revised
- GI
gastrointestinal
- IN
intranasal
- IV
intravenous
- KET
ketamine
- MAD
mucosal atomizer device
- MDZ
midazolam
- MRI
magnetic resonance imaging
- N2O
nitrous oxide
- PBO
placebo
- PO
enteral, oral
- RSS
Ramsey Sedation Score
- TM
transmucosal
- UMSS
University of Michigan Sedation Scale
- VAS
visual analog scale
- VCUG
voiding cystourethrogram
Footnotes
Disclosures Dr. Jamie Miller is on the speaker's bureau for Chiesi, USA, Inc. The other authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.American Academy of Pediatrics, American Academy of Pediatric Dentistry, Cote CJ, Wilson S for the Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118(6):2587–602. doi: 10.1542/peds.2006-2780. [DOI] [PubMed] [Google Scholar]
- 2.Johnson PN. Pain management. In: Nahata M, Benavides S, editors. Pediatric Pharmacotherapy. 1st ed. Lenexa, KS: American College of Clinical Pharmacy; 2013. pp. 834–857. [Google Scholar]
- 3.Louon A, Reddy VG. Nasal midazolam and ketamine for paediatric sedation during computerised tomography. Acta Anaesthsiol Scand. 1994;38(3):259–261. doi: 10.1111/j.1399-6576.1994.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 4.Fallah R, Nakhaei MH, Behdad S et al. Oral chloral hydrate vs. intranasal midazolam for sedation during computerized tomography. Indian Pediatr. 2013;50(2):233–235. doi: 10.1007/s13312-013-0065-5. [DOI] [PubMed] [Google Scholar]
- 5.Mekitarian Filho E, de Carvalho WB, Gilio AE et al. Aerosolized intranasal midazolam for safe and effective sedation for quality computed tomography imaging in infants and children. J Pediatr. 2013;163(4):1217–1219. doi: 10.1016/j.jpeds.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Lami RO, Pereira ACP. Transmucosal dexmedetomidine for computed tomography sedation. Pediatr Anesth. 2008;18(4):349–350. doi: 10.1111/j.1460-9592.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 7.Cengiz M, Baysal Z, Ganidagli S. Oral sedation with midazolam and diphenhydramine compared with midazolam alone in children undergoing magnetic resonance imaging. Pediatr Anesth. 2006;16(6):621–626. doi: 10.1111/j.1460-9592.2005.01820.x. [DOI] [PubMed] [Google Scholar]
- 8.Elder JS, Longenecker R. Premedication with oral midazolam for voiding cystourethrography in children: safety and efficacy. AJR Am J Roentgenol. 1995;164(5):1229–1232. doi: 10.2214/ajr.164.5.7717236. [DOI] [PubMed] [Google Scholar]
- 9.Herd DW, McAnulty KA, Keene NA, Sommerville DE. Conscious sedation reduced distress in children undergoing voiding cystourethrography and does not interfere with the diagnosis of vesicoureteric reflux: a randomized controlled study. AJR Am J Roentgenol. 2006;187(6):1621–1626. doi: 10.2214/AJR.05.1216. [DOI] [PubMed] [Google Scholar]
- 10.Sorkhi H, Bakhshandeh-Bali MK, Nooreddini HG. Randomized clinical trial of sedation with oral midazolam for voiding cystourethrography in children. Med J Islam Repub Iran. 2010;24:67–71. [Google Scholar]
- 11.Azarfar A, Esmaeeili M, Farrokh A et al. Oral midazolam for voiding dysfunction in children undergoing cystourethrography: a controlled randomized clinical trial. Nephrourol Mon. 2014;6(3):e17168. doi: 10.5812/numonthly.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson GG, Chen C, Yan Y et al. The efficacy of oral midazolam for decreasing anxiety in children undergoing voiding cystourethrogram: a randomized, double-blind, placebo controlled study. J Urol. 2011;185(suppl 6):2542–2546. doi: 10.1016/j.juro.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Akil I, Ozkol M, Ikizoglu OY et al. Premedication during micturating cystourethrogram to achieve sedation and anxiolysis. Pediatr Nephrol. 2005;20(8):1106–1110. doi: 10.1007/s00467-005-1874-0. [DOI] [PubMed] [Google Scholar]
