Abstract
OBJECTIVES: The purpose of this study was to assess the rate of prescribing errors, resulting adverse events, and patient outcomes associated with sedation and analgesia in the pediatric intensive care unit (PICU) before and during a national shortage of fentanyl and injectable benzodiazepines.
METHODS: A retrospective chart review was performed of patients admitted to the PICU with at least 1 prescribed order for a sedative or analgesic agent during the time periods of January to February of 2011 and 2012. Initial orders for sedative and analgesic agents were identified and investigated for appropriateness of dose and were assessed for error-associated adverse events. Orders were stratified by timing in regard to clinical pharmacist on-site availability. Demographic and outcome information, including unintended extubations, ventilator days, and PICU length of stay, were gathered.
RESULTS: One hundred sixty-nine orders representing 72 patients and 179 orders representing 75 patients in 2011 and 2012, respectively, were included in analysis. No differences were found in the rate of prescribing errors in 2011 and 2012 (33 errors in 169 orders vs. 39 errors in 179 orders, respectively, p=0.603). No differences were found in rates of prescribing errors in regard to clinical pharmacist on-site availability. A significant increase was seen in unintended extubations per 100 ventilator days, with 0.15 in 2011 vs. 1.13 in 2012, respectively (p<0.001). A significant decrease was seen in ventilator days per patient (p<0.001) and PICU length of stay per patient (p=0.019).
CONCLUSIONS: There were no differences in rates of prescribing errors before versus during the fentanyl and benzodiazepine shortage.
INDEX TERMS: benzodiazepines, drug substitution, medication errors, opioid analgesic, pediatric intensive care units
INTRODUCTION
Medication errors harm 1% to 2% of patients, with most errors originating during the prescribing process.1 The most common prescribing error is prescribing the wrong dose, which may be associated with significant adverse events.2 The potential for medication errors increases during drug shortages due to increased prescribing of unfamiliar agents.3 The use of an alternative drug, dosage form, or dosage strength accounted for up to 27% of reported harmful outcomes related to drug shortages in hospitalized patients.4
American Society of Health System Pharmacists reported a shortage of fentanyl and the injectable benzodiazepines midazolam, lorazepam, and diazepam beginning in 2011. Reports of resolution of the shortages were unclear, due in part to discontinued production of particular preparations, increased demand, and manufacturing issues.5,6 Opioids are often prescribed for analgesia in the pediatric intensive care unit (PICU).7,8 Fentanyl is often chosen over other opioid agents because of its cardiovascular stability, positive effects on pulmonary vascular resistance, and ability to reduce the sympathetic stress response.7–9 Benzodiazepines are among the most common sedatives initiated first in the PICU.10,11 They enhance the effect of the inhibitory neurotransmitter gamma-aminobutyric acid and have sedative and amnesiac properties but offer no intrinsic analgesia.7,8
The national shortage of fentanyl and injectable benzodiazepines was most prevalent at the study institution in January and February of 2012. With an uncertain supply of these commonly prescribed agents, allocation was prioritized to specific patient populations, and limitations were placed on the use of these agents in the PICU. Clinical pharmacists and critical care physician leadership identified potential therapeutic alternatives for initial management of pain and sedation and provided standardized algorithms to the critical care teams (Appendix Figure). This included use of other opioids, such as morphine and hydromorphone. It also necessitated use of less familiar agents for sedation including propofol, pentobarbital, ketamine, and dexmedetomidine. The purpose of this study was to evaluate the change in prescribing errors during the fentanyl and injectable benzodiazepine shortage, to assess adverse events associated with prescribing errors, and to evaluate patient outcomes related to sedation, including unintended extubations, ventilator duration, and PICU length of stay.
