Abstract
Background
Visceral leishmaniasis (VL) is a neglected tropical disease and is fatal if untreated. There is no vaccine available against leishmaniasis. The majority of patients with cutaneous leishmaniasis (CL) or VL develop a long-term protective immunity after cure from infection, which indicates that development of an effective vaccine against leishmaniasis is possible. Such protection may also be achieved by immunization with live attenuated parasites that do not cause disease. We have previously reported a protective response in mice, hamsters and dogs with Leishmania donovani centrin gene knock-out parasites (LdCen-/-), a live attenuated parasite with a cell division specific centrin1 gene deletion. In this study we have explored the effects of salivary protein LJM19 as an adjuvant and intradermal (ID) route of immunization on the efficacy of LdCen-/- parasites as a vaccine against virulent L. donovani.
Methodology/Principal Findings
To explore the potential of a combination of LdCen-/- parasites and salivary protein LJM19 as vaccine antigens, LdCen-/- ID immunization followed by ID challenge with virulent L. donovani were performed in hamsters in a 9-month follow up study. We determined parasite burden (serial dilution), antibody production (ELISA) and cytokine expression (qPCR) in these animals. Compared to controls, animals immunized with LdCen-/- + LJM19 induced a strong antibody response, a reduction in spleen and liver parasite burden and a higher expression of pro-inflammatory cytokines after immunization and one month post-challenge. Additionally, a low parasite load in lymph nodes, spleen and liver, and a non-inflamed spleen was observed in immunized animals 9 months after the challenge infection.
Conclusions
Our results demonstrate that an ID vaccination using LdCen-/-parasites in combination with sand fly salivary protein LJM19 has the capability to confer long lasting protection against visceral leishmaniasis that is comparable to intravenous or intracardial immunization.
Author Summary
Leishmaniasis is a disease with a wide spectrum of clinical manifestations caused by different species of protozoa belonging to the Leishmania genus that are transmitted by sand fly vectors. Visceral infections of Leishmania cause significant mortality and morbidity and development of a vaccine to prevent leishmaniasis has become a high priority. We have previously reported that intravenous immunization with a live attenuated parasite vaccine comprised of Leishmania donovani parasites lacking the centrin gene conferred protection in mice, hamsters and dogs. In the current report, we describe the immunological response and associated protection to the ID immunization with attenuated parasites in combination with a sand fly salivary protein (LJM19). We observe that protection against experimental ID challenge with L. donovani resulting from ID immunization with live attenuated parasites in combination with LJM19 is comparable to intracardial immunization and offers improved protective immunity compared to immunization with salivary protein alone and non-immunized hamsters. This study supports the potential use of the genetically attenuated vaccine and a recombinant sand fly salivary protein for control of visceral leishmaniasis.
Introduction
Leishmaniasis is a disease with a wide spectrum of clinical manifestations caused by different species of protozoa belonging to the Leishmania genus that are transmitted by sand fly vectors [1]. The disease causes high morbidity and significant mortality throughout the world, where 350 million people in 98 countries are at risk of contracting the infection. Moreover, approximately 1.0 to 1.5 million cases of cutaneous leishmaniasis (CL), and 200,000 to 500,000 cases of visceral leishmaniasis (VL), are registered annually [2].
VL is fatal if not treated [2]. The treatment of leishmaniasis is still based on the use of the parenteral administration of pentavalent antimonial compounds. However, side effects associated with the treatment and increased parasite resistance have made control and elimination of VL a serious challenge [3,4]. Therefore, the development of new strategies to prevent leishmaniasis has become a high priority [5].
The development of a vaccine for VL has been the focus of several research groups. Among the various types of vaccines, genetically modified live-attenuated vaccines provide the immunized host with diverse and complex antigens and induce a potent protective immunity in murine models [5,6]. Importantly live attenuated parasites cause no pathology in experimental infections [7–14], while inducing protection reflected by a significant reduction of parasite burden in animals challenged with virulent wild type strains [10,12,14–18].
We have previously reported on the LdCen-/- parasites as a live attenuated candidate vaccine in several animal models [12,14,18]. Infection with LdCen-/- was non-pathogenic i.e., safe and highly immunogenic in mice, hamsters and dogs [12,13,18]. In addition, immunization with LdCen-/- induced protection against homologous challenge with wild type L. donovani and conferred cross-protection against infection with a heterologous challenge with L. braziliensis, L. mexicana and L. infantum [14,18]. However, previous studies with LdCen-/- parasites as immunogens were performed without any adjuvants. Since the adjuvants can activate a range of innate immune pathways it is difficult to predict on an empirical basis which adjuvant will work most effectively with live attenuated parasites. Since the adaptive response is the primary determinant of protective immunity generated by vaccination, immunomodulatory reagents that could supplement LdCen-/- induced immunity without causing rapid elimination of the vaccine antigen due to innate immune reactions could make LdCen-/- more effective as an anti-Leishmania vaccine.
Saliva from sand flies contains potent pharmacologic components that facilitate blood meal acquisition and modulates the host inflammatory and immune responses [19,20]. Arthropod vector saliva also plays an important role in pathogen transmission from the sand fly to the vertebrate host [21]. Recent reports have shown the importance of some salivary proteins from sand fly vectors such as LJM19, LJM11 or LJM17 as potential targets for vaccine development against Leishmania infection [11,19,22–30]. A specific immune response against salivary proteins has been reported in various animal models. For example, hamsters immunized with plasmid DNA coding for LJM19, a Lu. longipalpis salivary protein, protected them from disease after challenge with wild type Leishmania infantum chagasi parasites plus saliva through the induction of a LJM19-specific immune response [26]. By comparison, salivary protein LJM11 provided partial protection that was not long lasting against virulent challenge [26]. Importantly, immunization with LJM19 induced higher ratios of IFN-γ/IL-10 and IFN- γ/TGF-β in the spleen, conditions consistent with a Th1 polarization [26,28]. These results suggested that salivary gland proteins such as LJM19 could be a potent supplement to the protective immunity with live attenuated Leishmania parasites.
In our previous studies, we have tested different routes of immunization, including intravenous (tail vein), intracardial and subcutaneous [12,13]. However, intradermal immunization can offer improved protective immunity and simplify the logistics of delivery as was previously demonstrated [26,28,29,31,32]. Therefore, it is of value to evaluate live attenuated parasite vaccines for their efficacy following intradermal immunization.