- 14.Keidan I, Zaslansky R, Weinberg M et al. Sedation during voiding cystourethrography: comparison of the efficacy and safety of using oral midazolam and continuous flow nitrous oxide. J Urol. 2005;174(4 part 2):1598–1601. doi: 10.1097/01.ju.0000176595.49213.13. [DOI] [PubMed] [Google Scholar]
- 15.Zier JL, Kvam KA, Kurachek SC, Finkelstein M. Sedation with nitrous oxide compared with no sedation during catheterization for urologic imaging in children. Pediatr Radiol. 2007;37(7):678–684. doi: 10.1007/s00247-007-0508-z. [DOI] [PubMed] [Google Scholar]
- 16.Stokland E, Andreasson S, Jacobsson B et al. Sedation with midazolam for voiding cystourethrography in children: a randomised double-blind study. Pediatr Radiol. 2003;33(4):247–249. doi: 10.1007/s00247-003-0874-0. [DOI] [PubMed] [Google Scholar]
- 17.Chung S, Lim R, Goldman RD. Intranasal fentanyl versus placebo for pain in children during catheterization for voiding cystourethrography. Pediatr Radiol. 2010;40(7):1236–1240. doi: 10.1007/s00247-009-1521-1. [DOI] [PubMed] [Google Scholar]
- 18.Patel VJ, Ahmed SS, Nitu ME, Rigby MR. Vasovagal syncope and severe bradycardia following intranasal dexmedetomidine for pediatric procedural sedation. Pediatr Anesth. 2014;24(4):446–8. doi: 10.1111/pan.12368. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler DS, Jensen RA, Poss WB. A randomized, blinded comparison of chloral hydrate and midazolam sedation in children undergoing echocardiography. Clin Pediatr. 2001;40(7):381–387. doi: 10.1177/000992280104000704. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim SV, Guc BU, Bozdogan N, Tokel K. Oral versus intranasal midazolam pred-medication for infants during echocardiographic study. Adv Ther. 2006;23(5):719–724. doi: 10.1007/BF02850311. [DOI] [PubMed] [Google Scholar]
- 21.Michaud L, Gottrand F, Ganga-Zandzou PS et al. Nitrous oxide sedation in pediatric patients undergoing gastrointestinal endoscopy. J Pediatr Gastroenterol Nutr. 1999;28(3):310–314. doi: 10.1097/00005176-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Rafeey M, Ghojazadeh M, Feizo Allah Zadeh H, Majidi H. Use of oral midazolam in pediatric upper gastrointestinal endoscopy. Pediatr Int. 2010;52(2):191–195. doi: 10.1111/j.1442-200X.2009.02936.x. [DOI] [PubMed] [Google Scholar]
- 23.Chiaretti A, Barone G, Rigante D et al. Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child. 2011;96(2):160–163. doi: 10.1136/adc.2010.188433. [DOI] [PubMed] [Google Scholar]
- 24.Tobias JD. Applications of nitrous oxide for procedural sedation in the pediatric population. Pediatr Emerg Care. 2013;29(2):245–265. doi: 10.1097/PEC.0b013e318280d824. [DOI] [PubMed] [Google Scholar]
- 25.Babl FE, Puspitadewi A, Barnett P et al. Preprocedural fasting state and adverse events in children receiving nitrous oxide for procedural sedation and analgesia. Pediatr Emerg Care. 2005;21(11):736–43. doi: 10.1097/01.pec.0000186427.07636.fc. [DOI] [PubMed] [Google Scholar]
- 26.Zier JL, Liu M. Safety of high-concentration nitrous oxide by nasal mask for pediatric procedural sedation: experience with 7802 cases. Pediatr Emerg Care. 2011;27(12):1107–1112. doi: 10.1097/PEC.0b013e31823aff6d. [DOI] [PubMed] [Google Scholar]