MATERIALS AND METHODS
A retrospective chart review was approved as exempt by review of the university's institutional review board to compare outcomes before (January to February 2011) and during (January to February 2012) the drug shortage. Initial orders for a sedative or analgesic agent were identified through the pharmacy order entry system. During the study period, the institution did not use computerized provider order entry; orders were written by prescribers in a paper chart, scanned to the pharmacy, and then entered by the pharmacist into the electronic medical record. Patients were included if they were admitted to the PICU and had an active order for a sedative or analgesic agent. Only the initial order for each agent was assessed for accuracy. Patients were excluded if they were admitted to the cardiovascular surgery or cardiac intensive care services, as the shortage agents were preferentially reserved for this population. Patients who were not mechanically ventilated were also excluded. For secondary clinical outcomes, patients were excluded if they were chronically ventilator-dependent.
The primary endpoint was the rate of prescribing errors before versus during the drug shortage. Secondary medication error outcomes included error-associated differences in adverse events before and during the drug shortage and the rate of medication errors during clinical pharmacist on-site availability (Monday through Friday from 8 am to 5 pm) compared to off-site availability (all other times). At the study institution, clinical pharmacists were available to their subspecialty service by pager at all hours of the day, even if the pharmacist was not available on-site. Clinical pharmacists did not keep a log of calls received during off-site hours. Secondary clinical outcomes investigated included unintended extubations, ventilator days, and PICU length of stay, defined as the calendar days from PICU admission to transfer to floor, discharge home, or death.
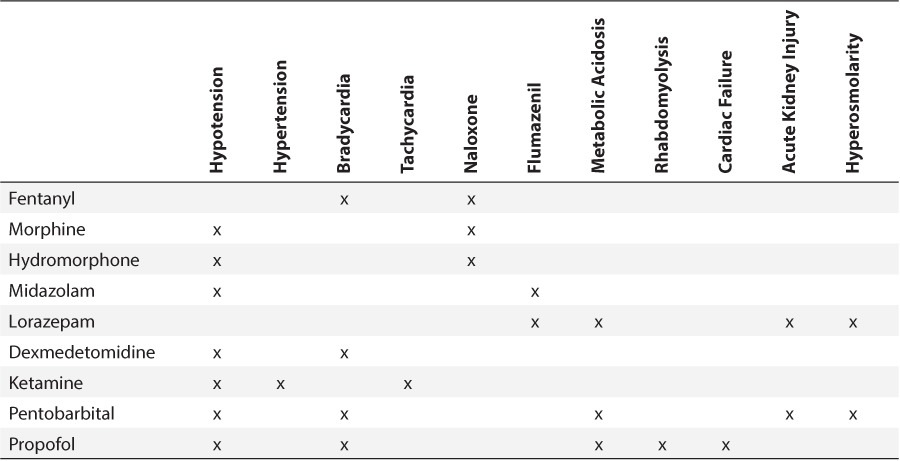
Prescribing errors included any orders outside the predefined appropriate dosing for each medication (Table 1). Opioid conversions were assessed with any prior opioid use.12,13 Prescribing errors were classified as underdosing, prescribing a dose lower than an appropriate weight-based dose, or overdosing, prescribing a dose greater than an appropriate weight-based or absolute maximum dose. After a prescribing error was identified, the patient's chart was assessed for predefined adverse events specific to the medication in error (Table 2). Hypotension, hypertension, bradycardia, and tachycardia were defined using the Pediatric Algorithm for Life Support guidelines and recorded if the event occurred within fifteen minutes after an intravenous administration or 2 hours after an enteral administration.14 Metabolic acidosis was defined as a pH less than 7.35 with a bicarbonate concentration below 22 mmol/L and a partial pressure of carbon dioxide (pCO2) between 35 and 45 mm Hg that occurred 24 hours after an initial dose through medication discontinuation. Naloxone and flumazenil administration were recorded if the patient received the medication during any time of their original prescribed therapy. Any additional actions required, including dose increase or decrease or the need for an additional sedative or analgesic medication, were also recorded.
Table 1.