Since our previous studies have shown that exposure to live attenuated parasites injected by intravenous or intracardial routes and without an adjuvant induced a strong protective immunity, we asked whether an intradermal immunization with LdCen-/- parasites in combination with LJM19 could further enhance vaccine induced protection. In the present study, we report for the first time the immunogenicity and protection outcome in hamsters intradermally primed with salivary protein LJM19 and boosted with genetically modified live attenuated L. donovani parasites (LdCen-/-) in combination with recombinant LJM19. Immunized hamsters demonstrated a strong immune response comparable to that of intracardial immunization with LdCen-/- and resulted in long term protection against infection with virulent L. donovani parasites.
Materials and Methods
Animals
Two-month-old female Syrian golden hamsters (Mesocricetus auratus) were obtained from the Harlan Laboratories and kept in the Food and Drug Administration (FDA) animal facility. The experimental procedures used in this study were reviewed and approved by the Animal Care and Use Committees of the FDA and the National Institute of Allergy and Infectious Diseases (NIAID).
Ethics statement
The animal protocol for this study has been approved by the Institutional Animal Care and Use Committee at the Center for Biologics Evaluation and Research, US FDA (ASP 1995#26). Further, the animal protocol is in full accordance with ‘The guide for the care and use of animals’ as described in the US Public Health Service policy on Humane Care and Use of Laboratory Animals 2015 (http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf).
Sand flies and preparation of SGH
Lu. longipalpis sand flies, Jacobina strain were reared at the Laboratory of Malaria and Vector Research, NIAID. Salivary glands were dissected from 5- to 7-day-old females and stored in PBS at -70°C. Before use, salivary glands were sonicated and centrifuged at 12,000×g for 2 min. The supernatant was collected and used immediately.
Intradermal immunization (LdCen-/- ± LJM19) and challenge (parasites and SGH)
LdCen-/- and L. donovani (Ld1S) promastigotes were grown at 26°C in medium 199 supplemented with 20% FCS. Three- to 4-month-old hamsters were immunized intradermally in the ear at two-week intervals between immunizations, using a 29-gauge needle (BD Ultra-Fine) in a volume of 20μl, using the following protocols. Group 1: prime with 2μg of LJM19 protein and boost with 107 stationary phase LdCen-/-promastigotes plus 2μg of LJM19. Group 2: 107 stationary phase LdCen-/- promastigotes via intracardial injection. Group 3: 2μg of LJM19 protein (two times). Group 4: BSA (control) (S1 Fig). Each experimental group consisted of 6 hamsters. Five weeks after the last immunization, the animals were challenged ID with 105 stationary phase Ld1S promastigotes in combination with 0.5 pairs SGH.
Determination of parasite burden
At 5 weeks post immunization, and 1 and 9 months post challenge, the parasite load was measured in the ear, lymph node (from challenged ear), spleen and liver by the limiting dilution assay as previously described [33].
Antibody detection
Whole blood from immunized hamsters was collected at the indicated time points before sacrifice in 1.1mL microcentrifuge tubes with serum gel-clotting activator (Sartesdt, GE). The serum was separated by centrifugation and used in IgG assays. Total IgG, IgG1 and IgG2 responses to L. donovani soluble antigens were measured by ELISA as described [34]. The following clones were used in the study. IgG cocktail (Catalog# 554010); IgG1 (clone-G94-56); IgG2 (clone-G192-3) (BD Biosciences).The cut-off value of reactivity for SLA antigens was calculated as the mean plus 2 SD of the OD values observed in naive controls. Sera from 9 naïve hamsters were used for determining cut-off values.
Cytokine determination by real-time PCR
Splenocytes were collected from hamsters, macerated, lysed in Trizol for RNA extraction. Total RNA was extracted from the ear, lymph node (superficial parotid lymph node), spleen and liver of infected hamsters using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed with ≈1–2μg of RNA using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche). Amplification conditions consisted of an initial pre-incubation at 95°C for 10 min, followed by amplification of the target DNA for 40 cycles of 95°C for 15 s and 60°C for 1 min with the LightCycler 480 (Bio-Rad). The efficiency of each reaction was determined. The expression levels of genes of interest were normalized to β-Actin levels. The results are expressed in fold change of 2-ΔCt over control.
Oligonucleotide primers and probes
Oligonucleotide primers used for real time PCR were: β Actin, reverse, ACA GAG AGA AGA TGA CGC AGA TAA TG, forward, GCC TGA ATG GCC ACG TAC A; IFN-γ, reverse, TGT TGC TCT GCC TCA CTC AGG, forward, AAG ACG AGG TCC CCT CCA TTC; TNF-α, reverse, TGA GCC ATC GTG CCA ATG, forward AGC CCG TCT GCT GGT ATC AC; IL-10, reverse, GGT TGC CAA ACC TTA TCA GAA ATG, forward, TTC ACC TGT TCC ACA GCC TTG; IL-4, reverse, ACA GAA AAA GGG ACA CCA TGC A, forward, GAA GCC CTG CAG ATG AGG TCT; IL-12/IL-23p40, reverse, AAT GCG AGG CAG CAA ATT ACT C forward, CTG CTC TTG ACG TTG AAC TTC AAG, iNOS, reverse, ACC ACA CAG CCT CCG AGT CC, forward, CTG CCA GAT GTG GGT CTT CC. The primers and probes were synthesized at the Center for Biologics and Evaluation and Research, FDA Core facility.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software Inc., USA). Non-parametric Kruskal-Wallis test followed by Dunns test were used to compare data from four groups (G1, G2, G3 and G4). Differences were considered significant when a p value ≤ 0.05 was obtained.
Results
Persistence of LdCen-/- in intradermally immunized animals
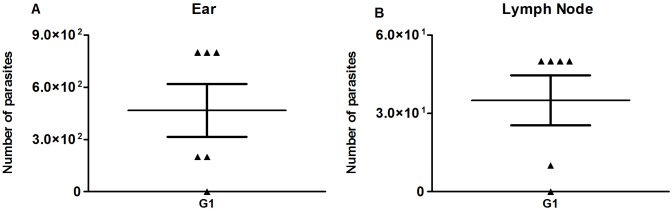
Since the efficacy of genetically modified Leishmania parasites in general, and LdCen-/- parasites in particular have not been tested through an intradermal route, we sought to determine the persistence of LdCen-/- parasites at the site of injection and its dissemination to other organs where relevant immune reactions might occur. Thus, we measured the parasite load in the ear, lymph node, spleen and liver 5 weeks post immunization (5wpi) in hamsters primed with LJM19 and boosted with LJM19 plus LdCen-/- (G1). We observed an average of 300 and 30 viable LdCen-/- parasites in the ear and draining lymph node, respectively, measured by limiting dilution (Fig 1A and 1B). However, we could not recover any LdCen-/- parasite from the spleen and liver from either G1 or from animals immunized with LdCen-/- intracardially.
