Summary
Osteoporosis and pathological increased occurrence of fractures are an important public health problem. They may affect patients’ quality of life and even increase mortality of osteoporotic patients, and consequently represent a heavy economic burden for national healthcare systems.
The adoption of simple and inexpensive methods for mass screening of population at risk may be the key for an effective prevention.
The current clinical standards of diagnosing osteoporosis and assessing the risk of an osteoporotic bone fracture include dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) for the measurement of bone mineral density (BMD).
Micro-computed tomography (micro-CT) is a tomographic imaging technique with very high resolution allowing direct quantification of cancellous bone microarchitecture.
The Authors performed micro-CT analysis of the femoral heads harvested from 8 patients who have undergone surgery for hip replacement for primary and secondary degenerative disease to identify possible new morphometric parameters based on the analysis of the distribution of intra-subject microarchitectural parameters through the creation of parametric images.
Our results show that the micro-architectural metrics commonly used may not be sufficient for the realistic assessment of bone microarchitecture of the femoral head in patients with hip osteoarthritis.
The innovative micro-CT approach considers the entire femoral head in its physiological shape with all its components like cartilage, cortical layer and trabecular region.
The future use of these methods for a more detailed study of the reaction of trabecular bone for the internal fixation or prostheses would be desirable.
Keywords: osteoporosis, quantitative micro-CT, DEXA
Introduction
Osteoporosis and pathological increased occurrence of fractures are an important public health problem (1). In fact, they may affect patients’ quality of life and even increase mortality of osteoporotic patients (2), and consequently represent a heavy economic burden for national healthcare systems (1). The adoption of simple and inexpensive methods for mass screening of population at risk may be the key for an effective prevention (3, 4).
The current clinical standards of diagnosing osteoporosis and assessing the risk of an osteoporotic bone fracture include dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) for the measurement of bone mineral density (BMD) at the spine and hip, the two skeletal locations most prone to fracture. DXA determines BMD in two dimensions, including both trabecular and cortical bone, with the result expressed as areal density (grams per square centimeter). Conversely, QCT allows the measurement of volumetric trabecular bone density without superimposition of cortical bone and other tissues, with the result expressed in milligrams per cubic centimeter of calcium hydroxyapatite.
DXA and QCT findings cannot be compared directly and sometimes the diagnosis based on BMD findings differs between the two modalities (5).
Li et al. showed a significant difference in osteoporosis detection rates between DXA and QCT. They demonstrated with clinical evidence that QCT has a greater diagnostic sensitivity than DXA (5).
Yu et al. reported that BMD measured by DXA was significantly higher in patients with spinal degenerative joint diseases than in those without such anatomical alterations. This evidence has been linked to the presence of osteophytes at the vertebral bodies and facet joints (6).
At the same time Ito et al. indicated that the presence of osteophytes is associated with higher BMD when measured with DXA (7).
Despite its 3D nature, QCT can only provide indirect estimation of cancellous bone parameters, due to the insufficient spatial resolution of clinical CT scanners as compared to the typical size of trabeculae. Micro-computed tomography (micro-CT) is a tomographic imaging technique with very high resolution (< 100 micron in three dimensions) allowing direct quantification of cancellous bone microarchitecture. In the preclinical field, micro-CT can be used for in vivo investigations in small animals, such as mice and rats. Clinical applications of micro-CT can be also found, such as imaging of biological samples ex vivo and in vivo imaging of extremities of patients (e.g., wrist and ankle), which is also known as peripheral quantitative CT (pQCT).
The capability of micro-CT as non destructive, fully 3D alternative to the tedious serial staining of specimen required by histomorphometric analysis of thin sections appears as the most attractive feature of this methodology for osteoporosis research (8).
The study of bone microarchitecture and its changes, caused by physiological aging and/or external traumatic or iatrogenic stress is thought to provide additional, independent information about the materials used in the prosthesis and in internal fixation. It may help to better predict fracture risk and assess response to drug intervention (9, 10).
In this study, the trabecular microarchitecture of the femoral head-neck post-surgery was evaluated both quantitatively and qualitatively by micro-CT with a resolution < 100 micron. The quantitative study is used to identify possible new morphometric parameters based on the analysis of the distribution of intra-subject microarchitectural parameters (thickness, density and trabecular spacing, etc.) through the creation of parametric images.
Material and methods
The Authors performed micro-CT analysis of the femoral heads harvested from 8 patients who have undergone surgery for hip replacement for primary and secondary degenerative disease. Both male and female have been included in the study, without any limit of age.
