Abstract
Despite the vital importance of Fgf for otic induction, previous attempts to study otic induction through Fgf misexpression have yielded widely varying and contradictory results. There are also discrepancies regarding the ability of Fgf to induce otic tissue in ectopic locations, raising questions about the sufficiency of Fgf and the degree to which other local factors enhance or restrict otic potential. Using heat shock-inducible transgenes to misexpress Fgf3 or Fgf8 in zebrafish, we found that the stage, distribution and level of misexpression strongly influence the response to Fgf. Fgf misexpression during gastrulation can inhibit or promote otic development, depending on context, whereas misexpression after gastrulation leads to expansion of otic markers throughout preplacodal ectoderm surrounding the head. Elevated Fgf also expands expression of the putative competence factor Foxi1, which is required for Fgf to expand other otic markers. Misexpression of downstream factors Pax2a or Pax8 also expands otic markers but cannot bypass the requirement for Fgf or Foxi1. Co-misexpression of Pax2/8 with Fgf8 potentiates formation of ectopic otic vesicles expressing a full range of otic markers. These findings document the variables critically affecting the response to Fgf and clarify the roles of foxi1 and pax2/8 in the otic response.
Keywords: zebrafish, Fgf, Pax2/5/8, Foxi1, cranial placodes, otic competence, heat shock
INTRODUCTION
The vertebrate inner ear develops from a simple epithelial thickening called the otic placode. In all vertebrate species examined to date, the otic placode is induced from uncommitted ectoderm lateral to the developing hindbrain in response to localized Fgf signaling (Reviewed by Ladher et al., 2010; Ohyama et al., 2007; Schimmang, 2007). In zebrafish embryos, for example, fgf3 and fgf8 are expressed in the hindbrain primordium during gastrulation and serve as the principal inducers of otic development (Léger and Brand, 2002; Liu et al., 2003; Maroon et al., 2002; Phillips et al., 2001). Subotic mesoderm also expresses fgf3 and fgf8 and contributes to induction and maintenance of the otic placode (Mendonsa and Riley, 1999; Nechiporuk et al., 2007; Nikaido et al, 2007). Disruption of fgf3 and fgf8 blocks the earliest known steps in otic development. Moreover, application of the Fgf-inhibitor SU5402 after the onset of otic induction shows that Fgf signaling must continue through mid-somitogenesis stages to maintain otic fate (Léger and Brand, 2002).
Although there is widespread acceptance that Fgf is required for otic induction, there have been contradictory findings regarding the sufficiency of Fgf. In zebrafish embryos, application of Fgf-coated beads can moderately expand the endogenous otic domain but does not lead to production of otic tissue in ectopic locations (Léger and Brand, 2002). Similar findings have been found following global activation of a heat shock-inducible transgene expressing fgf8 (Hans et al., 2007). In contrast, injection of plasmid expression-vectors at the 8-cell stage to achieve mosaic misexpression of fgf3 or fgf8 can expand endogenous otic domains and induce ectopic otic placodes in cranial ectoderm from the level of anterior somites to the front of the head (Phillips et al., 2004). A similar range of outcomes has been reported following Fgf-misexpression in chick and Xenopus. In chick Fgf19 alone cannot induce otic markers in explants of prospective otic ectoderm (Ladher et al., 2000), whereas applying Fgf-coated beads to intact embryos can induce expression of a subset of early otic markers, albeit only in regions near the endogenous otic placode (Adamska et al., 2001). There are also reports that Fgf can impair otic development: In one such study, Fgf misexpression reduced the size of the otic vesicle while stimulating production of microvesicles expressing lens markers (Domíngues-Frutos et al., 2009). In another study, electroporation of Fgf-expression vectors initially expanded the otic domain of Pax2 but blocked all subsequent stages of otic development (Freter et al., 2008). In contrast, viral misexpression of Fgf3 can induce formation of ectopic otic vesicles expressing a full range of otic markers (Vendrell et al., 2000), and cultured explants of head ectoderm show that the entire preplacodal ectoderm surrounding the head is competent to express early otic markers in response to exogenous Fgf2 (Martin and Groves, 2006). In Xenopus, Fgf2-coated beads can induce formation of ectopic otic vesicles in a wide region between the eyes and anterior somites (Lombardo and Slack, 1998). The reason for the different outcomes in these experiments is not clear, but the varied techniques used likely produce marked differences in the stage, duration, spatial distribution, and amount of Fgf signaling. Any or all of these variables could influence the response to Fgf signaling.
Members of the Pax2/5/8 family of transcription factors are important mediators of Fgf signaling during otic induction. Expression of pax8 marks the earliest known response to Fgf during late gastrulation and is critical for setting the size of the otic placode (Pfeffer et al., 1998; Phillips et al., 2001). Knockdown or loss of pax8 reduces the size of the otic placode by nearly half (Ikenega et al., 2011; Mackereth et al, 2005). pax2a and pax2b expression normally begin during early somitogenesis stages and are partially redundant with pax8 (Hans et al., 2004; Mackereth et al., 2005). Knockdown of all Pax2/8 function leads to loss of otic fate by 24 hpf, indicating that these genes are needed to maintain otic fate. Whether Pax2/8 function is sufficient as a downstream response to Fgf has not been previously examined.
In addition to Fgf signaling, the transcription factor Foxi1 is required for induction of pax8 in prospective otic tissue (Hans et al., 2004; Solomon et al., 2003; Solomon et al., 2004). Although otic expression of pax2a and pax2b is induced independently of Foxi1, their expression domain is much smaller in foxi1 mutants. Despite the importance of Foxi1, the functional relationship between Fgf and Foxi1 remains unclear. For example, there are discrepancies as to whether Fgf inhibits or enhances foxi1 expression (Hans et al., 2007; Nechiporuk et al., 2007; Phillips et al., 2004), possibly reflecting differences in misexpression technique. Additionally, because pax2a/b expression depends on Fgf but not Foxi1, appropriate misexpression of Fgf might be expected to expand the domain of pax2a and bypass the need for Foxi1.
Here we used heat-shock inducible transgenes to examine key parameters that influence the ability of Fgf to induce otic development. The effects of transient misexpression of Fgf were dependent on the stage and level of misexpression. Global transient activation of hs:fgf3 or hs:fgf8 at mid-late gastrula stages (7–8 hpf) severely impaired otic induction, in part by disrupting formation of the principal signaling centers in the hindbrain. Additionally, mosaic studies showed that high-level misexpression blocks otic fate cell-autonomously, whereas low to moderate levels promote otic development. At later stages high-level Fgf misexpression, either global or local, was no longer inhibitory but instead caused a dramatic expansion in the expression of otic markers into preplacodal ectoderm surrounding the anterior neural plate. At all stages examined, Fgf misexpression upregulated foxi1 expression in ectoderm abutting the anterior neural plate. Moreover, the ability of Fgf to expand otic tissue required foxi1. Misexpression of hs:pax2a or hs:pax8 also expanded endogenous otic domains but was not sufficient to bypass the requirement for Fgf or Foxi1. Co-misexpression of Fgf with pax2a or pax8 led to production of ectopic otic tissue in a broad range of cranial ectoderm rostral to somites. Our data document the extent to which even small changes in the timing, distribution and level of Fgf signaling and its downstream effectors can influence otic induction. Furthermore, the data clarify functional relationships between Fgf, foxi1 and pax2/8 genes.
