Abstract
Neutrophil infiltration is the characteristic pathological feature of M. pneumoniae pneumonia (MPP). This study aimed to explore the associations among neutrophil activity, clinical presentation, and role of the M. pneumoniae/interleukin-8 (IL-8)/neutrophil axis in the pathogenesis of MPP. A total of 42 patients with MPP were prospectively enrolled in the study. Neutrophil activity, including matrix metalloproteinase-9 (MMP-9), myeloperoxidase (MPO), and neutrophil elastase (NE), were measured. Clinical information was collected for all patients and control group. In vitro, IL-8 production was measured at different time points after M. pneumoniae infection of bronchial epithelial cells, and neutrophil activity was analyzed after IL-8 stimulation. The percentage of neutrophil in the bronchoalveolar lavage fluid was higher in the group of patients with high levels of M. pneumoniae DNA than in those with low levels of M. pneumoniae DNA (P < 0.05). IL-8, MMP-9, and NE in patients with MPP significantly increased compared with controls and decreased after treatment (P < 0.05). MPO and MMP-9 were associated with duration of fever (r = 0.332, P < 0.05) and length of stay (r = 0.342, P < 0.05), respectively. In vitro, M. pneumoniae induced IL-8 production by bronchial epithelial cells in a time dependent manner. MPO, MMP-9 and NE production by neutrophils significantly increased compared with medium controls after IL-8 stimulation. In summary, the M. pneumoniae/IL-8/neutrophil axis likely plays a vital role in the pathogenesis of MPP.
Introduction
Community-acquired pneumonia (CAP) is a major health problem in the world and is associated with substantial morbidity, mortality, and healthcare costs. Among all the infectious pathogens, Mycoplasma pneumoniae (M. pneumoniae) is one of the most important agents causing severe respiratory disease in children [1], accounting for up to 40% of CAP cases in children. Furthermore, as many as 18% of pediatric patients with M. pneumoniae pneumonia (MPP) require hospital admission [2].
The severity of MPP seems to depend on the host immune response to the infection through various mechanisms, including an allergic reaction to M. pneumoniae, M. pneumoniae virulence, host defenses, and polarization toward T-helper cell 1 or T-helper cell 2 predominance [3]. The characteristic pathological feature of human MPP is marked by lymphocytic infiltration in the peribronchovascular areas and accumulation of neutrophils and lymphocytes in the lung alveolar spaces. Neutrophils are sentinel cells of the innate immune system and are the principal cellular responders to acute inflammation [4]. Interleukin 8 (IL-8) production by bronchial epithelial cells can be induced by M. pneumoniae antigen or live M. pneumoniae, which then chemoattracts and activates neutrophils [5]. During acute pahse of MPP infection, the majority cells in bronchoalveolar lavage fluid of hamster are neutrophils which are replaced by lymphocytes in later phase of infection [6].
The present study aimed to explore the associations between neutrophil activity, clinical presentation and the role of the M. pneumoniae/IL-8/neutrophil axis in the pathogenesis of MPP in children who had been admitted to hospital. Our study also examined the process of IL-8 secretion by normal human bronchial epithelial (NHBE) cells and the activity of neutrophils stimulated by IL-8 in vitro.
Materials and Methods
Subjects
This study was approved by the Institutional Human Ethical Committee of the Children’s Hospital of Soochow University. Written consent was obtained from the guardians on behalf of the patients enrolled in this study. The patients were defined as MPP infection at following criteria: 1) Children with clinical symptoms of pneumonia (cough, fever, tachypnea, chest retractions, or abnormal auscultatory findings), confirmed by radiography and 2) M. pneumoniae DNA was detected in BALF by real-time polymerase chain reaction (PCR) and specific IgM and IgG antibodies against M. pneumoniae in paired sera were detected by enzyme-linked immunosorbent assays (ELISA). Patients were excluded if they were diagnosed as chronic lung disease, bronchopulmonary malformation, immunodeficiency, immunosuppression, cardiovascular disease, or were co-infected with other pathogens. Study was performed from January to December 2014 in 42 MPP patients. Fifteen of age matched control patients were selected from children who suffered from foreign body in the bronchus within 48 hours without secondary infection.