Appropriate Ranges for Initial Medication Doses *
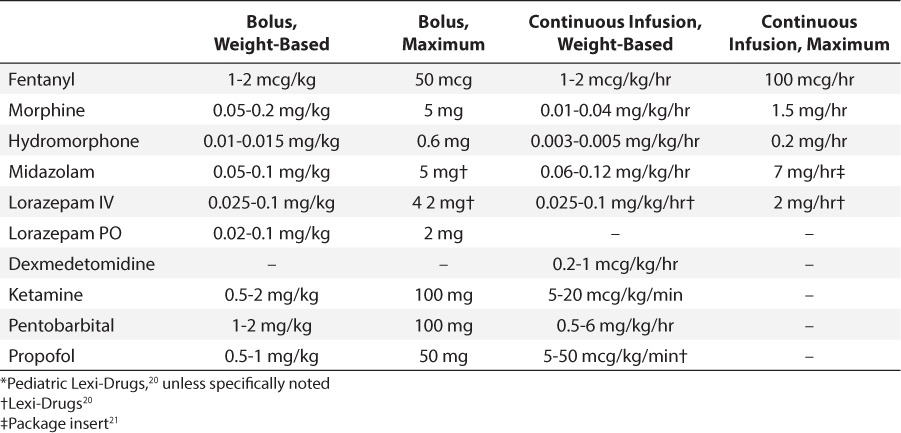
Table 2.
Reviewed Adverse Events by Medication
The chi-squared test was used to analyze sex, the rate of prescribing errors, error-associated adverse events, and clinical pharmacist on-site availability. The Mann-Whitney U test was used to compare age, weight, ventilator days, and PICU length of stay. Hospital mortality, defined as death at any point during the same hospital admission, and unintended extubations were compared using the Fisher exact test. The a priori level of significance was <0.05.
RESULTS
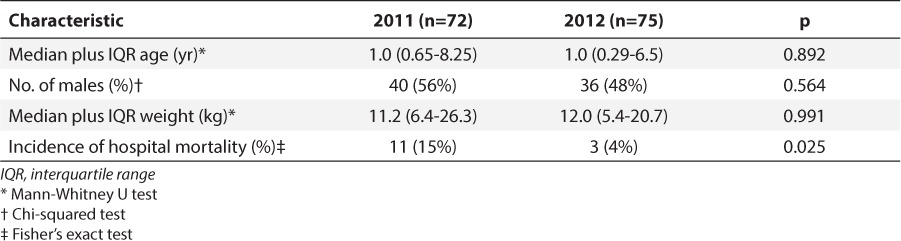
Seven hundred fifty orders representing 208 patients were identified from January to February 2011. Five hundred eighty-one orders were excluded, whereas 169 orders were included, representing 72 patients (Figure). In 2012, 785 orders representing 226 patients were identified. Six hundred six orders were excluded, resulting in 179 orders representing 75 patients being included (Figure). Patient characteristics were similar between the groups, except for hospital mortality, which was statistically higher in the 2011 group (Table 3).
Figure.
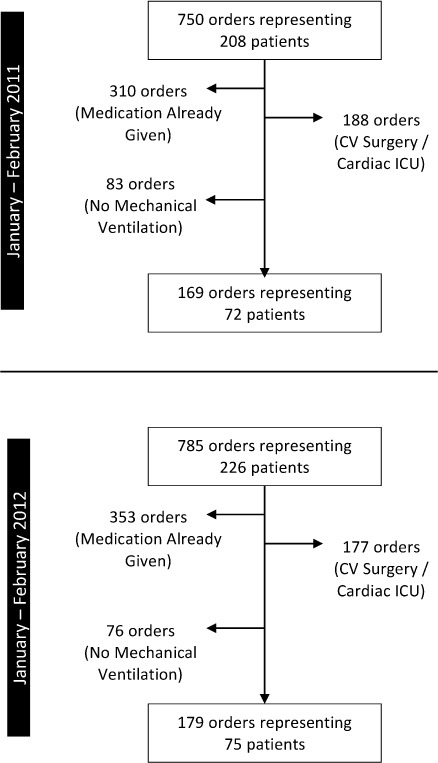
Patient enrollment.
CV, cardiovascular; ICU, intensive care unit.