Fig 1. Parasite burden 5 weeks after immunization.
Detection of parasites by limiting dilution assays and expressed as number of parasites per organ after 5 weeks of immunization with LdCen-/- in the ear (A) and lymph node (B) of group 1 (G1).
Prime/boost intradermal immunization with LJM19 and LdCen-/- plus LJM19 induced a strong anti-Leishmania antibody response
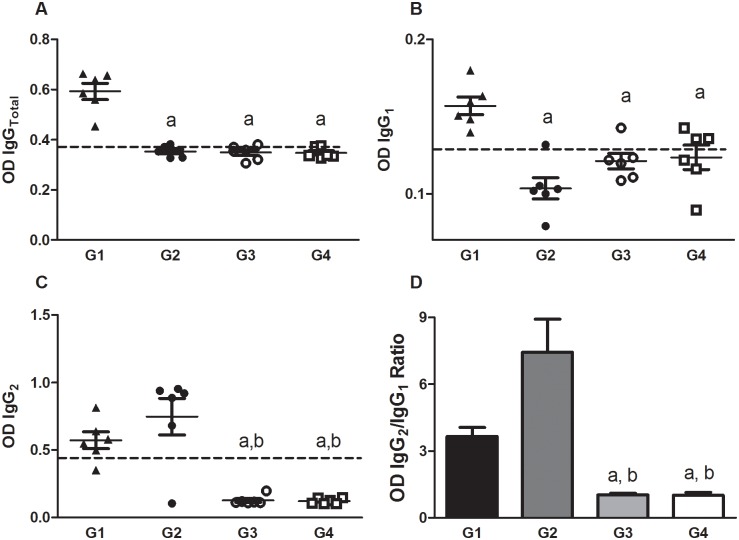
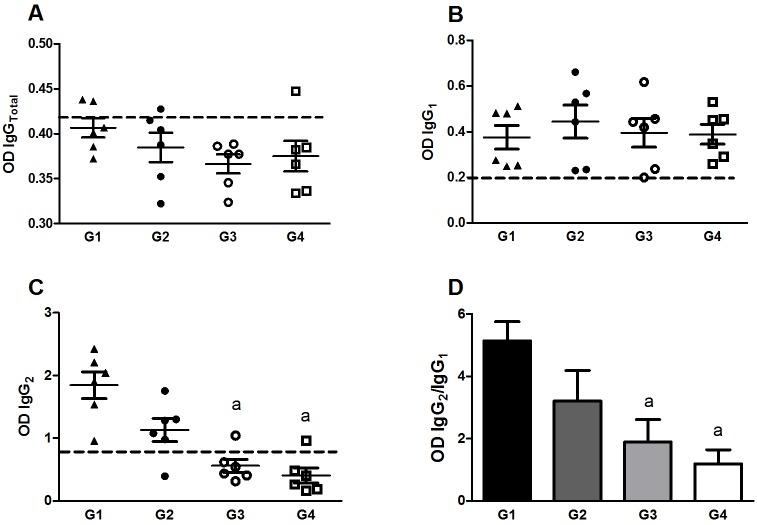
The analysis of sera from hamsters primed with LJM19 and boosted with LdCen-/- plus LJM19 indicated the occurrence of strong Leishmania-specific antibody responses. The SLA-specific IgGTotal and IgG1 production was significantly higher for immunized animals in G1 when compared to animals immunized IC with LdCen-/- (IC, G2), at 5wpi (p<0.05) (Fig 2A and 2B). However, increased levels of IgG2 were detected in G1 and G2 groups compared to G3 and control group G4 (p<0.01 and p<0.001, respectively; Fig 2C) that show base line reactivity. Importantly, the IgG2/IgG1 ratio was significantly higher in G1 and G2 groups compared to G4, the control group (p<0.05) and to G3 (p<0.05) that received LJM19 alone (Fig 2D). Of note, the IgG2/IgG1 ratio was higher in G2 compared to G1 (p<0.05) (Fig 2D).
Fig 2. Specific antibody production 5 weeks after immunization.
ELISAs using plates coated with SLA from L. donovani were performed to detect production of total IgG (A), IgG1 (B), IgG2 (C) and the ratio IgG2/IgG1 (D). The OD values representing antibody concentration are shown on the y-axis, and the error bars indicate the standard deviation. Dotted lines represent the cut-off value. Statistical differences (p<0.05) are indicated in letters (a: G1 and b: G2).
Local expression of cytokines in the ear of immunized hamsters
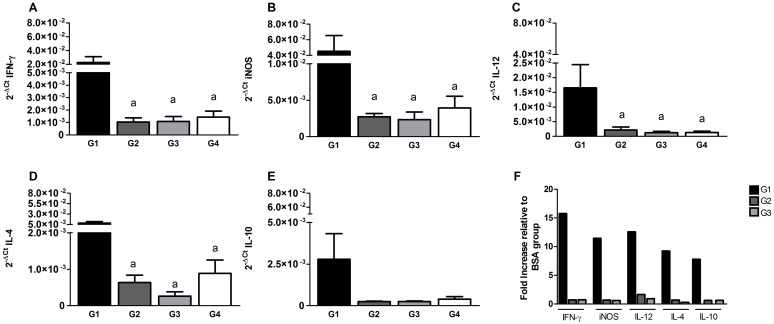
In order to evaluate the protective immunity induced after ID immunization, we analyzed the mRNA expression of both Th1 and Th2 cytokines (IFN-γ, iNOS, IL-12/IL-23p40, IL-4 and IL-10) in the ear, the site of injection at 5wpi. The IFN-γ, iNOS and IL-12 expression levels were significantly higher in immunized hamsters in G1 compared with G2, G3 and G4 groups (p<0.05; Fig 3A,3B and 3C, respectively). Expression of IL-4 and IL-10 cytokines was also higher in G1 animals after immunization, when compared to animals in groups G2, G3 and G4 (p<0.05; Fig 3D and 3E, respectively). However, the level of IFN-γ was higher than either IL-10 or IL-4 in G1 animals. Additionally, G1 animals showed up to a ~16-fold, ~12-fold and ~13-fold increase in IFN-γ, iNOS and IL-12 respectively, compared to BSA-immunized control group G4 (Fig 3F). Similarly, mRNA levels of IL-4 and IL-10 were up-regulated ~9- and 8-folds, respectively, in G1 compared to G4 animals.
Fig 3. Cytokine responses 5 weeks after immunization with LdCen-/- parasites and LJM19.
Immune response in the ear tissue of immunized hamsters was measured. The expression of mRNA encoding IFN-γ (A), iNOS (B), IL-12 (C), IL-4 (D) and IL-10 (E) was evaluated by qPCR. The data were normalized to β-Actin expression. (F) Fold increase of the cytokines compared to BSA (G4) group. Statistical differences (p<0.05) are indicated in letters (a: G1).