Demographic and clinical information were collected in all patients. Laboratory specimens were obtained including blood, nasopharyngeal aspirates (NPAs) and BALF. The following laboratory tests were conducted: C-reactive protein, alanine transaminase, L-lactate dehydrogenase and creatine kinase (type MB isoenzyme). Nine other viruses were detected by direct immunofluorescence assay and PCRs as previously described [7]. BALF cytology was also performed.
BALF collection
Fiber optic bronchoscopy and BALF collection were performed as described previously [8]. BALF samples were examined for M. pneumoniae DNA, IL-8, matrix metalloproteinase 9 (MMP-9), myeloperoxidase (MPO) and neutrophil elastase (NE). Cells in BALF were counted based on Giemsa and Wright staining after centrifugation at 200 × g for 10 min at 4°C.
Serology of M. pneumoniae
Specific IgM and IgG antibodies against M. pneumoniae were detected in serum samples of patients in the acute phase of MPP (on admission) and convalescent phase (on discharge) respectively, using a commercial ELISA kit (Serion ELISA classic M. pneumoniae IgG/IgM, Institute Virion/Serion, Würzburg, Germany) according to the manufacturer's instructions as previously described [9].
Real-time PCR for M. pneumoniae detection
A real-time PCR procedure (Daan Gene Co. Ltd, Guangzhou, China) approved by the State Food and Drug Administration of China was used for the detection of M. pneumoniae as described previously [8]. In brief, one of the equally divided samples of BALF was shaken for 30 s and centrifuged at 15,000 × g for 5 min. The sediment was collected and DNA extracted from a 400 μl sample in accordance with the manufacturer’s instructions. Then, PCR amplification was conducted using primers and probes purchased from Daan Gene Company. Quantification curves were plotted using several concentrations of standard control samples.
Examination of IL-8, MMP-9, MPO, and NE in BALF
The BALF samples were immediately centrifuged and preserved at -80°C for subsequent assays. IL-8, MMP-9, MPO and NE (R&D Company) levels in supernatant of BALF were measured by ELISA according to the manufacturer’s instructions.
IL-8 secretion by NHBE cells infected with M. pneumoniae in vitro
The normal human bronchial epithelial (NHBE) cells were purchased from American Type Culture Collection (Bethesda, MD) and used at culture passages 3–5. The cells were grown in serum-free bronchial epithelial cell growth medium (BEGM; Clonetics, Houston TX) containing the following supplements (all from Clonetics): bovine pituitary extract (52 μg/ml), hydrocortisone (0.5 μg/ml), human epidermal growth factor (0.5 ng/ml), epinephrine (0.5 μg/ml), transferrin (10 μg/ml), insulin (5 μg/ml), retinoic acid (0.1 ng/ml), triiodothyronine (6.5 ng/ml), gentamycin (50 μg/ml), and amphotericinB (50 ng/ml).
The M. pneumoniae strain M129 was purchased from the Institute of Pathogen Biology, Medical College of University of South China. M. pneumoniae was grown in SP4 broth for 72 h at 37°C, spun at 10,000 × g for 20 min, re-suspended in saline to yield 1 × 108 CFU/50μl and frozen at -80°C in aliquots that were subsequently used to infect epithelial cells. On the infection day, frozen M. pneumoniae aliquots were thawed, spun, resuspended in SP4 broth, and incubated for 2 h at 37°C. For infection with viable M. pneumoniae, the suspension of freshly harvested M. pneumoniae was diluted with supplement-free bronchial epithelial cell growth (BEGM) medium to obtain a designated infectious dose of 1–100 CFU/cell in six well plates (NHBE, 2×104 cells/well in 2ml of serum-free BEGM). The supernatants were collected for IL-8 protein measurement by using an IL-8 ELISA kit (R&D Systems) at time points 2, 6, 12, 24, 48, and 72h.