Table 3.
Patient Characteristics
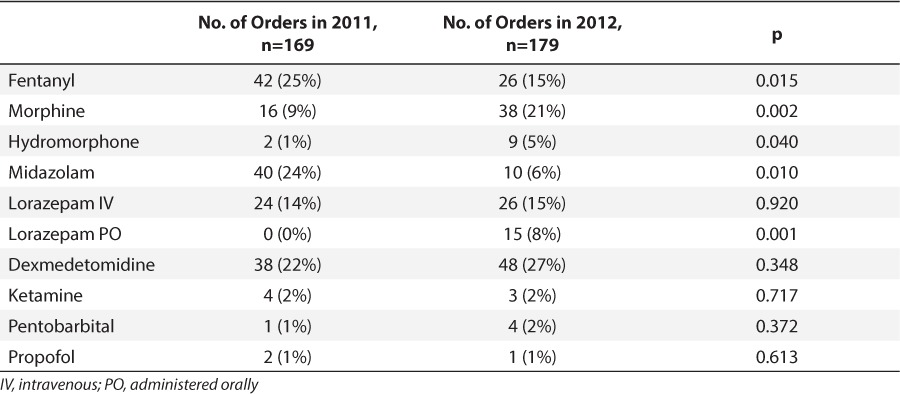
Fentanyl was the analgesic agent most commonly prescribed in 2011, representing 25% of total orders. During the shortage, fentanyl use decreased by 10%, and prescriptions of other opioids increased, including morphine and hydromorphone (Table 4). The benzodiazepines midazolam and lorazepam equaled 38% of orders in 2011 versus 29% in 2012.
Table 4.
Medication Orders Before and During Shortage
No differences were found between the rate of prescribing errors for initial orders in 2011 and that in 2012 (p=0.603) (Table 5). Fifty-eight-percent of all errors were due to underdosing, whereas 42% of errors were due to overdosing (Table 6). Morphine, midazolam, and enteral lorazepam were more commonly underdosed than overdosed.
Table 5.
Prescribing Errors for Initial Orders by Medication