MATERIALS & METHODS
Strains and developmental conditions
Wild type embryos were derived from AB line (Eugene, OR). Transgenic lines used in this study include Tg(hsp70:fgf8a)x17 (Millimaki et al., 2010), Tg(hsp70:fgf3)x27 and Tg(hsp70:pax2a)x23 (Sweet et al., 2011), Tg(hsp70:pax8)x22 (this publication) and Tg(brn3c:gfp)s356t (Xiao et al., 2005). For convenience, these transgenes are referred to in the remainder of the text as hs:fgf8, hs:fgf3, hs:pax8, hs:pax2a and brn3c:gfp respectively. Except for some experimental conditions noted below, embryos were developed under standard conditions at 28.5°C in fish water containing methylene blue and were staged based on standard protocols (Kimmel et al., 1995).
Misexpression
For standard misexpression studies, embryos heterozygous for heat shock inducible transgenes were incubated in a water bath at 39°C for 30 minutes at time points described in the results. Variations of the heat shock regimen included use of different temperatures and/or use of homozygous transgenic embryos, as noted in the text. After heat shock, embryos were incubated at 33°C in order to maintain elevated transgene expression for a longer period. Additionally, incubation at 33°C after heat shock eliminates problems with cell death sometimes observed when transgenic embryos are returned to lower temperatures following heat shock). Except where noted, phenotypes caused by trasngene activation were assessed in at least 20 embryos per experiment. The phenotypes described herein were fully penetrant unless otherwise stated.
Cell transplantation
A lineage tracer (lysine-fixable biotinylated dextran, 10000 MW, in 0.2 M KCl) was injected into the donor embryos at the one-cell stage. Labeled cells from donor embryos at blastula stages were transplanted into non-labeled hosts of the same stage. Transplanted cells were identified in the hosts by streptavidin-FITC antibody staining.
In situ hybridization
In situ hybridization was carried out as described previously (Jowett and Yan, 1996; Phillips et al., 2001).
Morpholinos
For gene knockdown experiments, 5 ng of morpholino oligomer directed against foix1 (foxi1-MO) (Gene Tools, Inc.) was injected into embryos at one-cell stage. The foxi1-MO sequence has been previously published (Solomon et al., 2003).
SU5402 treatment
SU5402 was dissolved in DMSO to make a stock solution of 20mM. Embryos are incubated with their chorions intact in a working concentration solution of 30μM SU5402 starting from 10.5 hpf, and then fixed at 13 hpf to examine the changes in pax8 or pax2a expression.
RESULTS
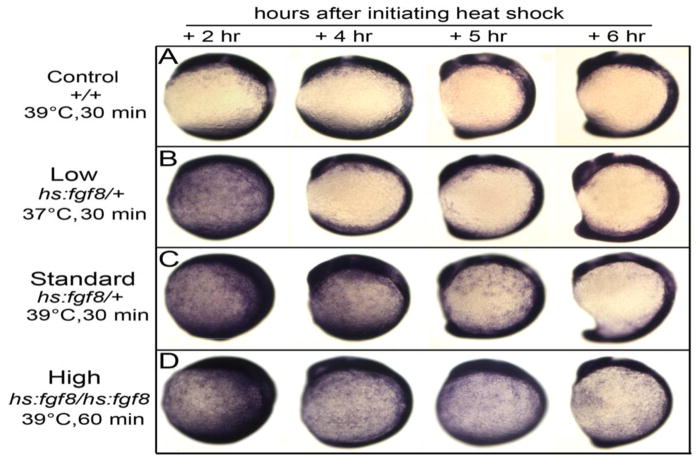
The temporal, spatial and genetic factors influencing the response to Fgf during otic induction are not fully established. To explore Fgf-responsiveness in more detail, we used heat shock inducible transgenic lines to misexpress fgf8 or fgf3 (Millimaki et al., 2010; Sweet et al., 2011) at various developmental stages and expression levels. Except where noted, our standard conditions for misexpression involved heat shocking heterozygous carriers at 39°C for 30 minutes (see Materials and Methods). Under these conditions, hs:fgf transcript levels peak by the end of the heat shock period, remain elevated for 90 minutes and then gradually decline over the next 2 hours. Expression of the Fgf-target genes erm and spry4 is slightly elevated by the end of the heat shock, increases to maximal expression one hour later and remains elevated for 4–5 hours after heat shock (Fig. 1C and data not shown).
Figure 1. Expression of erm in response to differential activation of hs:fgf8.
(A-D) Embryos were heat shocked at 10 hpf under conditions indicated to the left and fixed at times indicated across the top to examine expression of the Fgf-target gene erm. Images show lateral views with dorsal to the right and anterior up.
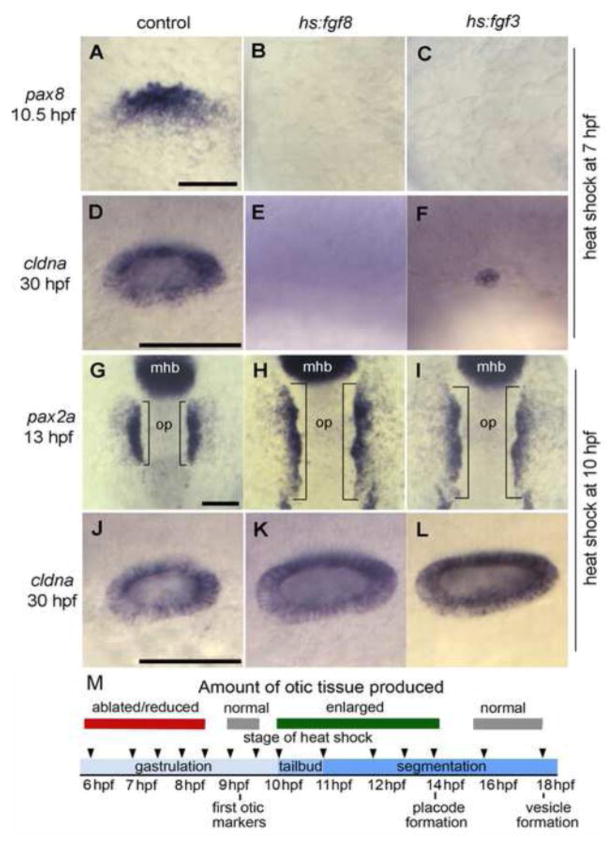
We began by testing the effects of misexpression of fgf8 or fgf3 at various developmental stages. Activation of hs:fgf3 or hs:fgf8 during late blastula/early gastrula stages (5 hpf or earlier) resulted in complete dorsalization of the embryo and was not informative (not shown). Activation of hs:fgf3 or hs:fgf8 at early to mid-gastrula stages (6–8 hpf) caused only partial dorsalization but severely impaired otic induction or blocked otic induction altogether (Table 1, Fig. 2A-F, M). Activation of hs:fgf8 or hs:fgf3 near the end of gastrulation (9–9.5 hpf), when otic markers are normally first detected, had no effect on otic development (Fig. 2M, Table 1). In contrast, activation of hs:fgf8 or hs:fgf3 after gastrulation (10–11 hpf) caused a dramatic enlargement of endogenous otic domains, though it did not induce formation of ectopic otic tissue anterior to the midbrain-hindbrain border (Table 1, Fig. 2G-L, M). Activating these transgenes at 12 hpf or 14 hpf also expanded otic tissue, though to a lesser degree, and activation at 16 hpf or later had no effect (Fig. 2M, Table 1). Similar results were previously reported using another hs:fgf8 line (Hans et al., 2007), though the transgenic lines used here appeared to show markedly stronger effects than the previous line. How Fgf misexpression causes such dramatic stage-dependent differences in otic development is not well understood.