Release of MPO, MMP-9, and NE by neutrophils after stimulation with IL-8 or M. pneumoniae in vitro
Blood neutrophils were isolated from leucocyte-enriched buffy coats by Ficoll-Paque Plus gradient centrifugation and dextran sedimentation, as previously described [10]. Erythrocytes were removed by hypotonic lysis. The final cell pellet was suspended in RPMI-1640 medium (Sigma-Aldrich, Shanghai, China) supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin to obtain 1×106 cell/well. Cell viability was determined by frequency of cells without annexin V staining determined by flow cytometry analysis. More than 99% of the blood neutrophils were viable immediately before the culture assays. Isolated neutrophils were stimulated by IL-8 (10 ng/ml) for 24h. The supernatants of culture were collected and stored at -80°C. The levels of MPO, MMP-9 and NE released by neutrophils were measured using commercially available ELISAs as mentioned above.
Data analysis
Numeration data were analyzed using the Chi-square test and measurement data were analyzed using the Student t-test or non-parametric test (Mann–Whitney U-test or Wilcoxon test) if the data distribution was non-normal. The Pearson or Spearman correlation test was used to assess correlations based on normal or abnormal distributed data. Associations between parameters and clinical profiles were analyzed using partial correlations. One-way analysis of variance (ANOVA) was used to identify differences between three or more groups. A two-sided p-value of < 0.05 was considered statistically significant. All analyses were performed using SPSS for Windows, version 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical data of children with MPP
The demographic data, clinical presentation, and laboratory findings of the study patients with MPP were shown in Table 1. The mean age of control patients was 4.4 ± 2.4 years, and the male percentage was 60% (9/15). There was no statistical significance in age and gender between children with MPP and control subjects (both P > 0.05).
Table 1. Demographic and clinical profiles of study patients (children) with MPP.
| Parameters | Patients with MPP |
|---|---|
| n = 42 | |
| Age (mean ± SD, year) | 5.6 ± 2.5 |
| Male (n, %) | 23 (54.8) |
| Duration of fever, (25th–75th percentile, d) | 15.0 (13.0–18.0) |
| Length of stay, (mean ± SD, d) | 10.9 ± 4.2 |
| White blood cell count (mean ± SD, ×109/L) | 9.5 ± 4.8 |
| Neutrophils (mean ± SD, ×109/L) | 6.8 ± 4.0 |
| C-reactive protein (25th–75th percentile, mg/L) | 20.9 (10.4–62.2) |
| Alanine transaminase increase (n, %) | 8 (19.0) |
| l-lactate dehydrogenase (mean ± SD, U/L) | 534.5 ± 227.3 |
| MB isoenzyme of creatine kinase (25th–75th percentile, U/L) | 16.4 (13.7–24.9) |
| Cytology of BALF (mean ± SD, %) | |
| Neutrophils | 62.5 ± 23.1 |
| Lymphocytes | 8.9 ± 7.5 |
| Macrophages | 27.4 ± 22.2 |
| Radiologic evaluation (n, %) | |
| Lobar or segmental opacity | 42 (100) |
| Opacity with pleural effusion | 13 (31.0) |
| Opacity with pulmonary atelectasis | 3 (7.1) |
| Macrolide medication (n, %) | 42 (100) |
| Methylprednisolone (n, %) | 42 (100) |
MPP: Mycoplasma pneumoniae pneumonia; BALF: bronchoalveolar lavage fluid; SD: standard deviation.