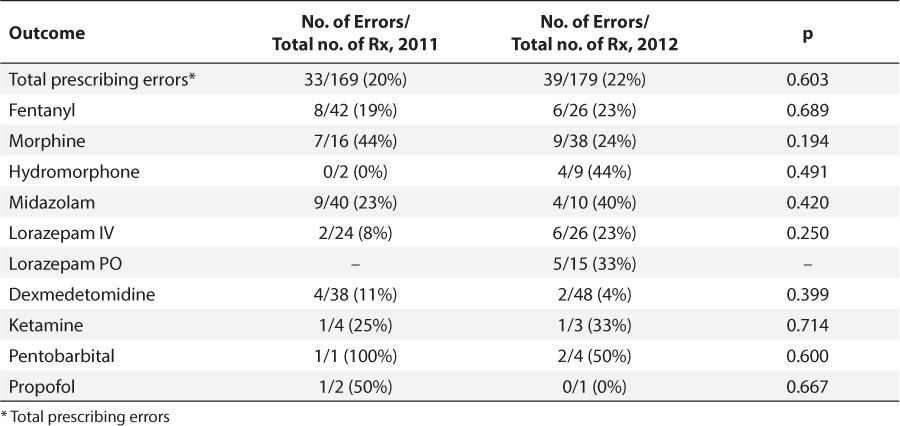
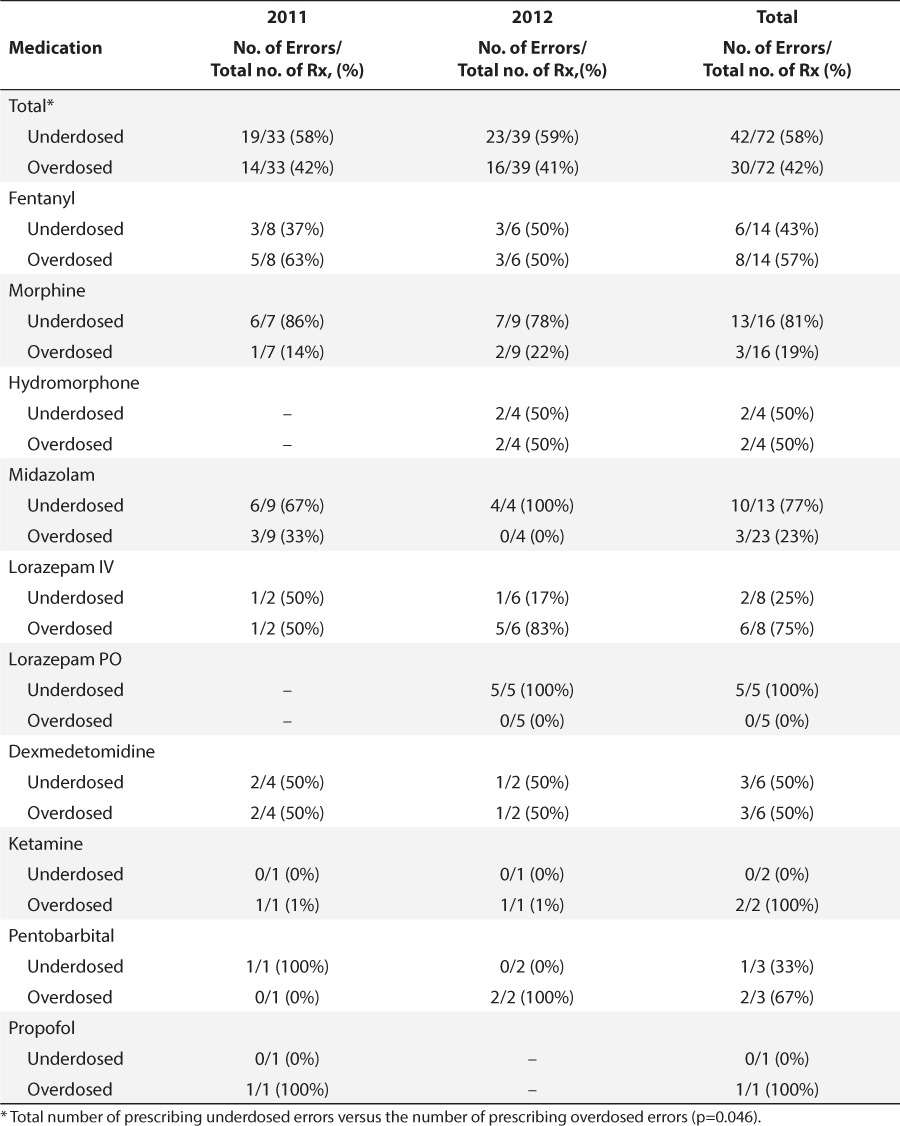
Table 6.
Prescribing Errors by Type
A total of 23% of patients experienced an error-associated adverse event in 2011 and 34% in 2012 (p=0.333) (Table 7). Hypotension was the most common adverse event followed by hypertension. One case of bradycardia occurred with pentobarbital administration, and 1 patient who received a ketamine bolus became tachycardic. One patient who was receiving lorazepam experienced metabolic acidosis. A patient receiving continuous infusion (CI) of fentanyl, 3 mcg/kg/hr, required naloxone administration when he became unresponsive. Flumazenil was administered to that same patient, who was also receiving CI midazolam, 0.1 mg/kg/hr. This dose was within the predefined appropriate CI rate, but the patient also received multiple midazolam boluses.
Table 7.