Table 1.
Stage-dependent effects on otic development following misexpression of fgf and/or pax genes.
| Trasngene | Heat Shock | Stage | Placodal Domain | Vesicle at 30 hpf |
|---|---|---|---|---|
| hs:fgf8/+ | 39°C, 30 min | 6 hpf | ablated | ablated |
| 7 hpf | ablated | ablated | ||
| 8 hpf | ablated | ablated | ||
| 9 hpf | normal | normal | ||
| 10 hpf | enlarged | enlarged | ||
| 11 hpf | enlarged | enlarged | ||
| 12 hpf | enlarged | enlarged | ||
| 14 hpf | enlarged | normal | ||
| 39°C, 60 min | 10 hpf | enlarged | enlarged | |
| 38°C, 30 min | 8 hpf | ablated | ablated | |
| 37°C, 30 min | 8 hpf | reduced | reduced | |
| 35°C, 18 hours | 6 hpf | normal | normal | |
| hs:fgf8/hs:fgf8 | 39°C, 30 min | 10 hpf | enlarged | enlarged |
| 39°C, 60 mine | 10 hpf | enlarged, ectopic | enlarged | |
| hs:fgf3/+ | 39°C, 30 min | 6 hpf | ablated | ablated |
| 7 hpf | ablated | ablated | ||
| 8 hpf | strongly reduced | strongly reduced | ||
| 9 hpf | normal | normal | ||
| 10 hpf | enlarged | enlarged | ||
| 11 hpf | enlarged | enlarged | ||
| hs:fgf3/hs:fgf3 | 39°C, 60 min | 10 hpf | enlarged, ectopic | enlarged |
| hs:pax8/+ | 39°C, 30 min | 8 hpf | enlarged | enlarged |
| 10 hpf | enlarged | enlarged | ||
| hs:pax2a/+ | 38°C, 30 min | 8 hpf | enlarged, ectopic | enlarged, ectopic |
| 10 hpf | enlarged, ectopic | enlarged, ectopic | ||
| hs:fgf8/+; hs:pax8/+ | 39°C, 30 min | 10 hpf | enlarged, ectopic | enlarged, ectopic |
| hs:fgf8/+ hs:pax2a/+ | 38°C, 30 min | 10 hpf | enlarged, ectopic | enlarged, ectopic |
Figure 2. Stage-dependent effects of Fgf misexpression.
(A-F) Embryos were heat shocked at 7 hpf and fixed at 10.5 hpf to examine expression of pax8 in the otic primordium (A-C) or 30 hpf to examine expression of cldna in the otic vesicle (D-F). (G-L) Embryos were heat shocked at 10 hpf and fixed at 13 hpf to examine otic expression of pax2a (G-I) or at 30 hpf to examine expression of cldna (J-L). Genotypes of wild-type controls and heterozygous transgenic embryos are indicated across the top of the figure. Images show lateral views with anterior to the left (A-F, J-L); dorsal views with anterior to the top (G-I). Scale bar, 50 μm. (M) Summary of the effects of activating hs:fgf8 at various developmental stages.
Distinct mechanisms of otic-impairment by early Fgf misexpression
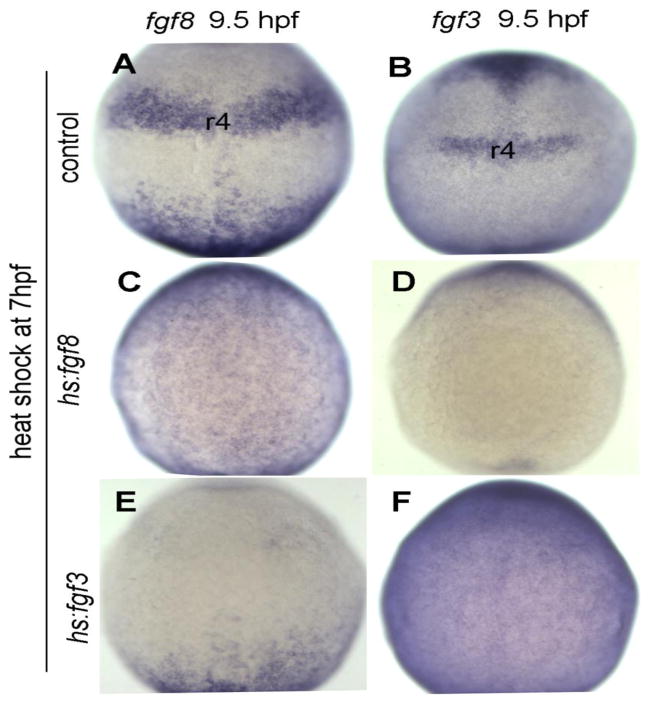
The observation that Fgf misexpression at mid gastrula stage blocks otic development is paradoxical because this is the stage when otic induction is thought to begin during normal development. We considered two possible explanations for this impairment: First, we hypothesized that early global activation of hs:fgf disrupts endogenous signaling centers needed to induce and maintain otic development. In support, global activation of hs:fgf8 or hs:fgf3 at 7 hpf prevented proper expression of endogenous fgf8 and fgf3 in the hindbrain through at least 9.5 hpf (Fig. 3C-F). This change is likely sufficient to disrupt otic development because, even if the transient pulse of transgenic Fgf were sufficient to initiate otic development, otic fate could not be maintained at later stages without endogenous signaling sources. Similar results were observed following heat shock at 8 hpf (not shown).
