Summary
E.F., female, age 58, mother of 4 children and otherwise healthy, had gone into menopause when she was 42. She had received hormone replacement therapy during 8 years. Due to low bone mass she had been treated with oral alendronate during 7 years. She had a normal calcium intake in her diet and engaged in regular physical activity. She did not smoke, and drank alcohol only occasionally. Her mother had sustained a hip fracture at age 90. Bone densitometry of her lumbar spine by DXA showed a T-score of −3.0; standardized bone mineral density (sBMD) had decreased by 11% in the previous 3 years. She was advised to start treatment with strontium ranelate (SrR) 2 g/day, plus oral cholecalciferol (1,000 IU/day). Three months later serum alkaline phosphatase had increased 10%, and serum osteocalcin was 18.9 ng/ml (upper normal limit 13.7). One year later her lumbar BMD had increased by 13.5%. After five years of treatment the BMD value was normal (1.357 g/cm2; T-score −0.3). The case presented here is noteworthy for two reasons. Firstly, the patient maintained low bone mass after several years of combined treatment with alendronate and hormone replacement; this combination usually induces greater densitometric responses than either treatment given alone. Secondly, she responded promptly and significantly to SrR in spite of the previous long exposure to alendronate. SrR is widely used for the treatment of osteoporosis. It is an effective and safe drug, provided the patients are properly selected. As shown here, it can help some patients to achieve a normal BMD.
Keywords: osteoporosis, treatment, strontium
Case presentation
E.F., female, age 58, mother of 4 children and otherwise healthy, had gone into menopause when she was 42. She had received hormone replacement therapy during 8 years. Due to low bone mass she had been treated with oral alendronate during 7 years. She had a normal calcium intake in her diet and engaged in regular physical activity (swimming). She did not smoke, and drank alcohol (wine or beer) only occasionally.
Her mother had sustained a hip fracture at age 90.
She consulted us in 2007, with bone densitometry of her lumbar spine showing a T-score of −3.0; standardized bone mineral density (sBMD) had decreased by 11% in the previous 3 years.
Weight was 76 kg, height was 1.72 m, and waist circumference was 85 cm. Her physical examination was unremarkable. Laboratory studies, including serum calcium, phosphorus, and total alkaline phosphatase, were normal.
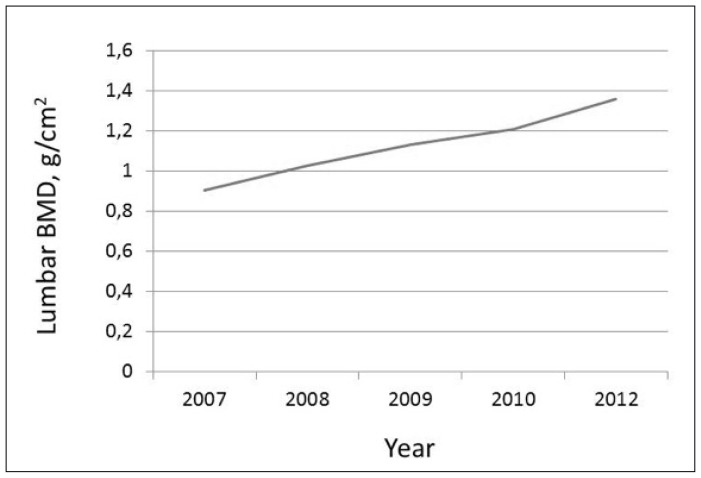
She was advised to start treatment with strontium ranelate 2 g/day, plus oral cholecalciferol (1,000 IU/day). Three months later serum alkaline phosphatase had increased 10%, serum osteocalcin was 18.9 ng/ml (upper normal limit 13.7) and she was tolerating the medication well. One year later her lumbar BMD by DXA (Lunar Prodigy) was 1.028 g/cm2; T-score −1.4; this represented an increase of 13.5%. Two years later the value was 1.131 (further 10% gain), and the femoral neck BMD was 0.853 g/cm2; T-score −1.1. After 3 years of treatment a new lumbar densitometry was performed with a Norland DXA equipment; the BMD was normal and the sBMD had increased by 6%. She suffered a fall at home, but did not fracture. The dose of strontium ranelate was decreased to 2 g three times weekly.
Two years later she repeated lumbar bone densitometry using a Lunar equipment, and again the value found was normal (1.357 g/cm2; T-score −0.3). She was told to stop strontium ranelate, but she was so happy with the therapeutic outcome that she requested permission to continue taking 1–2 envelopes weekly, as a supplement.
The evolution of her lumbar BMD is shown in Figure 1.
Figure 1.
Lumbar BMD values (Lunar) along 5 years of treatment.
Discussion
Strontium ranelate (SrR) is one of the medications presently used to treat osteoporosis (1). Its mechanism of action is considered dual: it has an antiresorptive action, while stimulating osteoblasts to increase bone mass (anabolic action) (2).