Intradermal immunization with LdCen-/- parasites results in control of the parasite load of virulent L. donovani early in infection
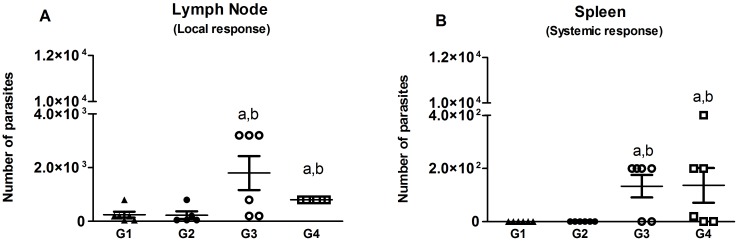
The number of viable L. donovani parasites was determined by the limiting dilution assay in the draining lymph node and spleen of immunized hamsters a month post challenge (mpc) (Fig 4). The number of live parasites in the lymph node (Fig 4A) and in the spleen (Fig 4B) were significantly lower (p<0.05) in the G1 and G2 groups either immunized with LJM19 then boosted with LdCen-/- plus LJM19 or immunized with LdCen-/- alone respectively, when compared with the groups of hamsters that received LJM19 alone or BSA alone.
Fig 4. Parasite burden 1 month after challenge.
Hamsters were challenged in the left ear with virulent L. donovani and presence of parasites was detected 1 month after challenge. The draining lymph node (A) and spleen (B) were used in limiting dilution assay and data expressed as number of parasites/organ. Statistical differences (p<0.05) are indicated in letters (a: G1 and b: G2).
Leishmania-specific IgG isotypes in immunized hamsters post challenge
We measured antibody levels in the sera of immunized animals one mpc. No difference was observed in the levels of IgGTotal and IgG1 between the groups (Fig 5A and 5B). The level of IgG2 was elevated in G1 immunized animals when compared to G3 and G4 hamsters (p<0.05 and p<0.01, respectively; Fig 5C). In addition, G1 group presented a significantly higher IgG2/IgG1 ratio in comparison to G3 and G4 control groups (p<0.05 and p<0.01, respectively) (Fig 5D), indicative of a Th1-type immune response post challenge.
Fig 5. Specific antibody production 1 month after challenge.
ELISAs using plates coated with SLA from L. donovani were performed to detect production of total IgG (A), IgG1 (B), IgG2 (C) and the ratio IgG2/IgG1 (D). The antibody OD values are shown on the y-axis, and the error bars indicate the standard deviation. Dotted lines represent the cut-off value. Statistical differences (p<0.05) are indicated in letters (a: G1).
The early immune response in lymph nodes, spleen and liver of immunized animals post challenge
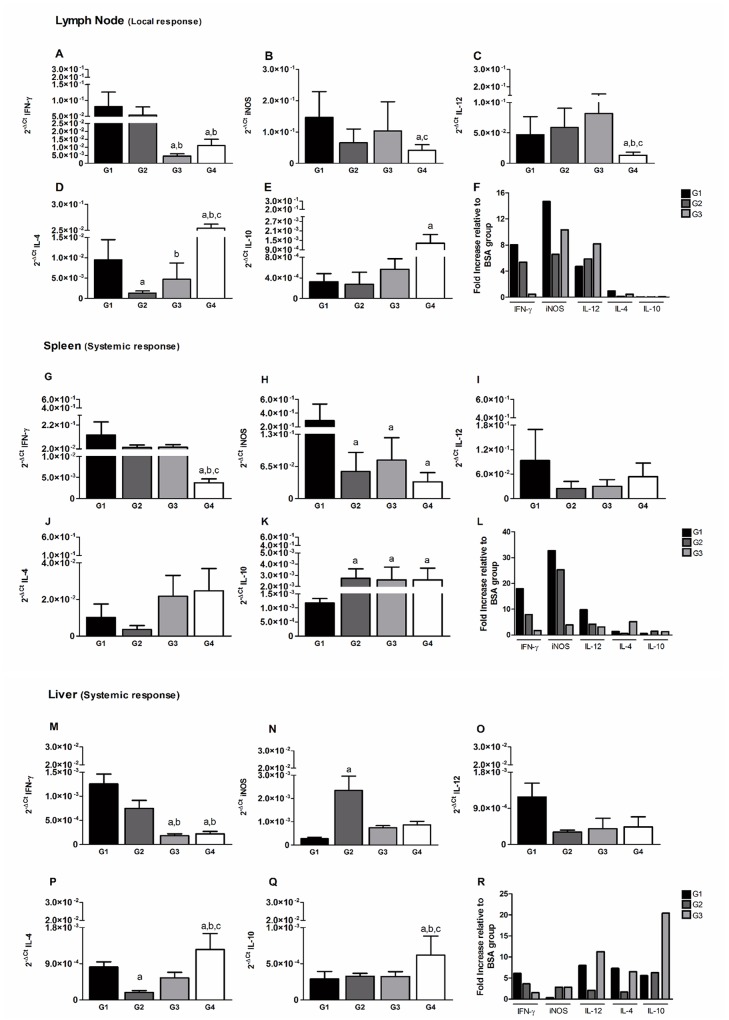
The mRNA expression level of Th1 and Th2 cytokines was estimated by qRT-PCR one mpc. In the lymph node (LN), G1 and G2 groups presented a high expression of IFN-γ, when compared to G3 and G4 groups (p<0.01) (Fig 6A). A moderate increase in the expression levels of iNOS mRNA transcripts in LN was observed only in G1, and G3 immunized hamsters compared to G4 group (p<0.05) (Fig 6B). Interestingly, cytokine IL-12 mRNA levels in LN were significantly higher in G1, G2 and G3 groups compared to the G4 control group (p<0.001) (Fig 6C). Concomitantly, the mRNA levels of the Th2 cytokines IL-4 and IL-10, primarily regulatory cytokine, were higher in LN of G4 control animals in comparison to G1, G2 and G3 immunized groups (p<0.05) (Fig 6D and 6E). Additionally, IFN-γ, iNOS and IL-12 from G1 were significantly up-regulated by ~8 folds, ~14 folds and ~5 folds, respectively, compared to G4 group (Fig 6F).
Fig 6. Cytokines response 1 month after challenge.