Figure 3. Misexpression of Fgf during gastrulation perturbs endogenous signaling centers.
Expression of fgf8 and fgf3 in rhombomere 4 (r4) of hindbrain at 9.5 hpf in control embryos (A, B), hs:fgf8 transgenic embryos (C, D) and hs:fgf3 transgenic embryos (E, F) respectively. Embryos were heat shocked at 7 hpf. Note that fgf8 expression is still globally elevated in hs:fgf8/+ embryos whereas fgf3 is globally elevated in hs:fgf3/+ embryos, but fgf genes are not detectably upregulated in the r4 region. All images show dorsal views with anterior to the top.
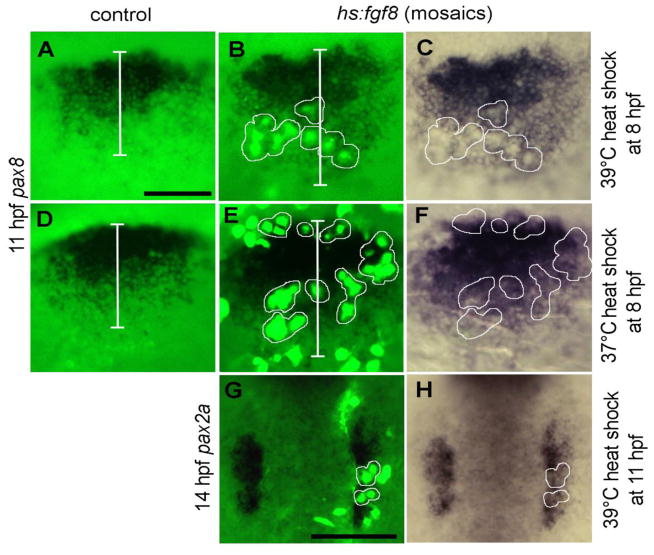
Second, because otic development normally occurs in cells near (but not within) domains of Fgf expression, we hypothesized that excess Fgf signaling might cell-autonomously block otic fate. To test this we generated mosaic embryos by transplanting hs:fgf8 transgenic cells into non-transgenic host embryos. The level of transgene-promoter activity can be regulated by adjusting the temperature from 35°C to 39°C (Adám et al., 2000; compare different responses in Figs. 1B and C). In one set of experiments, mosaic embryos were heat shocked under standard conditions (39°C for 30 minutes) at 8 hpf. This treatment caused cell-autonomous impairment of pax8 expression in transgenic cells within the otic region at 11 hpf (Fig. 4B, C). In these same embryos, the domain of pax8 expression was expanded non-autonomously in adjacent host cells (Fig. 4B, C). The same results were obtained following transgene activation at 38°C (not shown). In contrast, a lower level of activation of hs:fgf8 at 37°C did not repress pax8 expression in transgenic cells (Fig. 4E, F). In this case, too, the domain of pax8 expression expanded in adjacent host cells (Fig. 4E, F). These results indicate that high levels of Fgf inhibit otic fate cell-autonomously, whereas low to moderate levels promote otic fate. Together, these data show that strong early misexpression of Fgf impairs otic induction by at least two mechanisms: It acts directly by cell-autonomously blocking otic fate, and it acts indirectly by preventing establishment of endogenous Fgf-signaling centers.
Figure 4. Effects of mosaic misexpression of Fgf8.
(A-F) Lateral views (anterior to the left) showing expression of pax8 at 11 hpf in the otic domain in control embryos (A, D) or hs:fgf8/+ mosaic embryos (B, C, E, F) heat shocked at 39°C (A-C) or 37°C (D-F) at 8 hpf. Clusters of transgenic cells (green) are encircled with white borders to facilitate comparison of fluorescent images (B, E) with bright field images of the same specimens (C, F). White bars (A, B, D and E) mark the ML width of the otic domain. Note that transgenic cells express pax8 following heat shock at 37°C but not at 39°C, yet the otic domain is laterally expanded at both temperatures. (G, H) Dorsal views (anterior to the top) showing expression of pax2a at 14 hpf in a mosaic embryo heat shocked at 39°C at 11 hpf. Transgenic cells (green, with white borders) express pax2a and the otic domain is lengthened along the AP axis. Scale bar, 150 μm.
Effects of Fgf misexpression at later stages
To distinguish autonomous from non-autonomous effects at later stages, we next examined the effects of mosaic activation of hs:fgf8 at 11 hpf. In agreement with the effects of global activation of hs:fgf8 at later stages (Fig. 2G-M), activating the transgene at 11 hpf in mosaic embryos, even ones containing relatively few hs:fgf8/+ cells, also expanded the otic domain of pax2a (Fig. 4G, H). Unlike misexpression at earlier stages, however, strong activation at 11 hpf did not cell-autonomously block otic differentiation within transgenic cell. Thus, once the otic development has been initiated, strong misexpression of Fgf reinforces otic fate and efficiently expands the endogenous otic primordium.