Cytology and expressions of IL-8, MPO, MMP-9, and NE in BALF of children with MPP
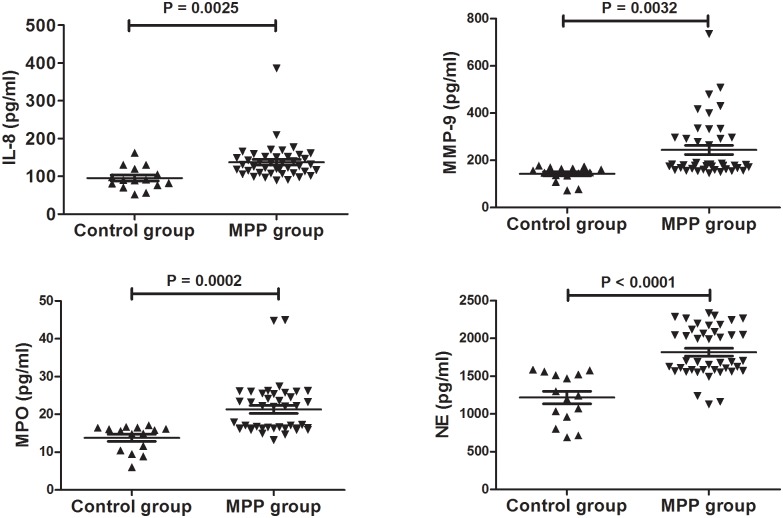
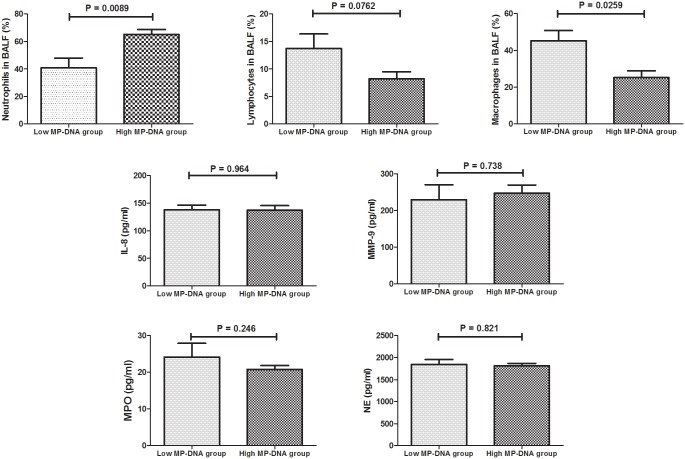
As shown in Fig 1, the levels of IL-8, MPO, MMP-9, and NE in BALF in patients with MPP were significantly higher than in the controls. According to the concentration of M. pneumoniae DNA in BALF, all MPP cases were divided into a low M. pneumoniae DNA group (< 107 copies/ml) and a high M. pneumoniae DNA group (≥107 copies/ml). BALF neutrophil percentage was higher in the high M. pneumoniae DNA group than in the low M. pneumoniae DNA group (Fig 2). However, the low M. pneumoniae DNA group had a higher percentage of macrophages in the BALF than the high M. pneumoniae DNA group. No significant difference between the two groups was found for levels of IL-8, MPO, MMP-9, and NE (Fig 2).
Fig 1. Comparison of IL-8, MPO, MMP-9, and NE levels in BALF between Patients with MPP and Controls.
The values in the graphs represent the mean ± SD. The P values were calculated using Student’s t test while Mann-Whitney U-test was used for comparison of MMP-9 between MPP and control groups. MPP: Mycoplasma pneumoniae pneumonia; IL: interleukin-8; MPO: myeloperoxidase; MMP-9: matrix metalloproteinase 9; NE: neutrophil elastase.
Fig 2. Comparison of Cytology, IL-8, MPO, MMP-9, and NE in BALF between the Low MP-DNA Group (< 107 copies/ml) and High MP-DNA Group (≥ 107 copies/ml).
The values in the graphs represent the mean ± SD. The P values were calculated using Student’s t test while Mann-Whitney U-test was used for comparison of MMP-9 between two groups. MPP: Mycoplasma pneumoniae pneumonia; IL: interleukin-8; MPO: myeloperoxidase; MMP-9: matrix metalloproteinase 9; NE: neutrophil elastase.