Immune response in the lymph nodes of hamsters immunized with LdCen-/- and LJM19 by qPCR (A to E). The mRNA expression of IFN-γ (A), iNOS (B), IL-12(C), IL-4 (D), and IL-10 (E) was evaluated. The data were normalized to β-actin expression. Fold increase of the cytokines compared to BSA (G4) group (F). Immune response in the spleens of hamsters immunized with LdCen-/- and LJM19 by qPCR (G to K). The mRNA expression of IFN-γ (G), iNOS (H), IL-12(I), IL-4 (J), and IL-10 (K) was evaluated. The data were normalized to β-actin expression. Fold increase of the cytokines compared to BSA (G4) group (L). Immune response in the livers of hamsters immunized with LdCen-/- and LJM19 by qPCR (M to Q). The mRNA expression of IFN-γ (M), iNOS (N), IL-12(O), IL-4 (P), and IL-10 (Q) was evaluated. The data were normalized to β-actin expression. Fold increase of the cytokines compared to BSA (G4) group (R). Statistical differences (p<0.05) are indicated in letters (a: G1, b: G2 and c: G3).
In the spleen, IFN-γ was up-regulated one mpc in groups G1 (p<0.001), G2 (p<0.01) and G3 (p<0.01), compared to control group (G4) (Fig 6G) In addition, G1 presented a higher expression of iNOS when compared to G2, G3 and G4 after challenge (p<0.001, p<0.005 and p<0.01, respectively) (Fig 6H). Interestingly, IL-12 mRNA levels were not significantly different in spleens from animals of the 4 groups though G1 animals exhibited a trend for increased IL-12 expression (Fig 6I). The IL-4 expression was decreased in G1 and G2 groups, but not significantly (Fig 6J) compared to G3 and G4. However, there was a significant reduction in IL-10 expression (p<0.05) in G1 when compared to G2, G3 and G4 groups (Fig 6K). The IFN-γ, iNOS and IL-12 mRNAs from G1 were significantly up-regulated by ~19 folds, ~32 folds and ~10 folds, respectively, compared to G4 group (Fig 6L).
In the liver, IFN-γ expression was upregulated one mpc in G1 and G2 groups in comparison with G3 and G4 animals (p<0.01) (Fig 6M). However, iNOS expression was significantly higher in G2 compared to G1, G3 and G4 (Fig 6N). Liver cells from group G1 immunized hamsters induced a significantly high expression of IL-12 (Fig 6O). IL-4 expression was significantly higher in G4 animals compared to G1, G2 and G3 hamsters (p<0.01), and lower in G2 group compared to G1 group, but was not significantly different compared to G3 group (Fig 6P). On the other hand, hamsters from control group G4 showed a significant up-regulation (p<0.001) in IL-10 expression compared to G1, G2 and G3 (Fig 6Q). The IFN-γ, iNOS and IL-12 mRNAs from G1 were moderately up-regulated by ~1.3 folds, ~0.39 folds and ~1.2 folds, respectively, compared to control group G4 (Fig 6R).
Prime/boost immunization with LJM19 and LdCen-/- plus LJM19 offered long term protection against a virulent L. donovani challenge in hamsters
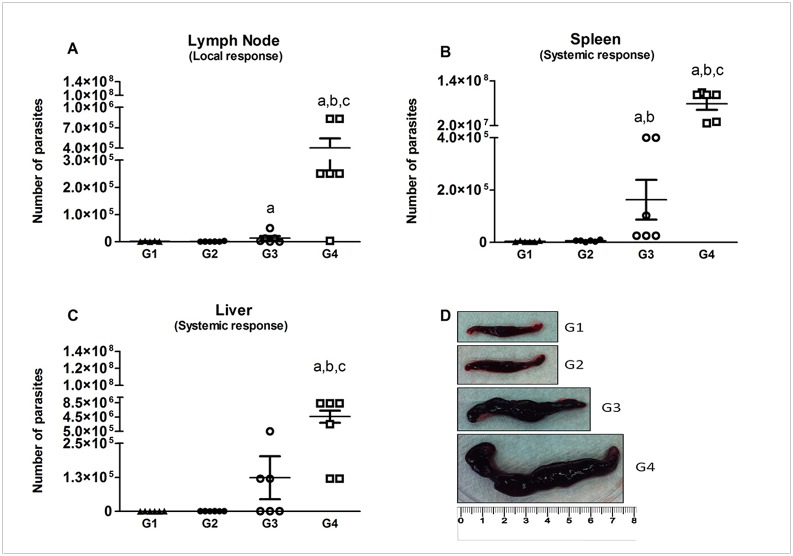
The animals in G1 and G2 groups showed robust protection 9 mpc as evident by a significant decrease in the lymph node parasite load (Fig 7A), spleen (Fig 7B) and liver (Fig 7C) in comparison to G4 animals (p<0.0001). Immunization with salivary gland protein LJM19 alone (G3) also provided significant protection (p<0.01), albeit weaker than that observed in animals in groups G1 and G2, compared to G4 BSA immunized animals.
Fig 7. Parasite burden 9 months after challenge.
Hamsters were challenged in the left ear with virulent L. donovani and presence of parasites was detected 9 months after challenge. The draining lymph node (A), spleen (B) and liver (C) were used in limiting dilution assay and data expressed as number of parasites/organ. Statistical differences (p<0.05) are indicated in letters (a: G1, b: G2 and c: G3). (D) Comparison of spleen size 9 months after challenge. The median of spleen size of hamsters of G1, G2, G3 and G4 was measured in centimeter. A representative sample from 6 immunized and challenged hamsters is shown.
Further, we wanted to test whether LdCen-/- immunization causes re-establishment of homeostatic conditions by comparing the spleen sizes. Both G1 and G2 presented a non-inflamed spleen (median 3.4 and 3.5cm, respectively), as compared to a highly inflamed spleen in the control group G4 (median 7.9cm) (Fig 7D). G3 animals showed an intermediate spleen size (median 5.2 cm).
Discussion
Previous work from our laboratories has shown that the LdCen-/- live attenuated vaccine is immunogenic in mice, hamsters and dogs [12,13,18]. Similarly, LJM19 protein from saliva of the vector Lu. longipalpis protected hamsters against challenge with L. infantum and L. braziliensis [26,28]. In the present work, we examined the value of combining the two immunization strategies for their potential to elicit protective immune responses in a hamster model of Leishmania donovani infection. ID needle inoculation of the ear has been extensively employed as the route of infection that most closely replicates the physiological ID and intra-epidermal deposition of parasites by the bite of an infected sand fly [33,35–37]. Additionally, the ID route presents the most practical route for vaccine delivery [26,28,29,31,32]. We hypothesized that a prime/boost strategy with LJM19 followed by LdCen-/- parasites plus LJM19, all delivered intradermally, would induce long-lasting protective immunity against L. donovani particularly since LdCen-/- parasites can undergo limited replication in the immunized host and provide an array of antigens very similar to those produced by a virulent parasite. As such, in this prime/boost protocol, priming with LJM19 would generate a specific adaptive immune response to the sand fly salivary gland protein as was observed in previous studies [26,28] that could result in a potent supplement to the specific adaptive immune response to antigens of the LdCen-/- parasites.