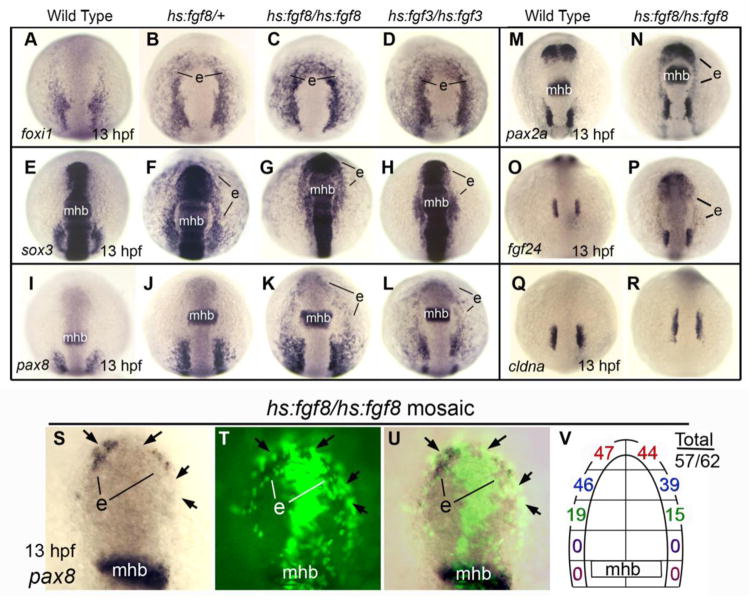
Despite enlargement of endogenous otic domains following Fgf misexpression at later stages, it is noteworthy that there were no signs of ectopic otic induction under standard misexpression conditions. This agrees with previous findings obtained with an earlier transgenic line (Hans et al., 2007). However, these results conflict with our previous findings that Fgf misexpression from injected plasmid vectors can induce otic placodes in ectopic locations around the front of the head (Phillips et al., 2004). Because injected plasmid frequently integrates into the genome as large concatemers, it is possible that the vectors used in our earlier study elicited stronger Fgf signaling than the transgenes utilized here. To boost the level of expression from our transgenic lines, hs:fgf3/+ and hs:fgf8/+ heterozygotes were heat shocked for 60 minutes beginning at 10 hpf. This resulted in dramatic upregulation of the otic competence factor foxi1 in preplacodal ectoderm surrounding the front of the head (Fig. 5B), mimicking the effects of plasmid-injection (Phillips et al., 2004). Additionally, the otic/epibranchial marker sox3 was ectopically expressed throughout the anterior preplacodal ectoderm (Fig. 5F). However, these conditions did not result in ectopic expression of pax8 (Fig. 5J). To further increase Fgf expression levels, we generated hs:fgf8/hs:fgf8 homozygotes and heat shocked them for 60 minutes beginning at 10 hpf. This resulted in significant, though spotty, ectopic expression of pax8 in anterior preplacodal ectoderm, as well as marked lateral expansion of endogenous otic domains (Fig. 5K). Domains of foxi1 and sox3 were also expanded (Fig. 5C, G). Note that the domain of sox3 was smaller in hs:fgf8/hs:fgf8 homozygotes compared to hs:fgf8/+ heterozygotes (Fig. 5F, G), consistent with previous finding that sox3 exhibits distinct thresholds for activation by moderate Fgf levels and downregulation by high Fgf levels (Bhat et al., 2011; Nikaido et al., 2007; Padanad and Riley, 2011; Sun et al., 2007). Similar results were obtained by heat shocking hs:fgf3/hs:fgf3 homozygotes for 60 minutes at 10 hpf (Fig. 5D, H, L). Analysis of additional early otic markers confirmed that strong global misexpression of hs:fgf8 induced ectopic expression of pax2a and, to a lesser extent, fgf24 (Fig. 5M-P). In contrast, expression of cldna was not induced ectopically (Fig. 5Q, R). Moreover, none of these transgenic embryos produced ectopic otic vesicles at later stages. Heat shocking hs:fgf8/hs:fgf8 homozygotes at 12 hpf or 14 hpf gave similar but weaker responses compared to activation at 10 hpf (not shown). Activation at 16 hpf was not effective, confirming that otic competence is gradually lost during mid-somitogenesis stages (Groves and Bronner-Fraser, 2000; Hans et al., 2007). Together, these data show that transient high-level misexpression of Fgf can induce expression of many early otic markers in anterior preplacodal ectoderm, but the conditions used here are not sufficient to sustain the full program of otic development in ectopic locations.
Figure 5. High level Fgf induces ectopic expression of otic markers in anterior preplacodal ectoderm after gastrulation.
(A-R) Dorsal views (anterior up) of embryos heat shocked at 39°C for 1 hour starting at 10 hpf and then fixed at 13 hpf to examine expression of foxi1 (A-D), sox3 (E-H), pax8 (I-J), pax2a, (M, N), fgf24 (O, P) and cldna (Q, R). Genotypes of embryos are indicated across the top of the figure. The midbrain-hindbrain border (mhb) and regions showing ectopic expression (e) are indicated. (S-U) A representative hs:fgf8/hs:fgf8 mosaic embryo that was heat shocked at 39°C for 1 hour at 10 hpf and fixed at 13 hpf to examine pax8 expression. Images show the same specimen viewed under bright field (S), fluorescence (T), and an overlay (U). Positions of hs:fgf8/hs:fgf8 transgenic cells (green, black arrows), the midbrain-hindbrain border (mhb), and ectopic patches of pax8 expression (e) are indicated. (V) A summary diagram showing the number of hs:fgf8/hs:fgf8 mosaic embryos with ectopic expression of pax8 in different regions of the preplacodal ectoderm. The total number of mosaic embryos showing any ectopic pax8/the total number examined is also indicated.
To achieve maximal Fgf misexpression in a more localized manner, we generated mosaic embryos containing scattered hs:fgf8/hs:fgf8 cells and heat shocked them for 60 minutes at 10 hpf. The majority (57/62) of mosaic embryos showed patches of robust pax8 expression in anterior preplacodal ectoderm in regions near transgenic cells (Fig. 5S-V). Interestingly, transgenic cells themselves tended not to express pax8 (arrows in Fig. 5S-U), similar to results obtained with plasmid-injection (Phillips et al., 2004). This suggests that high-level misexpression of Fgf8 can to some extent cell-autonomously impair otic differentiation in anterior preplacodal ectoderm, possibly accounting for the less robust ectopic expression of pax8 seen after global misexpression of Fgf8 (Fig. 5K, L). To more fully analyze the effects of mosaic misexpression, we took advantage of the variable distribution of transgenic cells to assess whether different regions of anterior preplacodal ectoderm are equally responsive to localized Fgf8 signaling. To facilitate quantitative analysis, we divided the anterior preplacodal ectoderm into five equal domains along the AP axis. As summarized in Fig. 5V, the highest frequency of ectopic pax8 expression was observed in the first two (anterior-most) regions, with right or left sides showing pax8 expression in up to 75% of mosaic embryos. The third (middle) region showed pax8 expression less than half as often, affecting no more than 31% of mosaic embryos on the right or left side (Fig. 5V). In no case did we observe ectopic pax8 expression in the last two (posterior-most) regions (n=0/62), despite the fact that transgenic cells were often observed there. Nevertheless, it is unlikely these regions lack otic-competence because global Fgf misexpression was able to induce ectopic pax8 throughout the anterior preplacodal ectoderm (Fig. 5K, L). It is possible that signals emitted by the midbrain-hindbrain border or other nearby tissues normally restrict otic development but are disrupted by global Fgf misexpression. Together, these data show that the entire preplacodal region is competent to express otic markers in response to Fgf, but responsiveness is not uniform, with some regions appearing somewhat resistant. Presumably, other signals or intrinsic factors can locally modulate the response to Fgf, as hypothesized by others (McCabe and Bronner-Fraser, 2009; Schlosser, 2006).
Misexpression of pax2/8 expands the otic field
Previous studies have shown that pax2/8 genes are important mediators of Fgf signaling and are required for normal induction and maintenance of otic tissue (Hans et al., 2004; Mackereth et al., 2005). We therefore generated heat shock inducible transgenic lines to test the effects of misexpression of pax8 or pax2a (Sweet et al., 2011, and this work). These transgenes have similar effects on embryonic patterning, though in the experiments reported here hs:pax8 was activated under standard conditions whereas hs:pax2a was activated by heat shocking at 38°C for 30 minutes (see Materials and Methods).