Comparisons of IL-8, MPO, MMP-9, and NE expressions in BALF between children with and without pleural effusion
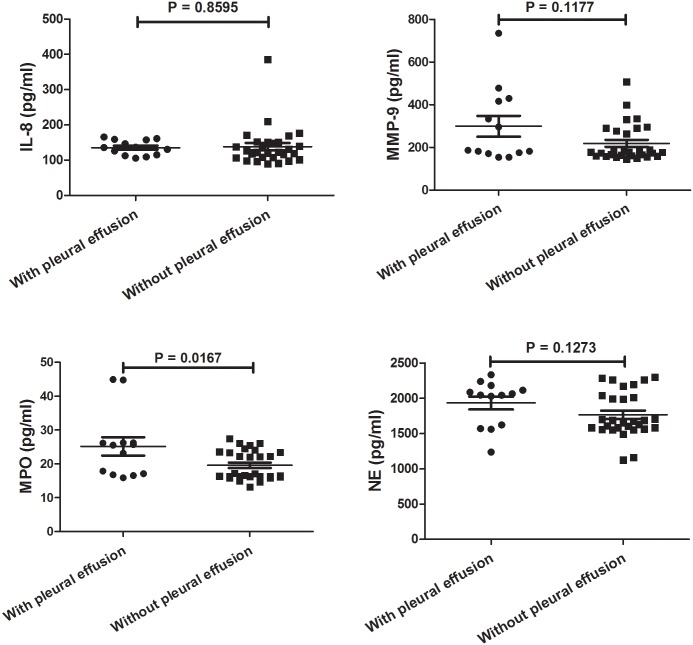
Interestingly, the level of MPO in children with pleural effusion was significantly higher than in children without pleural effusion. No significant difference was found in IL-8, MMP-9, and NE between children with and without pleural effusion as shown in Fig 3.
Fig 3. Comparison of IL-8, MPO, MMP-9, and NE in BALF between Patients with and without Pleural Effusion.
The values in the graphs represent the mean ± SD. The P values were calculated using Student’s t test while Mann-Whitney U-test was used for comparison of MMP-9 and MPO between two groups. IL: interleukin-8; MPO: myeloperoxidase; MMP-9: matrix metalloproteinase 9; NE: neutrophil elastase.
Relationships between IL-8, MPO, MMP-9, NE, and clinical profiles
The MPO level in children with MPP was associated with duration of fever (r = 0.332, P = 0.032) and MMP-9 level was associated with length of stay (r = 0.342, P = 0.026). No association was found between IL-8, MPO, MMP-9, NE, and other clinical aspects (P > 0.05). Because of interaction between MPO and NE (r = 0.472, P = 0.002), partial correlation was conducted and MPO was still associated with duration of fever (r = 0.353, P = 0.024).
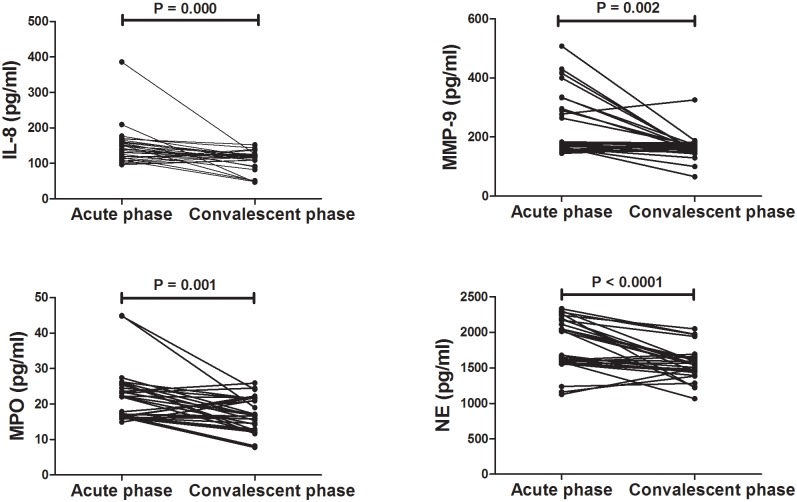
Levels of IL-8, MPO, MPP-9, and NE decreased after treatment
Convalescent BALF samples were obtained from 30 MPP patients and concentrations of IL-8, MPO, MPP-9, and NE significantly decreased after treatment shown in Fig 4.
Fig 4. Comparison of IL-8, MPO, MMP-9, and NE in BALF before and after Treatment.
The P values were calculated using Paired t test while Wilcoxon test was used for comparison of IL-8 and MMP-9 before and after treatment. IL: interleukin-8; MPO: myeloperoxidase; MMP-9: matrix metalloproteinase 9; NE: neutrophil elastase.