Error-Associated Adverse Events and Dose Adjustments
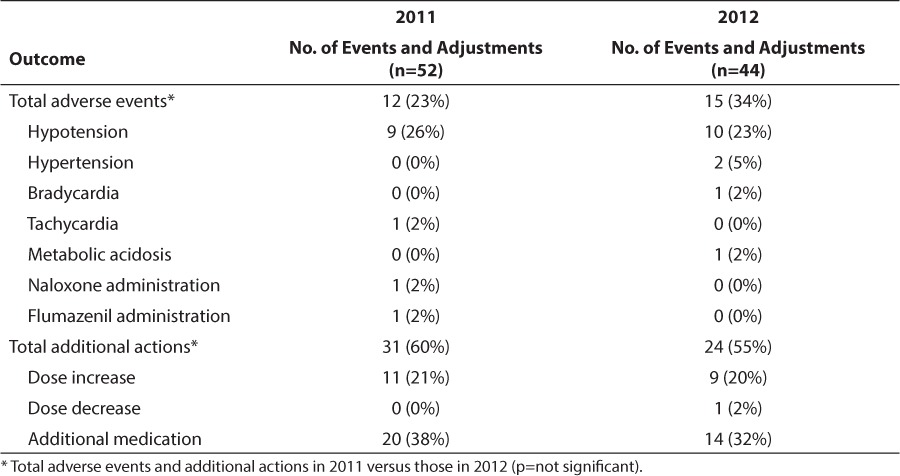
There were no differences between 2011 and 2012 in orders requiring additional actions, with 60% and 55% of orders requiring an adjustment, respectively (Table 5). When orders were stratified based upon clinical pharmacist on-site versus off-site availability, no differences in rates of prescribing errors were noted (21 errors in 143 orders vs. 51 errors in 222 orders, respectively, p=0.152). For the secondary clinical outcomes, patients who were chronically ventilator-dependent were excluded (n=9 in 2011, n=10 in 2012). There was a significant increase in unintended extubations per 100 ventilator days, with 0.15 extubations in 2011 vs. 1.13 extubations in 2012 (p<0.001). Significant decreases were seen in ventilator days per patient, with 6 days in 2011 vs. 2.5 days in 2012 (p<0.001) and PICU length of stay per patient from 7.5 days to 6.0 days (p=0.019) in 2011 to 2012, respectively.
DISCUSSION
The drug shortage crisis has affected all healthcare organizations, with an ever-growing number of critically important medications in short supply. When assessing a drug shortage, position statements from several organizations recommend identifying therapeutic alternatives early, placing limitations on use by prioritizing patients, establishing ongoing communication with staff, and proactively monitoring adverse outcomes.3,15,16 In the PICU, the recent shortage of fentanyl and injectable benzodiazepines required many substitutions for these commonly prescribed agents.
At the study institution, the clinical pharmacists identified potential therapeutic alternatives for initial management of sedation and, in conjunction with the critical care physician leadership, developed a standardized approach to conservation of shortage medications. The clinical pharmacists prepared printed references containing algorithms for use, dosing recommendations, and monitoring parameters for the alternative sedative and analgesic agents. They also provided in-person prescriber education. Both fentanyl and midazolam were preferentially prioritized to the cardiovascular surgery and cardiac intensive care unit patients. Fentanyl was reserved for hemodynamically unstable patients requiring opioid analgesia, thereby causing an overall increase in morphine and hydromor-phone use during the shortage. Midazolam was reserved for specific populations as well, including patients requiring neuromuscular blockade who could not receive another appropriate agent (see Appendix).
Overall rates of prescribing errors were similar to the rates found in a tertiary children's hospital PICU, reported as 21% in a 2012 study by Maat et al.17 However, our rates reflected only the initial orders for each medication and do not account for dose titrations after initial prescribing. Only initial orders were assessed in order to limit variability secondary to clinical titrations due to patient response or adverse events. We hypothesized an increase in prescribing errors of these initial orders during the shortage, though no difference was seen. Ongoing communication by the clinical pharmacist with provision of detailed medical references as well as routine face-to-face education to prescribers both during patient care rounds and throughout the day might have decreased errors and contributed to the similarity in the rates of prescribing errors in 2011 and 2012. Availability of the clinical pharmacist by pager at all hours of the day might have further mitigated this difference. Although clinical pharmacists were available by pager at all times during both time periods, no formal call log was maintained; therefore, it was not possible to objectively assess whether the number sedation-related calls increased during this time.