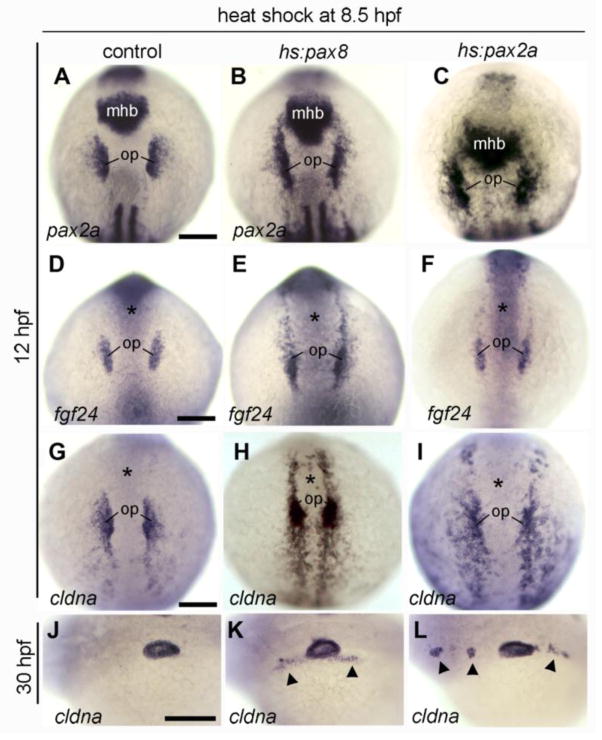
Activation of hs:pax8 at mid to late gastrula stage (8.5 hpf) caused expansion of early otic markers into anterior preplacodal ectoderm to the level of the midbrain-hindbrain border (pax2a) or to the level of the eye (fgf24, cldna) (Fig. 6B, E, H). When these embryos were examined at 30 hpf, diffuse ectopic staining of cldna was observed in ectoderm just anterior and posterior to the otic vesicle (Fig. 6K). However, no ectopic otic vesicles were ever observed under these conditions. Similar results were obtained after misexpression of pax8 at tailbud stage (10 hpf), except that cldna expression at 30 hpf was less diffuse, often appearing in microvesicles near the endogenous otic vesicle (Table 2, and data not shown). Activation of hs:pax2a at 8.5 hpf or 10 hpf led to similar expansion of early otic markers (Fig. 6C, F, I). In this line, however, production of cldna-positive microvesicles was more common and was observed after heat shock at either 8.5 or 10 hpf (Fig. 6L, Table 2). In rare cases, ectopic microvesicles were observed beyond the midbrain-hindbrain border up to the level of the eye (Fig. 6L, Table 2). The microvesicles produced under these conditions were not normal otic structures, however, because they did not express other otic patterning genes such as pax2a, dlx3b, otx1 or atoh1a (not shown). These data indicate that misexpression of pax2/8 genes can expand the endogenous domain of early otic markers but is usually not sufficient to induce otic development elsewhere in the preplacodal ectoderm. Additionally, these genes are not sufficient to induce a full program of otic differentiation even in cases where extra-otic microvesicles were observed.
Figure 6. Expansion of otic markers following activation of hs:pax8 or hs:pax2a.
(A-L) Dorsal views (anterior up) or lateral views (anterior to the left) of embryos heat shocked at 8.5 hpf and fixed at 12 hpf to examine expression of pax2a (A-C), fgf24 (D-F) and cldna (G-I), or embryos were fixed at 30 hpf to examine expression of cldna in the otic vesicle (J-L). Genotypes of wild-type or heterozygous transgenic embryos are indicated across the top of the figure. Expression in the otic placode (op) and midbrain-hindbrain border (mhb) is indicated. Alternatively, the position of the midbrain-hindbrain border is marked by an asterisk in (D-I). Arrowheads in K, L mark regions with ectopic expression of cldna. Scale bar, 150 μm.
Table 2.
Production of microvesicles following global misexpression of hs:fgf8, hs:pax2a or hs:pax8 beginning at 10 hpf.
| Transgene | No. of embryos | Mean no. of microvesicles per embryo* | Fraction of embryos with ectopic microvesicles† |
|---|---|---|---|
| hs:fgf8 | 17 | 0 | 0/17 |
| hs:pax2a | 10 | 6.7 ± 2 | 2/10 |
| hs:pax8 | 12 | 4.4 ± 2 | 0/12 |
| hs:fgf8 + hs:pax2a | 10 | 17.4 ± 3.7 | 9/10 |
| hs:fgf8 + hs:pax8 | 15 | 9.7 ± 2.3 | 9/15 |
Mean ± SD of the total number of microvesicles.
Refers to microvesicles forming anterior to the midbrain-hindbrain border or posterior to the first somite.
Misexpression of pax2/8 cannot bypass the need for Fgf signaling
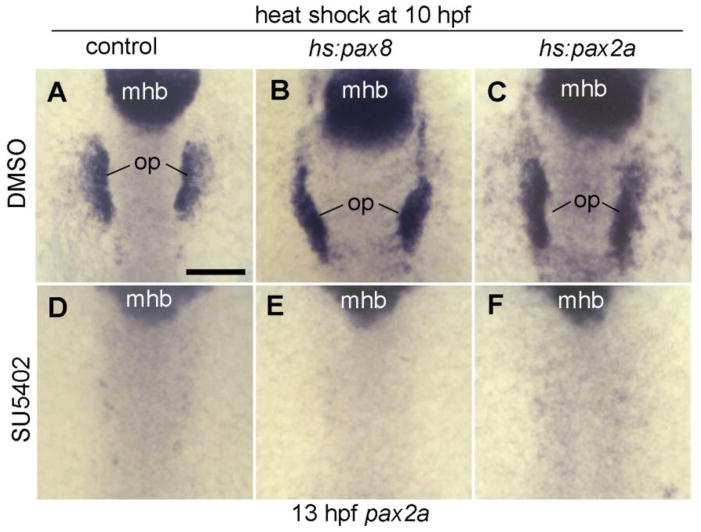
The extent to which Pax2a and Pax8 can mediate the full effect of Fgf signaling is not clear. We therefore tested whether activation of hs:pax2a or hs:pax8 can bypass the need for Fgf during otic induction. Embryos carrying hs:pax8 or hs:pax2a were heat shocked at 10 hpf, just after the onset of otic induction, and afterwards Fgf signaling was blocked using the pharmacological inhibitor SU5402. Under these conditions, otic development was completely abolished by 13 hpf (Fig. 7D-F). These results show that pax2/8 genes are not sufficient to expand or maintain otic development in the absence of Fgf signaling, suggesting that additional Fgf-target genes are essential for otic induction.
Figure 7. Pax2/8 misexpression cannot bypass the need for Fgf.