M. pneumoniae induced IL-8 release from NHBE
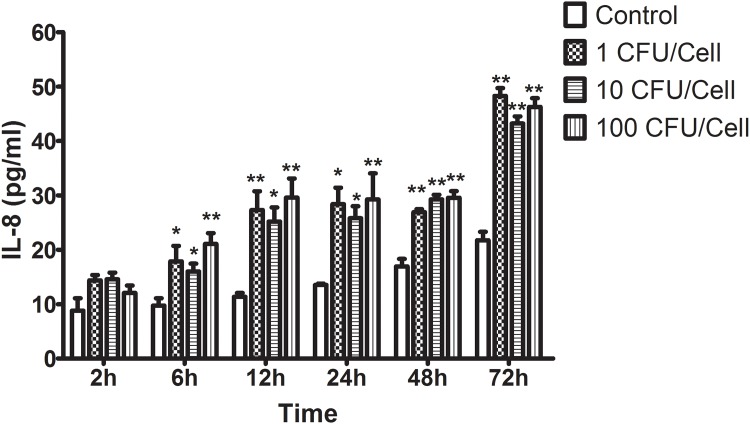
In vitro, IL-8 release from NHBE was detected after M. pneumoniae stimulation. As seen in Fig 5, M. pneumoniae induced IL-8 production in a time-dependent manner in the 72-h period when compared with medium controls. However, there was no difference among various multiplicities of infection from 1 CFU/cell to 100 CFU/cell.
Fig 5. Concentrations of IL-8 Secreted by NHBE Cells after M. pneumoniae Infection.
M. pneumoniae induced IL-8 production in a time-dependent manner. Data are represented as means ± SEMs from two independent experiments (* P < 0.05, ** P < 0.01 vs. controls). No significant difference was found between various multiplicities of infection (1–100 CFU/cell). The P values were calculated using ANOVA. IL: interleukin-8; NHBE: normal human bronchial epithelium.
Neutrophil activity induced by IL-8
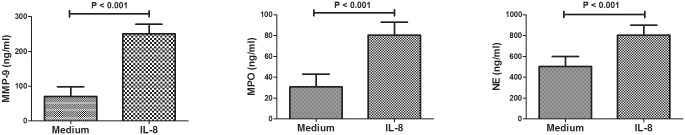
In vitro, neutrophil activity including MPO, MMP-9, and NE release was analyzed after IL-8 stimulation. As shown in Fig 6, concentrations of MPO, MMP-9, and NE in the supernatant significantly increased compared with medium controls.
Fig 6. MPO, MMP-9, and NE Production Induced by Neutrophils after IL-8 Stimulation Significantly Increased Compared with Medium Controls.
Data are represented as means ± SEMs. The P values were calculated using Student’s t test. IL-8: interleukin-8; MPO: myeloperoxidase; MMP-9: matrix metalloproteinase 9; NE: neutrophil elastase.
Discussion
Inflammation is a fundamental innate immune response to environmental factors, including infections. Excessive release of proinflammatory cytokines can occur following infection that skews the host response to "hyperinflammation" with exaggerated tissue damage. An excessive inflammation response induced by the host’s innate and adaptive immune systems is one of the main causes of the immunopathogenesis of M. pneumoniae infection and contributes to clinical presentations [11]. Various cells are involved in the inflammation response, such as macrophages, lymphocytes, bronchial epithelial cells as well as neutrophils. Macrophages rather than neutrophils are essential for the clearance of M. pneumoniae from the lungs [12]. On the contrary, neutrophil accumulation might lead to “hyperinflammation” due to MPO, MMP-9, and NE release.
The present study focused on the neutrophil activity induced by high expression of IL-8 and associations with clinical characteristics and laboratory findings. The findings are evidence that the M. pneumoniae/IL-8/neutrophil axis likely plays an important role in the pathogenesis of MPP based on both BALF analyses from children with MPP and experiments in vitro. We presumed that M. pneumoniae attaches to bronchial epithelial cells and induces the release of IL-8, which in turn drives the recruitment and activation of neutrophils.
However, our study did not show significance difference of IL-8 between patients with low M. pneumoniae DNA and high M. pneumoniae DNA, neither did show difference among various multiplicities of infection in vitro. There was no dose-response relationship between M. pneumoniae and IL-8 expression. Nevertheless, children with high M. pneumoniae load presented more neutrophils in BALF compared to children with low M. pneumoniae load. Taken together, other chemokines of neutrophils might take part in neutrophil accumulation in lungs.