We had previously observed LdCen-/- parasites in the spleen up to 5 wpi after intracardial (IC) injection [12]. In the current study, we observed parasites in the immunized ear and the draining lymph node but not in the spleen, at 5wpi after ID injection, suggesting that either the parasites take a longer time to disseminate to the viscera and reach the spleen or alternatively they do not visceralize. Of interest, recent studies with dermotropic parasite strains (L. donovani isolated from a cutaneous lesion and L. major) that fail to persistently visceralize nevertheless produced protective immunity in a low-dose infection followed by challenge with L. donovani and L. infantum in mouse models [38,39]. This suggests that visceralization may not be a necessary pre-condition for protective immunity against VL to develop in the immunized mice. Consistent with this hypothesis, our results indicate that since the draining lymph nodes represent the immunological niche where relevant reactions between APCs that acquired the antigens and naïve T cells could occur, recovery of attenuated parasites from the lymph nodes 5 weeks post immunization suggested that parasite persistence, i.e. antigen availability, was adequate for protective immunity to be established.
Immunization of hamsters with attenuated parasites associated with LJM19 protein elicited a biased Th1-type immune response at 5wpi at the site of injection. As expected, immunization by LJM19 alone provided protection against L. donovani parasites, however, it was considerably weaker compared to the one observed following a prime/boost ID immunization with LJM19 followed by LJM19 and LdCen-/- or an IC immunization with LdCen-/-. Of note, boosting with LdCen-/- along with LJM19 protein through the ID route resulted in a higher pro-inflammatory response compared to immunization with LdCen-/-alone through the IC route. Indeed, immunization with LdCen-/- through the intravenous (IV) route in mice and IC route in hamsters [12] and subcutaneous route in dogs [13] has been shown to promote a pro-inflammatory response, with the presence of IL-12p40, IFN-γ, iNOS and TNF-α. Additionally, our finding that increased levels of IL-4, IFN-α, iNOS and IL-12/IL-23p40 in immunized hamsters would suggest a mixed immune response (Th1 biased) triggered by LdCen-/- vaccination, as we observed in dogs immunized previously [18,38,40]. Our results suggest that the ID mode of immunization, at least in combination with LJM19, is equally efficacious compared to the IC mode. Additionally, the high ratio of IgG2/IgG1 observed in groups G1 and G2 is considered an additional immune biomarker of protection [5,41–43].
During natural transmission, an infected sand fly deposits saliva and parasites into the skin of the host while feeding. To mimic the natural mode of infection with Leishmania, we injected L. donovani wild type parasites into the ear of hamsters along with sand fly salivary gland extract. After one month of infection, hamsters immunized with LdCen-/- either alone (IC) or with LJM19 protein (ID) demonstrated a reduced parasite burden in the lymph node and spleen. In our study, similar to the response observed post-immunization, challenged hamsters presented a significant increase of IgG2 production, and a high ratio of IgG2/IgG1, as well as an enhanced production of IFN-γ and iNOS. Higher levels of IgG2 might also contribute to pathogen clearance in vaccinated animals [44,45].
Previously, it was reported that iNOS and concomitant high levels of NO were produced by macrophages in protected mice vaccinated with attenuated parasites after challenge with L. donovani [12,14]. In the present study hamsters immunized with either LdCen-/- alone or in association with recombinant LJM19 displayed increased IFN-γ and iNOS expression in lymph nodes and spleen one month after challenge with wild type parasites. Of importance, five weeks post-immunization we observed a higher production of IL-12 only in animals immunized with LdCen-/- in association with recombinant LJM19. It can be speculated that LJM19 might be pre-conditioning the innate immune arm and thus allowing the antigen presenting cells such as DCs to produce IL-12 that is necessary for initiating a strong adaptive Th1 cell immunity. Certainly, the ability of LJM19 to produce a Th1 response in hamsters has been previously demonstrated [26]. As such, it may be argued that LJM19 might be enhancing the immunogenicity of LdCen-/- as a vaccine. An increased IFN-γ/IL-10 ratio has been observed when DNA vectors expressing KMP11 along with LJM19 were used as immunogens compared to either KMP11 or LJM19 alone at 5 months post challenge [46]. This increased IFN-γ/IL-10 ratio did not result in reduced splenic parasite burden between KMP11+LJM19 and either antigen or LJM19 alone groups at 5 months post challenge. Further in their study the authors also observed increased IFN-γ/IL-10 ratio after 5 months of infection in non-immunized animals which does not explain the role of increased IFN-γ levels in protection. The observed differences between da Silva [46] and our study in splenic parasite burden could be due to the ability of the live attenuated parasites to induce sustained immunological reactions (Fig 6G and 6K) because of a longer availability of a multitude of antigens compared to recombinant antigens that tend to have limited availability and diversity that is reflected in parasite control up to 9 months post challenge. Further, in the LdCen-/- immunized animals (G1 and G2) down-regulation of IL-10 and a concomitant increase in IL-12 in lymph node, spleen and liver may explain the greater parasite killing observed at the challenge site. In murine and human VL, production of Th1 cytokines is desirable for resolution of infection [47–50]. In addition, IL-12 results in the generation of Th1 cells that produce both IFN-γ and IL-12, thus favoring the development of a protective cellular immune response against Leishmania [51–53]. An important consideration is that the sustained Th1-type immune response and long-term protection generated here against L. donovani after ID injection of LdCen-/- parasites in combination with LJM19 is comparable to that observed following IC or intravenous immunization with LdCen-/- parasites alone [12,14]. A similar induction of Th1 immunity was also observed in dogs immunized subcutaneously with LdCen-/- parasites and challenged with L. infantum [18]. At 9 months post challenge, the parasite load in the lymph nodes and in the spleen was significantly reduced in all the immunized groups compared to the control group. The control of parasitemia in the spleen translated into lack of splenomegaly in immunized and challenged animals compared to control challenged animals. Importantly, the significant reduction of parasitemia after immunization with LJM19 alone in our current study argues that LJM19 contributes to the observed protection in G1 hamsters. This is corroborated by Gomes at al. [26] who observed a decrease in parasite load in the spleen and liver in hamsters immunized with LJM19 after 2 and 5 months post I.D. inoculation of L. infantum chagasi with sand fly salivary gland homogenate. Additionally, in an independent study, Tavares et.al [28] showed that hamsters immunized with LJM19 induced protection against infection with L. braziliensis. Taken together our data indicate the induction of a long-lasting protective immune response in the spleen, liver and lymph nodes in hamsters immunized intradermally with LJM19 and LdCen-/- after challenge with virulent parasites and reveal that a stronger immune response is elicited when Leishmania donovani live attenuated parasites are combined with a salivary gland protein. In summary, we have demonstrated the capability of a combined vaccine composed of live attenuated LdCen-/- parasite and a defined salivary gland protein from Lu. Longipalpis (LJM19) delivered intradermally to confer strong long-lasting protection against L. donovani infection in a hamster model.