(A-F) Dorsal views (anterior up) of pax2a expression at 13 hpf. Embryos were heat shocked at 10 hpf and then incubated in water containing 0.15% DMSO or 30 μM SU5402 and 0.15% DMSO. Genotypes of wild-type and heterozygous transgenic embryos are indicated across the top of the figure. Positions of midbrain-hindbrain boundary (mhb) and otic placode (op) are indicated. Scale bar, 150 μm.
Effects of co-misexpression of Fgf8 and Pax8/Pax2a
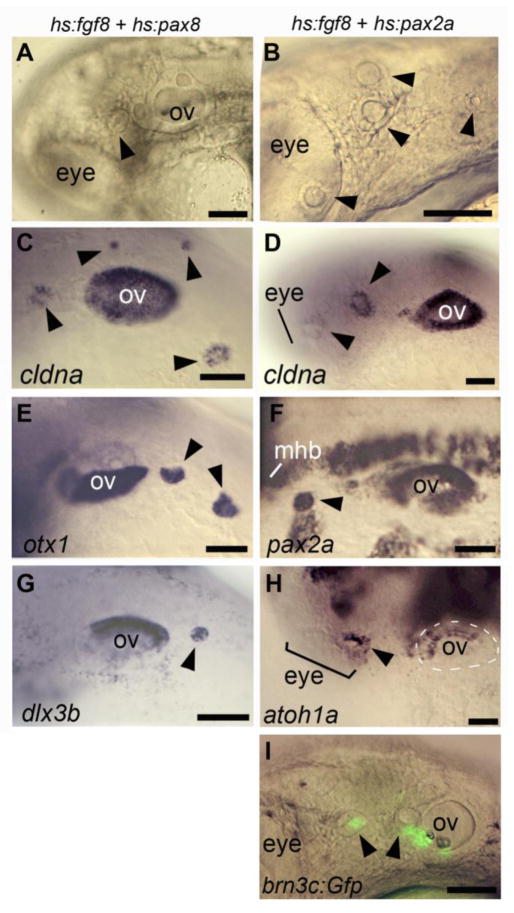
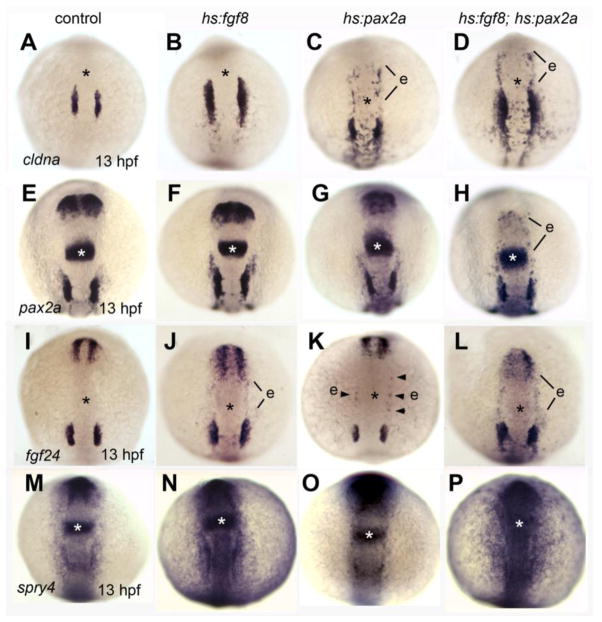
Given the lag between Fgf misexpression and activation of endogenous pax8 and pax2a genes, we speculated that co-misexpression of transgenic Fgf8 with transgenic Pax2a/8 might accelerate and enhance early steps inotic development and thereby stabilize production of ectopic otic vesicles. In support, co-activation of hs:fgf8 with hs:pax2a or hs:pax8 at 10 hpf (in embryos heterozygous for relevant transgenes) strongly enhanced production of ectopic microvesicles compared to activation of individual transgenes (Fig. 8A, B and Table 2). For example, there was a three-fold increase in the number of microvesicles produced in hs:fgf8-hs:pax2a embryos compared to hs:pax2a alone (Table 2). Moreover, 90% (9/10) of double transgenic embryos produced ectopic microvesicles in the anterior head and/or adjacent to anterior somites (Fig 8B, D, F, H, I and Table 2). Similarly, 60% (9/15) of hs:fgf8-hs:pax8 double transgenic embryos produced ectopic otic vesicles in these regions (Fig. 8A, C, E, G, and Table 2). Another difference between single vs. double transgenic embryos was in the range of otic markers expressed within microvesicles. While microvesicles induced by hs:pax8 or hs:pax2a alone expressed only cldna, microvesicles produced in hs:fgf8-hs:pax8 or hs:fgf8-hs:pax2a embryos expressed a full range of otic vesicle markers including cldna, dlx3b, otx1, pax2a and atoh1a at 30 hpf (Fig. 8C-H, and data not shown). Additionally, ectopic vesicles were observed to express brn3c:gfp (Fig. 8I), indicating the presense of sensory hair cells (Xiao et a., 2005). To better understand the effects of co-misexpression, we heat shocked hs:fgf8-hs:pax2a double transgenic embryos at 10 hpf and analyzed expression of early otic markers at 13 hpf. Double transgenic embryos showed a number of changes in gene expression compared to single transgenic embryos. First, expression of cldna was more robust in anterior preplacodal ectoderm in double transgenic embryos (Fig. 9A-D). Second, scattered patches pax2a-positive of cells were observed in anterior preplacodal ectoderm only in double transgenic embryos (Fig. 9E-H), despite significant downregulation of pax2a in the optic stalk (Fig. 9H). Third, expression of fgf24 in anterior preplacodal ectoderm was stronger in double-transgenic embryos (Fig. 9I-L). Likewise, upregulation of the Fgf-target gene spry4 was also more pronounced in preplacodal ectoderm in double transgenic embryos (Fig. 9M-P). Together, these results indicate that co-misexpression of hs:fgf8 with hs:pax2a or hs:pax8 can synergistically promote stable induction of ectopic otic placodes and vesicles expressing a host of otic patterning genes.
Figure 8. Formation of ectopic otic vesicles following co-misexpression of Fgf8 with Pax2a or Pax8.
(A-L) Lateral views (anterior to the left) of embryos at 30 hpf, following heat shock at 10 hpf. Images show live embryos (A, B, L) or fixed specimens showing expression of cldna (C, D), otx1b (E), pax2a (F), dlx3b (G), or atoh1a (H). The specimen in (L) also carries brn3c:Gfp transgene to mark sensory hair cells. Embryos heterozygous for the indicated inducible transgenes are labeled across the top of the figure. Positions of the eye, midbrain-hindbrain border (mhb), endogenous otic vesicle (ov) and ectopic otic vesicles (arrowheads) are indicated. Scale bar, 50 μm.
Figure 9. Effects of co-misexpression of Fgf8 with Pax2a on early otic development.