Interestingly, MPO, MMP-9, and NE levels increased in the BALF of all patients with M. pneumoniae infection and decreased after treatment. MPO and MMP-9 might be effective biomarkers to predict disease severity in the present study. Previous studies have reported several biomarkers in serum or BALF such as soluble B7-H3 [13], IL-18 [14], MUC18 [15] as well as the community-acquired respiratory distress syndrome (CARDS) toxin which is an unique M. pneumoniae virulence factor regulating inflammasome activity [16, 17]. However, further studies in large, well-characterized patient samples are needed to confirm and explore the clinical applications of these observations.
IL-8 is a mediator between M. pneumoniae and neutrophils. It is reported that M. pneumoniae components (whole organism lysate or membrane extracts) could induce IL-8 release in the bronchial epithelium through ERK or NF-κB in a time and dose-dependent manner [18, 19]. Study showed NHBE cells infected with live M. pneumoniae might induce CARDS toxin production which causes IL-8 secretion [20]. IL-8 release could also be induced in macrophages by microbes [21]. Moreover, the M. pneumoniae extract could induce IL-17 release and subsequently cause neutrophil accumulation in the lung [22].
Meanwhile, IL-8 production by NHBE infected with M. pneumoniae, acts on neutrophils induce MPO, MMP-9, and NE release that leads to inflammation and tissue damage. A previous study showed that IL-8-induced MMP-9 release from neutrophils is mediated through CXCR2 and involves two distinct pathways, one involving PKC and ERK1/2 and the other involving Src-family kinases [23]. MPO and NE release in neutrophils stimulated by IL-8 from younger individuals significantly increased compared to medium controls [24].
However, some limitations of this study should be noted. First of all, this study only included 42 MPP cases which do not conform to a large samples study. Secondly, the data analysis alone may not serve as a conclusive interpretation because of lacking of the study for M. pneumoniae infection model in Vivo. What’s more, our study was based on a single center for data, which might have potential biases.
Conclusion
Our study elucidates that bronchial epithelial cells infected by M. pneumoniae overexpressed IL-8, which subsequently enhanced neutrophils activity through MPO, MMP-9, and NE release. Consequently, the M. Pneumoniae/IL-8/neutrophil axis likely plays a vital role in the pathogenesis of MPP.
Supporting Information
(XLS)
Acknowledgments
This manuscript has been edited by Editage of language editing service.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Zhengrong Chen, grant number 81401296; Wei Ji, grant number 81570016), Science and Technology Projects for the youth of Suzhou (Zhengrong Chen, grant number KJXW2012019), and Suzhou Science and Technology Projects (Zhengrong Chen, grant number SYS201350; Yongdong Yan, grant number SYS201435), Science and Technology Projects of Suzhou sanitary bureau (Yongdong Yan, grant number lczx201409). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.HuongPle T, Hien PT, Lan NT, Binh TQ, Tuan DM, Anh DD. First report on prevalence and risk factors of severe atypical pneumonia in Vietnamese children aged 1–15 years. BMC Public Health. 2014;14:1304 10.1186/1471-2458-14-1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waites KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol. 2003;36:267–278. [DOI] [PubMed] [Google Scholar]
- 3.Saraya T, Kurai D, Nakagaki K, Sasaki Y, Niwa S, Tsukagoshi H, et al. Novel aspects on the pathogenesis of Mycoplasma pneumoniae pneumonia and therapeutic implications. Front Microbiol. 2014;5:410 10.3389/fmicb.2014.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Looney MR, Matthay MA. Neutrophil sandwiches injure the microcirculation. Nat Med. 2009;15:364–366. 10.1038/nm0409-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pease JE, Sabroe I. The role of interleukin-8 and its receptors in inflammatory lung disease: implications for therapy. Am J Respir Med. 2002;1:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yano T, Komatsu S, Araki K, Kuboshiro M, Ichikawa Y, Ohizumi K, et al. Role of transiently accumulated neutrophils in the lung of hamster in development of pneumonia due to Mycoplasma pneumoniae. KansenshogakuZasshi. 