Supporting Information
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported in part by the Intramural Research Program at the National Institute of Allergy and Infectious Diseases, NIH (JGV SK FO MA) and Center for Biologics Evaluation and Research, FDA (JAF RD SG HLN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–18. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One [Internet]. 2012;7(5):e35671 Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0035671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft SL, Coombs GH. Leishmaniasis—Current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19(11):502–8. [DOI] [PubMed] [Google Scholar]
- 4.Minodier P, Jurquet AL, Noel G, Uters M, Laporte R, Garnier JM. [Leishmaniasis treatment]. Arch Pediatr. 2010;17(6):838–9. 10.1016/S0929-693X(10)70137-8 [DOI] [PubMed] [Google Scholar]
- 5.Costa CHN. How effective is dog culling in controlling zoonotic visceral leishmaniasis? A critical evaluation of the science, politics and ethics behind this public health policy. Rev Soc Bras Med Trop [Internet]. 2011;44(2):232–42. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Okwor I, Uzonna J. Vaccines and vaccination strategies against human cutaneous leishmaniasis. Hum Vaccin. 2009;5(May):291–301. [DOI] [PubMed] [Google Scholar]
- 7.Buxbaum LU, Denise H, Coombs GH, Alexander J, Mottram JC, Scott P. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J Immunol. 2003;171(7):3711–7. [DOI] [PubMed] [Google Scholar]
- 8.Späth GF, Lye L-F, Segawa H, Sacks DL, Turco SJ, Beverley SM. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science. 2003;301(2003):1241–3. [DOI] [PubMed] [Google Scholar]
- 9.Stewart J, Curtis J, Spurck TP, Ilg T, Garami A, Baldwin T, et al. Characterisation of a Leishmania mexicana knockout lacking guanosine diphosphate-mannose pyrophosphorylase. Int J Parasitol [Internet]. 2005;35(8):861–73. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Silvestre R, Cordeiro-Da-Silva A, Santarém N, Vergnes B, Sereno D, Ouaissi A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. J Immunol [Internet]. 2007;179(5):3161–70. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Cabral SM, Silvestre RL, Santarém NM, Tavares JC, Silva AF, Cordeiro-da-Silva A. A Leishmania infantum cytosolic tryparedoxin activates B cells to secrete interleukin-10 and specific immunoglobulin. Immunology [Internet]. 2008;123(4):555–65. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2433312&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvapandiyan A, Dey R, Duncan R, Nylén S, Nakhasi HL, Sacks DL, et al. Intracellular Replication-Deficient Leishmania donovani Induces Long Lasting Protective Immunity against Visceral Leishmaniasis. J Immunol [Internet]. 2009;183(3):1813–20. Available from: 10.4049/jimmunol.0900276 [DOI] [PubMed] [Google Scholar]
- 13.Fiuza JA, Santiago HDC, Selvapandiyan A, Gannavaram S, Ricci ND, Bueno LL, et al. Induction of immunogenicity by live attenuated Leishmania donovani centrin deleted parasites in dogs. Vaccine [Internet]. 2013;31(14):1785–92. Available from: 10.1016/j.vaccine.2013.01.048 [DOI] [PubMed] [Google Scholar]
- 14.Dey R, Natarajan G, Bhattacharya P, Cummings H, Dagur PK, Terrazas C, et al. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J Immunol [Internet]. 2014;193(7):3513–27. Available from: 10.4049/jimmunol.1303145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titus RG, Gueiros-Filho FJ, de Freitas L a, Beverley SM. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci U S A. 1995;92(October 1995):10267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulou B, Roy G, Breton M, Kündig C, Dumas C, Fillion I, et al. Reduced infectivity of a Leishmania donovani biopterin transporter genetic mutant and its use as an attenuated strain for vaccination. Infect Immun [Internet]. 2002;70(1):62–8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=127620&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saravia NG, Escorcia B, Osorio Y, Valderrama L, Brooks D, Arteaga L, et al. Pathogenicity and protective immunogenicity of cysteine proteinase-deficient mutants of Leishmania mexicana in non-murine models. Vaccine [Internet]. 2006;24(19):4247–59. Available from: [DOI] [PubMed] [Google Scholar]
- 18.Fiuza JA, Gannavaram S, Santiago HDC, Selvapandiyan A, Souza DM, Passos LSA, et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine [Internet]. 2015;33(2):280–8. Available from: 10.1016/j.vaccine.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 19.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med [Internet]. 2001;194(3):331–42. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2193460&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade BB, De Oliveira CI, Brodskyn CI, Barral a., Barral-Netto M. Role of sand fly saliva in human and experimental leishmaniasis: Current insights. Scand J Immunol. 2007;66(2–3):122–7. [DOI] [PubMed] [Google Scholar]
- 21.Chagas AC, Oliveira F, Debrabant A, Valenzuela JG, Ribeiro JMC, Calvo E. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog [Internet]. 2014;10(2):e1003923 Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=24516388&retmode=ref&cmd=prlinks\npapers2://publication/doi/10.1371/journal.ppat.1003923 10.1371/journal.ppat.1003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239(4845):1306–8. [DOI] [PubMed] [Google Scholar]
- 23.Kamhawi S. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2000;2:1765–73. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira F, Lawyer PG, Kamhawi S, Valenzuela JG. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLoS Negl Trop Dis [Internet]. 2008;2(4):e226 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2291569&tool=pmcentrez&rendertype=abstract 10.1371/journal.pntd.0000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collin N, Gomes R, Teixeira C, Cheng L, Laughinghouse A, Ward JM, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog [Internet]. 2009;5(5):e1000441 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2677456&tool=pmcentrez&rendertype=abstract 10.1371/journal.ppat.1000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci U S A. 2008;105(22):7845–50. 10.1073/pnas.0712153105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes R, Oliveira F, Teixeira C, Meneses C, Gilmore DC, Elnaiem D-E, et al. Immunity to sand fly salivary protein LJM11 modulates host response to vector-transmitted leishmania conferring ulcer-free protection. J Invest Dermatol [Internet]. 2012;132(12):2735–43. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3461249&tool=pmcentrez&rendertype=abstract 10.1038/jid.2012.