Before surgery, the patients were evaluated both clinically and with blood tests to exclude the presence of pathologies that may have affected the bone metabolism.
In addition, patients enrolled, except one, were subjected to bone densitometry at the site of prosthesis implants before surgery.
The machine used for the densitometry examination was a GE Lunar DPX Bravo; it analyzes the femoral region of interest (ROI) of neck, Ward’s triangle, trochanter, and total femur; the parameter used for the study was the BMD (g/cm2) of the femoral neck.
In order to assess retrospectively the bone quality from the point of view of the microarchitecture, each bone sample was immersed in formalin (4% v/v) immediately after excision and for at least 24 hours and stored in a sealed recipient, and afterwards it was analyzed through a high-resolution IRIS micro-CT scanning system (Raytest GmbH, Straubenhardt, Germany), obtaining volumetric acquisitions of the whole sample at isotropic voxel size of 60 μm.
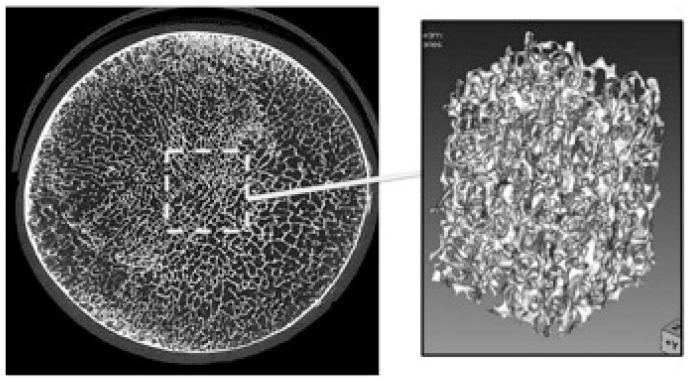
The micro-CT data analysis consisted in three steps: (1) segmentation of the bone component by mean of local thresholding; (2) selection of a cubic ROI in the segmented volume; (3) 3D morphometric analysis of microarchitectural parameters in the ROI. The proposed approach used in this study allowed to generate 3D parametric maps of each morphometric parameter for the whole sample, by using an ad-hoc developed software for the automatic translation of the cubic ROI through the segmented volume. With this approach, we sought to overcome the limitation of the analysis of small fragments of cancellous bone, whose result might depend on the choice of the extracted fragment selected for micro-CT imaging (Figure 1). Variability of microarchitecture through the sample is well evident with the new approach, possibly adding clinically meaningful information to the conventional single-ROI analysis.
Figure 1.
Micro-CT image of the transverse plane of the bone sample. Conventional approach for cancellous bone quantification consists in either a destructive core drilling and subsequent very high resolution micro-CT scanning or quantification on a single ROI placed at the center of the sample.
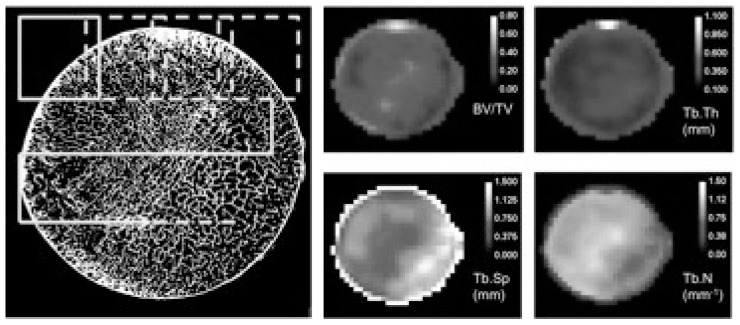
In our study, the moving ROI (Figure 2) had a volume of 53 mm3 and was translated in 3 dimensions through the segmented volume at steps of 2 mm. For each ROI position, the BoneJ software (7–8) was used to calculate the following trabecular bone parameters: the voxel-based bone volume fraction (BV/TV), representing the volume of mineralized bone per unit volume of the sample; the trabecular bone thickness (Tb.Th in mm) and the trabecular bone spacing (Tb.Sp). Average value and standard deviation (SD) of all parameters were computed for all ROI position, and the coefficient of variation (COV) defined as the ratio of SD/Avg was used as a metric of variability for each parameter.
Figure 2.
New approach of whole-sample micro-CT quantitative analysis. A cubic ROI (dashed yellow rectangle) is moved through the segmented bone volume and the quantitative parameters are calculated for each ROI position. After scanning the entire volume, the parametric maps of the microarchitectural parameters are obtained (color-coded images, shown in the right panel). The variability of the bone quality parameters are clearly evidenced in the parametric maps.