1991;65:365–373. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZR, Mize M, Wang YQ, Yan YD, Zhu CH, Wang Y, et al. Clinical and epidemiological profiles of lower respiratory tract infection in hospitalized children due to human bocavirus in a subtropical area of China. J Med Virol. 2014;86:2154–2162. 10.1002/jmv.23952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Li S, Chen Z, Du L. Detection of Mycoplasma pneumoniae in different respiratory specimens. Eur J Pediatr. 2011;170:851–858. 10.1007/s00431-010-1360-y [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Wang Y, Yan Y, Zhu C, Huang L, Shao X, et al. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int J Infect Dis. 2014;29:18–23. 10.1016/j.ijid.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 10.Gasparoto TH, Vieira NA, Porto VC, Campanelli AP, Lara VS. Differences between salivary and blood neutrophils from elderly and young denture wearers. J Oral Rehabil. 2011;38:41–51. 10.1111/j.1365-2842.2010.02126.x [DOI] [PubMed] [Google Scholar]
- 11.Waites KB, Balish MF, Atkinson TP. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol. 2008;3:635–648. 10.2217/17460913.3.6.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai JF, Zindl CL, Duffy LB, Atkinson TP, Jung YW, van Rooijen N, et al. Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to Mycoplasma pneumoniae. PLoS One. 2010;5:e14417 10.1371/journal.pone.0014417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ZR, Zhang GB, Wang YQ, Yan YD, Zhou WF, Zhu CH, et al. Soluble B7-H3 elevations in hospitalized children with Mycoplasma pneumoniae pneumonia. Diagn Microbiol Infect Dis. 2013;77:362–366. 10.1016/j.diagmicrobio.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 14.Narita M, Tanaka H, Abe S, Yamada S, Kubota M, Togashi T. Close association between pulmonary disease manifestation in Mycoplasma pneumoniae infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon. Clin Diagn Lab Immunol. 2000;7:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q, Case SR, Minor MN, Jiang D, Martin RJ, Bowler RP, et al. A novel function of MUC18: amplification of lung inflammation during bacterial infection. Am J Pathol. 2013;182:819–827. 10.1016/j.ajpath.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muir MT, Cohn SM, Louden C, Kannan TR, Baseman JB. Novel toxin assays implicate Mycoplasma pneumoniae in prolonged ventilator course and hypoxemia. Chest. 2011;139:305–310. 10.1378/chest.10-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose S, Segovia JA, Somarajan SR, Chang TH, Kannan TR, Baseman JB. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. Mbio. 2014;5:e02186–14. 10.1128/mBio.02186-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn MH, Lee KE, Choi SY, Kwon BC, Chang MW, Kim KE. Effect of Mycoplasma pneumoniae lysate on interleukin-8 gene expression in human respiratory epithelial cells. Chest. 2005;128:322–326. [DOI] [PubMed] [Google Scholar]
- 19.Chmura K, Bai X, Nakamura M, Kandasamy P, McGibney M, Kuronuma K, et al. Induction of IL-8 by Mycoplasma pneumoniae membrane in BEAS-2B cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L220–230. 10.1152/ajplung.90204.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009;4:e7562 10.1371/journal.pone.0007562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohnet S, Kötschau U, Braun J, Dalhoff K. Role of interleukin-8 in community-acquired pneumonia: relation to microbial load and pulmonary function. Infection. 1997;25:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurai D, Nakagaki K, Wada H, Saraya T, Kamiya S, Fujioka Y, et al. Mycoplasma pneumoniae extract induces an IL-17-associated inflammatory reaction in murine lung: implication for mycoplasmal pneumonia. Inflammation. 2013;36:285–293. 10.1007/s10753-012-9545-3 [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol. 2005;78:279–288. [DOI] [PubMed] [Google Scholar]
- 24.Dalboni TM, Abe AE, de Oliveira CE, Lara VS, Campanelli AP, Gasparoto CT, et al. Activation profile of CXCL8-stimulated neutrophils and aging. Cytokine. 2013;61:716–719. 10.1016/j.cyto.2013.01.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.