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavares NM, Silva R a, Costa DJ, Pitombo M a, Fukutani KF, Miranda JC, et al. Lutzomyia longipalpis saliva or salivary protein LJM19 protects against Leishmania braziliensis and the saliva of its vector, Lutzomyia intermedia. PLoS Negl Trop Dis [Internet]. 2011;5(5):e1169 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3104964&tool=pmcentrez&rendertype=abstract 10.1371/journal.pntd.0001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roatt BM, Aguiar-Soares RDDO, Vitoriano-Souza J, Coura-Vital W, Braga SL, Corrêa-Oliveira R, et al. Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: immunogenicity and parasitological evaluation. PLoS One [Internet]. 2012;7(11):e49780 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3506642&tool=pmcentrez&rendertype=abstract 10.1371/journal.pone.0049780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahedifard F, Gholami E, Taheri T, Taslimi Y, Doustdari F, Seyed N, et al. Enhanced Protective Efficacy of Nonpathogenic Recombinant Leishmania tarentolae Expressing Cysteine Proteinases Combined with a Sand Fly Salivary Antigen. PLoS Negl Trop Dis. 2014;8(3):e2751 10.1371/journal.pntd.0002751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MOHEBALI M. Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr district, I.R. Iran. Vaccine [Internet]. 2004;22(29–30):4097–100. Available from: http://www.sciencedirect.com/science/article/pii/S0264410X04003275 [DOI] [PubMed] [Google Scholar]
- 32.Mutiso JM, Macharia JC, Gicheru MM. Immunization with Leishmania vaccine-alum-BCG and montanide ISA 720 adjuvants induces low-grade type 2 cytokines and high levels of IgG2 subclass antibodies in the vervet monkey (Chlorocebus aethiops) model. Scand J Immunol. 2012;76(5):471–7. 10.1111/j.1365-3083.2012.02764.x [DOI] [PubMed] [Google Scholar]
- 33.Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, et al. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol [Internet]. 2002;168(8):3992–4000. Available from: [DOI] [PubMed] [Google Scholar]
- 34.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. J Infect Dis [Internet]. 2002;186(10):1530–4. Available from: [DOI] [PubMed] [Google Scholar]
- 35.Méndez S, Gurunathan S, Kamhawi S, Belkaid Y, Moga M a, Skeiky Y a, et al. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J Immunol [Internet]. 2001;166(8):5122–8. Available from: [DOI] [PubMed] [Google Scholar]
- 36.Peters a M, Fowler JC, Britton TB, Solanki CK, Ballinger JR, Ravichandran D, et al. Functional variation in lymph node arrangements within the axilla. Lymphat Res Biol [Internet]. 2009;7(3):139–44. Available from: 10.1089/lrb.2008.1021 [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro-Gomes FL, Roma EH, Carneiro MBH, Doria N a., Sacks DL, Peters NC. Site-dependent recruitment of inflammatory cells determines the effective dose of Leishmania major. Infect Immun. 2014;82(7):2713–27. 10.1128/IAI.01600-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCall L-I, Zhang W-W, Matlashewski G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog [Internet]. 2013;9(1):e1003053 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3536654&tool=pmcentrez&rendertype=abstract 10.1371/journal.ppat.1003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romano A, Doria NA, Mendez J, Sacks DL PN. No Title. J Immunol. 2015;195(8):3816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh A, Zhang WW, Matlashewski G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine. 2001;20(1–2):59–66. [DOI] [PubMed] [Google Scholar]
- 41.Garg R, Gupta SK, Tripathi P, Hajela K, Sundar S, Naik S, et al. Leishmania donovani: identification of stimulatory soluble antigenic proteins using cured human and hamster lymphocytes for their prophylactic potential against visceral leishmaniasis. Vaccine [Internet]. 2006;24(15):2900–9. Available from: [DOI] [PubMed] [Google Scholar]
- 42.Chávez-Fumagalli M a., Costa M a F, Oliveira DM, Ramírez L, Costa LE, Duarte MC, et al. Vaccination with the Leishmania infantum ribosomal proteins induces protection in BALB/c mice against Leishmania chagasi and Leishmania amazonensis challenge. Microbes Infect. 2010;12(12–13):967–77. 10.1016/j.micinf.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 43.Maroof A, Brown N, Smith B, Hodgkinson MR, Maxwell A, Losch FO, et al. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J Infect Dis [Internet]. 2012;205(5):853–63. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3274377&tool=pmcentrez&rendertype=abstract 10.1093/infdis/jir842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int Immunol. 2003;15(8):937–44. [DOI] [PubMed] [Google Scholar]
- 45.Guha R, Gupta D, Rastogi R, Vikram R, Krishnamurthy G, Bimal S, et al. Vaccination with leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci Transl Med [Internet]. 2013;5(202):202ra121 Available from: 10.1126/scitranslmed.3006406 [DOI] [PubMed] [Google Scholar]
- 46.Da Silva R a a, Tavares NM, Costa D, Pitombo M, Barbosa L, Fukutani K, et al. DNA vaccination with KMP11 and Lutzomyia longipalpis salivary protein protects hamsters against visceral leishmaniasis. Acta Trop [Internet]. 2011;120(3):185–90. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3747832&tool=pmcentrez&rendertype=abstract 10.1016/j.actatropica.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghalib HW, Whittle JA, Kubin M, Hashim FA, el-Hassan AM, Grabstein KH, et al. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J Immunol. 1995;154:4623–9. [PubMed] [Google Scholar]
- 48.Kenney RT, Sacks DL, Gam a a, Murray HW, Sundar S. Splenic cytokine responses in Indian kala-azar before and after treatment. J Infect Dis. 1998;177(3):815–8. [DOI] [PubMed] [Google Scholar]
- 49.Dey R, Majumder N, Bhattacharyya Majumdar S, Bhattacharjee S, Banerjee S, Roy S, et al. Induction of host protective Th1 immune response by chemokines in Leishmania donovani-infected BALB/c mice. Scand J Immunol [Internet]. 2007;66(6):671–83. Available from: [DOI] [PubMed] [Google Scholar]
- 50.Banerjee A, De M, Ali N. Complete cure of experimental visceral leishmaniasis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favorable T cell responses. J Immunol. 2008;181(2):1386–98. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170(3):827–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14(7):335–8. [DOI] [PubMed] [Google Scholar]
- 53.Trinchieri G. Interleukin-12 and interferon-gamma. Do they always go together? Am J Pathol. 1995;147(6):1534–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.