Results
From our preliminary data we found a strong inhomogeneity of the parameters considered in the various regions of interest (ROI) of the sample analyzed between the various cases, especially those of primary and secondary osteoarthritis.
Quantitative analysis (Table 1) showed that in patients with aseptic necrosis of the femoral head (patient number 2) there was an increase in apparent bone fraction (BV/TV.AVG = 46%) along with a high variability in the trabecular spacing. Patients number 1 and number 6 had a similar BMD, but the analysis based on parametric mapping showed a strong variability of trabecular bone density in post-traumatic patient (BV/TV.COV = 56%) against the case of primary osteoarthritis (BV/TV.COV = 28%).
Table 1.
Results of the quantitative analysis performed by DXA (BMD) and micro-CT with moving ROI (all other parameters). BMD: bone mineral density; Tb.Th: trabecular thickness; Tb.Sp: trabecular spacing; BV/TV: bone volume fraction; AVG: average; COV: coefficient of variation.
| Patient n. | Sex | Age (y) | Weight (kg) | Height (cm) | BMD neck (g/cm2) | Tb.Th. AVG (mm) | Tb.Sp. AVG (mm) | Tb.N. AVG (mm-1) | BV/TV. AVG | Tb.Th. COV (mm) | Tb.Sp. COV (mm) | Tb.N.COV (mm-1) | BV/TV. COV | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 115 | 174 | 0,892 | 0,269 | 1,166 | 0,790 | 0,208 | 0,267 | 0,642 | 0,284 | 0,313 | |
| 2 | F | 84 | 60 | 150 | 1,135 | 0,548 | 0,955 | 0,814 | 0,461 | 0,551 | 0,911 | 0,329 | 0,401 | Aseptic necrosis |
| 3 | F | 58 | - | - | N/D | 0,298 | 1,156 | 0,852 | 0,277 | 0,157 | 0,864 | 0,342 | 0,261 | |
| 4 | F | 72 | 40 | 145 | 0,657 | 0,325 | 1,589 | 0,674 | 0,208 | 0,218 | 0,780 | 0,402 | 0,401 | |
| 5 | M | 75 | 87 | 173 | 1,056 | 0,403 | 1,174 | 0,786 | 0,276 | 0,676 | 0,694 | 0,354 | 0,388 | |
| 6 | F | 41 | 54 | 154 | 0,896 | 0,628 | 1,997 | 0,485 | 0,285 | 0,561 | 0,687 | 0,563 | 0,428 | Post-traumatic |
| 7 | M | 61 | 115 | 180 | 1,109 | 0,342 | 1,087 | 0,823 | 0,298 | 0,287 | 0,731 | 0,303 | 0,342 | |
| 8 | F | 87 | 75 | 155 | 1,05 | 0,434 | 1,629 | 0,623 | 0,274 | 0,318 | 0,786 | 0,388 | 0,317 |
The value of average trabecular thickness of the patient post-traumatic is falsely high (0.63 mm) due to the inclusion of peri-infarcted bone (thick), with a strong decrease in trabecular density media (Tb.N.AVG = 0.48 mm−1 in posttraumatic against 0.79 mm−1 in the case of primary osteoarthritis).
The Authors also did a Quality Assessment which showed that in patient number 1, affected by primary osteoarthritis, a thinning of the mean trabecular size was evident with homogeneous distribution over the whole sample as evidenced by the relatively low coefficient of variation of this parameter (Tb.Th.COV = 27%).
In patient number 2, an alteration of the morphology of the femoral head was found, which was compatible with the aseptic necrosis pathology reported by the patient in anamnesis.
Moreover, they showed the presence of areas of strongly reduced trabecular density surrounded by areas of much greater density.
Although the trabecular collapse of the necrotic area caused an increase in apparent bone fraction (BV/TV.AVG = 46%, min = 10%, max = 86%) was detected a high variability of trabecular spacing (Tb.Sp.COV = 91%)
In patient number 6, having post-traumatic osteoarthritis, there was a high inhomogeneity in the trabecular distribution mainly interested the peripheral portion of the femoral head. In particular, it showed an area of bigger subchondral rarefaction in correspondence of the osteophyte of the femoral head.
In this area, an increased trabecular spacing and a reduced number of trabeculae has been found (Tb.N.COV = 56%).
Discussion
Bone is a living tissue that adapts to various loading conditions and mechanical stress, and the use of drugs. In particular, the ability of the bone to “resist fracture” and “respond” to mechanical stimulation or drug depends on its microarchitecture.