After SrR treatment, significantly higher mineral apposition rate in cancellous bone has been observed by histomorphometry. Using μCT of bone biopsies collected from humans receiving long-term treatment with SrR over 5 years, increase in cortical thickness and trabecular number has been demonstrated, with no change in cortical porosity (3). Also, greater effects on distal tibia cortical thickness and trabecular volumetric density were observed with SrR versus alendronate over 2 years using HR-pQCT (4).
After a 36-month treatment of postmenopausal osteoporotic women with SrR, strontium ions partially replace calcium in the hydroxyapatite crystals, only in newly formed bone (5, 6). Besides, strontium (Sr) content in bone reaches a plateau at 3 years, representing 1.5–2.0% of tissue weight in the latter biopsy study (6). However, in a study of a single osteoporotic volunteer taking oral supplements of strontium citrate, Sr content in bone was determined by in vivo X-ray fluorescence; continuous increases were found at the finger and ankle, with values after 800 days representing 7 and 15 times, respectively, the basal levels (7). Regarding the “overestimation” of BMD the molar correction factor for Sr is 10% per 1% relative content of Sr in hydroxyapatite almost irrespectively of the DXA device used (8).
The case presented here is noteworthy for two reasons. Firstly, the patient maintained low bone mass after several years of combined treatment with alendronate and hormone replacement; this combination usually induces greater densitometric responses than either treatment given alone (9). Secondly, she responded promptly and significantly to SrR in spite of the previous long exposure to alendronate. We and others have shown that the densitometric response to SrR in patients with prior bisphosphonate treatment is less than in patients not previously exposed to bisphosphonates (10, 11). SrR is widely used for the treatment of osteoporosis. It is an effective and safe drug, provided the patients are properly selected (12). As shown here, it can help some patients to achieve a normal BMD.
References
- 1.Schurman L, Bagur A, Claus-Hermberg H, Messina OD, Negri AL, Sánchez A, González C, Diehl M, Rey P, Gamba J, Chiarpenello J, Moggia MS, Mastaglia S. Guidelines 2012 for the diagnosis, prevention and treatment of osteoporosis. Medicina (B Aires) 2013;73:55–74. [PubMed] [Google Scholar]
- 2.Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 3.Arlot ME, Jiang Y, Genant HK, Zhao J, Burt-Pichat B, Roux JP, Delmas PD, Meunier PJ. Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J Bone Miner Res. 2008;23:215–222. doi: 10.1359/jbmr.071012. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoli R, Laroche M, Krieg MA, Frieling I, Thomas T, Delmas P, Felsenberg D. Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int. 2010;30:1341–1348. doi: 10.1007/s00296-010-1542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CH, Paris O, Siegel S, Roschger P, Paschalis EP, Klaushofer K, Fratzl P. Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res. 2010;25:968–975. doi: 10.1359/jbmr.091038. [DOI] [PubMed] [Google Scholar]
- 6.Boivin G, Farlay D, Khebbab MT, Jaurand X, Delmas PD, Meunier PJ. In osteoporotic women treated with strontium ranelate, strontium is located in bone formed during treatment with a maintained degree of mineralization. Osteoporos Int. 2010;21:667–677. doi: 10.1007/s00198-009-1005-z. [DOI] [PubMed] [Google Scholar]
- 7.Moise H, Adachi JD, Chettle DR, Pejović-Milić A. Monitoring bone strontium levels of an osteoorotic subject due to self-administration of strontium citrate with a novel diagnostic tool, in vivo XRF: a case study. Bone. 2012;51:93–97. doi: 10.1016/j.bone.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Pors Nielsen S, Slosman D, Sørensen OH, Basse-Cathelinat B, De Casin P, Roux C, Meunier PJ. Influence of strontium on bone mineral density and bone mineral content measurements by dual-X-ray absorptiometry. J Clin Densit. 1999;2:371–9. doi: 10.1016/s1094-6950(06)60402-2. [DOI] [PubMed] [Google Scholar]
- 9.Eviö S, Tiitinen A, Laitinen K, Ylikorkala O, Välimäki MJ. Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. J Clin Endocrinol Metab. 2004;89:626–631. doi: 10.1210/jc.2003-030198. [DOI] [PubMed] [Google Scholar]
- 10.Brun L, Galich AM, Vega E, Salerni H, Maffei L, Premrou V, Costanzo PR, Sarli MA, Rey P, Larroudé MS, Moggia MS, Brance ML, Sánchez A. Strontium ranelate effect on bone mineral density is modified by previous bisphosphonate treatment. SpringerPlus. 2014;3:676. doi: 10.1186/2193-1801-3-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton ET, Steel SA, Aye M, Doherty SM. The effect of prior bisphosphonate therapy on the subsequent therapeutic effects of strontium ranelate over 2 years. Osteoporos Int. 2012;23:295–303. doi: 10.1007/s00198-011-1547-8. [DOI] [PubMed] [Google Scholar]
- 12.Reginster JY, Brandi ML, Cannata-Andía J, Cooper C, Cortet B, Feron JM, Genant H, Palacios S, Ringe JD, Rizzoli R. The position of strontium ranelate in today’s management of osteoporosis. Osteoporos Int. 2015;26:1667–1671. doi: 10.1007/s00198-015-3109-y. [DOI] [PubMed] [Google Scholar]