The significant differences in mortality between the groups might have affected the data relating to clinical outcomes. Additional investigation of deceased patients found that, in 2011, several patients were admitted with severe underlying chronic diseases such as spinal muscular atrophy, metabolic origin dilated cardiomyopathy, and severe aplastic anemia. These likely contributed to increased hospital mortality, a longer ventilator duration, and increased PICU length of stay seen in 2011. Due to the retrospective nature of the study, severity of illness scores could not be calculated for all patients. However, differences in hospital mortality indicate there might have been differences in the severity of illnesses between the 2 study periods, which also could have influenced the differences in PICU length of stay and ventilator days.
There were several other limitations to this study. First, the retrospective nature of the study limited the ability to identify adverse events due to variability in charting. Furthermore, causality of each adverse event could not be assigned to a specific agent. We could not account for all additional factors that could have affected duration of mechanical ventilation, including delirium or use of neuromuscular blocking agents. However, dexmedetomidine has been associated with decreased ventilator days compared with midazolam in the adult ICU.18,19 Increased prescribing of dexmedetomidine during the benzodiazepine shortage, although not statistically significant, might have contributed to the decreased duration of mechanical ventilation in 2012. Additionally, bed availability can limit transfer out of the PICU, which might have influenced length of stay. Although the number and training level of prescribers remained consistent in the PICU, prescribers in the 2 periods were not identical. Standard hours were used to estimate clinical pharmacist on-site availability, but it was not possible to truly assess the clinical pharmacists' on-site presence. Furthermore, we were unable to determine interventions at the time each order was written from either the clinical or staff pharmacist.
There was a significant increase in unintended extubations per 100 ventilator days. This may indicate that, although appropriate therapy was initiated at similar rates before and after the shortage, when unfamiliar medications were used, prescribers were less able to titrate therapy to appropriate sedation, resulting in under-sedation and increased chance for unintended extubations. During the study period, validated sedation scores were not optimally and reliably recorded and could not be retrospectively evaluated to determine the appropriateness of sedative titration or the differences in achieving sedation goals before and during the shortage period.
CONCLUSIONS
In conclusion, we found no increase in prescribing errors for initial sedative and analgesic orders during the fentanyl and benzodiazepine shortage. These outcomes suggest that early awareness of drug shortage, identification of potential therapeutic alternatives, and appropriate communication and education may mitigate the potential for increased prescribing errors that may be seen during drug shortages. Future study and quality improvement work should focus on prospectively evaluating steps that can be implemented to mitigate the risk of prescribing errors, including mechanisms that can be implemented with computerized order entry systems to help guide prescribers in the use of unfamiliar medications.
Acknowledgments
Results were presented previously at the Great Lakes Pharmacy Resident Conference, April 2014. This work was completed while the authors were affliated with Riley Hospital for Children at Indiana University Health, Indianapolis, Indiana.
ABBREVIATIONS
- CV
cardiovascular
- CI
continuous infusion
- ICU
intensive care unit
- IQR
interquartile range
- IV
intravenous
- pCO2
partial pressure of carbon dioxide
- PICU
pediatric intensive care unit
- PO
administered orally
Appendix.
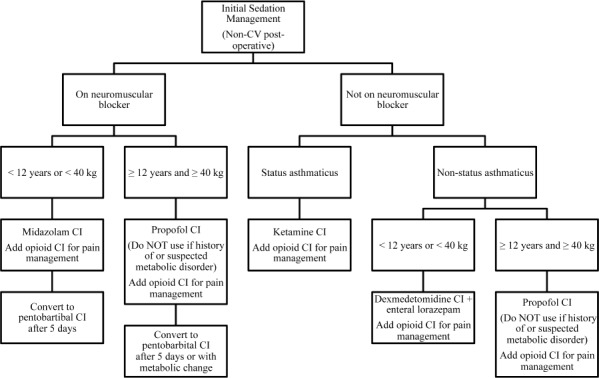
Initial sedation management algorithm during drug shortage.