To date, the methods used for the micro-architectural study make the analysis of samples obtained by coring and therefore are restricted to areas that may not reflect the real situation of the global skeletal segment considered. Our results show that the micro-architectural metrics commonly used, generally based on the analysis of the properties of trabecular bone small specimens limited to the center of the sample, may not be sufficient for the realistic assessment of bone microarchitecture of the femoral head in patients with hip osteoarthritis.
The innovative micro-CT approach considers the entire femoral head in its physiological shape with all its components like cartilage, cortical layer and trabecular region. A better understanding of the variability of microarchitectural parameters and their relationships with clinical conditions may lead to the definition of new imaging biomarkers of osteoporosis that, in future, could be translated into the clinical settings.
The use of micro-architectural parametric maps obtained by micro-CT, according to the above approach, seems promising to set new quantitative metrics for the retrospective assessment of bone quality and its response to drug therapies, although the use of mean values and COV seems to be an under-utilization of the totality of the information available.
Osteoporosis is often considered a disease of cancellous bone. This kind of bone has been related etiologically to osteoarthrosis, and long-term success of orthopedic implants depends on a sound cancellous bone stock. The explanation of the factors that determine the mechanical properties, how these properties are maintained, and how bone reacts to changes in its environment is the ultimate aim of most cancellous bone research (11).
Conclusions
New metrics of quantification based on the local and spacevariant analysis of the quality of trabecular bone, extended to areas biomechanically and functionally heterogeneous of femoral head and neck samples, has been presented and discussed as a generalization to current metrics of cancellous bone quality. The future use of these methods for a more detailed study of the reaction of trabecular bone for the internal fixation or prostheses would be desirable.
Footnotes
Disclosure
No benefits or funds were received in support of this study. None of the Authors has any conflict of interests to disclose.
References
- 1.Pike C, Birnbaum HG, Schiller M, et al. Economic burden of privately insured non-vertebral fracture patients with osteoporosis over a 2-year period in the US. Osteoporos Int. 2011;22:47. doi: 10.1007/s00198-010-1267-5. [DOI] [PubMed] [Google Scholar]
- 2.Brenneman SK, Barret-Connor E, Sajjan S, et al. Impact of recent fracture on health-related quality of life in postmenopausal women. J Bone Miner Res. 2006;21(6):809–816. doi: 10.1359/jbmr.060301. [DOI] [PubMed] [Google Scholar]
- 3.Liu JM, Ning G, Chen JL. Osteoporotic fractures in Asia: risk factors and strategies for prevention. J Bone Miner Metab. 2007;25:1–5. doi: 10.1007/s00774-006-0720-1. [DOI] [PubMed] [Google Scholar]
- 4.Marin F, Gonzalez-Macias J, Diez-Perez A, et al. Relationship between bone quantitative ultrasound and fractures: a meta-analysis. J Bone Miner Res. 2006;21:1126–1135. doi: 10.1359/jbmr.060417. [DOI] [PubMed] [Google Scholar]
- 5.Li Na, et al. Comparison of QCT and DXA: Osteoporosis Detection Rates in Postmenopausal Women. Int J Endocrinol. 2013;2013:895474. doi: 10.1155/2013/895474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu W, et al. Influence of degenerative joint disease on spinal bone mineral measurements in postmenopausal women. Calcified Tissue International. 1995;57(3):169–174. doi: 10.1007/BF00310253. [DOI] [PubMed] [Google Scholar]
- 7.Ito M, et al. Relationship of osteophytes to bone mineral density and spinal fracture in men. Radiology. 1993;189(2):497–502. doi: 10.1148/radiology.189.2.8210380. [DOI] [PubMed] [Google Scholar]
- 8.Graeff W, Engelke K. Microradiography and microtomography. In: Ebashi E, Koch M, Rubenstein E, editors. Handbook on synchrotron radiation. Amsterdam: North-Holland; 1991. pp. 361–405. [Google Scholar]
- 9.Brandi ML. Microarchitecture, the key to bone quality. Rheumatology. 2009;48:iv3–iv8. doi: 10.1093/rheumatology/kep273. [DOI] [PubMed] [Google Scholar]
- 10.Genant HK, et al. Advanced CT bone imaging in osteoporosis. Rheumathology. 2008;47:iv9–iv16. doi: 10.1093/rheumatology/ken180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odgaard A. Three-dimensional methods for quantification of cancellous bone architecture’. Orthopaedic Research Laboratory, Department of Orthopaedic Surgery Aarhus University Hospital; Aarhus, Denmark: 1996. [Google Scholar]