CV, cardiovascular; CI, continuous infusion.
Footnotes
Disclosures The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Coombes ID, Stowasser DA, Coombes JA et al. Why do interns make prescribing errors? A qualitative study. Med J Aust. 2008;30:161–168. doi: 10.5694/j.1326-5377.2008.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 2.Garbutt JM, Highstein G, Jeffe DB et al. Safe medication prescribing: training and experience of medical students and house staff at a large teaching hospital. Acad Med. 2005;80:594–599. doi: 10.1097/00001888-200506000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Institute for Safe Medication Practices. Weathering the storm: managing the drug shortage crisis. ISMP Medication Safety Alert! October 7, 2010;15(20):1–4. http://www.ismp.org. Accessed October 20, 2015. [Google Scholar]
- 4.Institute for Safe Medication Practices. A shortage of everything except errors: harm associated with drug shortages. ISMP Medication Safety Alert! April 19, 2012;17:1–3. http://www.ismp.org. Accessed October 20, 2015. [Google Scholar]
- 5.American Society of Health-System Pharmacists. Drug Shortages. Current Drugs. Current Drug Shortage Bulletins. Midazolam. ASHP. August 15, 2013. http://www.ashp.org/DrugShortages/Current. Accessed October 20, 2015.
- 6.American Society of Health-System Pharmacists. Drug Shortages. Current Drugs. Current Drug Shortage Bulletins: Fentanyl. ASHP. August 15, 2013. http://www.ashp.org/DrugShortages/Current. Accessed October 20, 2015.
- 7.Tobias JD, Rasmussen GE. Pain management and sedation in the pediatric intensive care unit. Pediatr Clin North Am. 1994;41(6):1269–1292. doi: 10.1016/s0031-3955(16)38873-3. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi J, Fraser GL, Coursin DB et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Tobias JD. Sedation and analgesia in the pediatric intensive care unit. Pediatr Ann. 2005;34(8):636–645. doi: 10.3928/0090-4481-20050801-12. [DOI] [PubMed] [Google Scholar]
- 10.Johnson PN, Miller JL, Hagemann TM. Sedation and analgesia in critically ill children. AACN Adv Crit Care. 2015;22(4):415–434. doi: 10.1097/NCI.0b013e31826b4dea. [DOI] [PubMed] [Google Scholar]
- 11.Curley MA, Wypij D, Watson RS et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313(4):379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lexi-Comp Online, Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc; 2013. Opioid Conversion Table. [Google Scholar]
- 13.Pereira J, Lawlor P, Vigano A et al. Equiaalgesic dose ratios for opioids: a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 14.Chameides L, Ralston M, editors. Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2011. [Google Scholar]
- 15.Butterfield L, Cash J, Pham K. Drug shortages and implications for pediatric patients. J Pediatr Pharmacol Ther. 2015;20(2):149–152. doi: 10.5863/1551-6776-20.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox ER, Birt A, James KB et al. ASHP guidelines on managing drug product shortages in hospitals and health systems. Arch Intern Med. 2009;66(15):1399–1406. doi: 10.2146/ajhp090026. [DOI] [PubMed] [Google Scholar]
- 17.Maat B, Bollen CW, vanVught AJ et al. Impact of computerized physician order entry (CPOE) on PICU prescribing errors. Intensive Care Med. 2014;40(3):458–459. doi: 10.1007/s00134-013-3193-4. [DOI] [PubMed] [Google Scholar]
- 18.Riker RR, Shehabi Y, Bokesch PM et al. Dexmedetomidine vs midazolam for sedation of critically ill patients. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 19.Jakob SM, Ruokonen E, Grounds M et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation. JAMA. 2012;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 20.Pediatric Lexi-Drugs Online. Hudson, OH: Lexi-Comp, Inc.; 2013. Lexi-Comp Online. [Google Scholar]
- 21.Midazolam [package insert] Lake Forest, IL: Hospira Inc.; 2010. [Google Scholar]