(A-P) Dorsal views (anterior up) of embryos heat shocked at 10 hpf and fixed at 13 hpf to examine expression of cldna (A-D), pax2a (E-H), fgf24 (I-L) and spry4 (M-P). Genotypes of wild-type and heterozygous transgenic embryos are indicated across the top of the figure. The position of the midbrain-hindbrain border is marked with an asterisk. Regions with ectopic gene expression (e) are indicated.
An indispensable role for foxi1
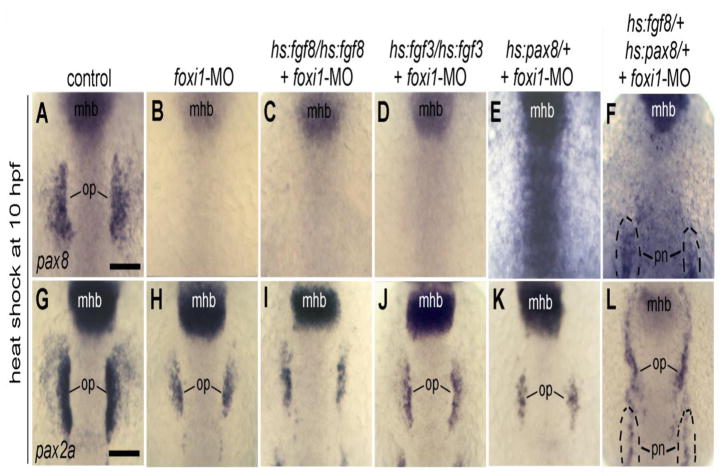
It was previously established that expression of pax8 requires foxi1 in the endogenous otic placode as well as in ectopic locations following Fgf misexpression (Hans et al., 2004; Solomon et al., 2003; Solomon et al, 2004). However, Fgf is still able to induce residual pax2a-positive otic placodes in the absence of foxi1. We therefore tested whether maximal misexpression of Fgf8 or Fgf3 could expand the otic domain of pax2a in foxi1 morphants. As expected, the otic domain of pax8 was eliminated under these conditions (Fig. 10A-D). Surprisingly, the otic domain of pax2a was no larger in foxi1 morphants after Fgf misexpression compared to non-transgenic foxi1 morphants (Fig. 10H-J). We also observed no ectopic pax2a expression under these conditions. Thus, despite the presence of a small domain of Fgf-dependent/Fox1- independent otic precursors, competence to respond to Fgf in other regions is absolutely dependent on foxi1.
Figure 10. Misexpression of Fgf or Pax8 cannot expand otic development without Foxi1.
(A-F) Expression of pax8 at 13 hpf. (G-L) Expression of pax2a at 13 hpf. Embryos were heat shocked at 10 hpf, and most embryos were injected at the one-cell stage with foxi1-MO (B-F, H-L) as indicated across the top of the figure. Genotypes of transgenic embryos, including hs:fgf8/hs:fgf8 (C, I), hs:fgf3/hs:fgf3 (D, J), hs:pax8/+ (E, K), and hs:fgf8/+; hs:pax8/+ (F, L) are indicated across the top of the figure. Positions of midbrain-hindbrain boundary (mhb), pronerphros (pn) and otic placode (op) are indicated. Note that the pronerphric domain of pax2a is also expanded anteriorly in double trasngenic embryos (L). All images show dorsal views with anterior to the top. Scale bar, 150 μm.
We next tested whether the requirement for foxi1 could be bypassed by misexpressing its downstream target, pax8. We focused on pax8 because this gene is especially critical for mediating the effects of foxi1 in establishing a normally sized otic placode (Ikenega et al., 2011; Mackereth et al., 2005). Despite the importance of pax8 for this function, activation of hs:pax8 alone was not sufficient to expand the otic domain in foxi1 morphants (Fig. 10E, K). In contrast, co-activation of hs:pax8 and hs:fgf8 expanded the otic domain of pax2a up to the level of the midbrain-hindbrain border (Fig. 10L), though there was still no otic expression of endogenous pax8 (Fig. 10F). Thus, Pax8 acting in concert with Fgf can partially compensate for loss of foxi1 to affect the size of the otic domain.
DISCUSSION
Previous studies investigating the effects of Fgf misexpression on otic induction have yielded widely varying, often contradictory results (Adamska et al., 2001; Domíngues-Frutos et al, 2009; Freter et al., 2008; Hans et al., 2007; Ladher et al., 2000; Léger and Brand, 2002; Lombardo and Slack, 1998; Martin and Groves, 2006; Phillips et al., 2004; Vendrell et al., 2000). We have identified a number of variables that critically influence how prospective otic cells respond to Fgf. First, sensitivity to Fgf varies according to developmental stage. Embryos are particularly vulnerable to inhibitory effects of Fgf over-expression during gastrulation stages, partly reflecting disruption of endogenous signaling centers. Second, the spatial distribution of Fgf expression strongly affects the outcome of Fgf signaling, with high levels blocking otic development within expressing cells while promoting otic development in neighboring cells. Third, delivering sufficiently high levels of Fgf just after gastrulation expands expression of early otic markers throughout preplacodal ectoderm surrounding the head. The ability of Fgf to expand otic development absolutely requires Foxi1, though our results suggest that Foxi1 acts downstream of Fgf rather than in an independent parallel pathway. Finally, misexpressing Pax2a or Pax8 expands otic development and potentiates the effects of misexpressing Fgf, leading to formation of enlarged and ectopic otic vesicles that express a full array of otic patterning genes. Nevertheless, Pax2/8 misexpression cannot bypass the need for either Fgf or Foxi1. Together these findings provide important insights into the conditional requirements for otic induction and potentially reconcile discrepancies in the literature regarding the effects of Fgf misexpression.
Response to Fgf is conditional
Otic placodes, like all other cranial placodes, develop from a contiguous zone of preplacodal ectoderm surrounding the anterior neural plate. Although preplacodal ectoderm is clearly multipotent, cells in different regions appear to have distinct biases reflecting their unique expression profiles of various transcription factors (McCabe and Bronner-Fraser, 2009; Schlosser, 2006). Nevertheless, sufficiently high levels of Fgf signaling can overcome regional biases to induce expression of early otic markers (pax8, pax2a, fgf24 and sox3) throughout the preplacodal ectoderm. At slightly lower Fgf levels, pax2/8 and fgf24 genes are not induced ectopically, yet expression of sox3 is expanded even more than with higher Fgf. This pattern mimics the gene expression profiles seen in otic and epibranchial placodes, respectively, which are induced in abutting domains by a lateral gradient of Fgf (Bhat and Riley, 2011; Padanad and Riley, 2011).
After initial otic induction, Fgf signaling continues from endogenous signaling centers and is initially required to maintain otic fate (Léger and Brand, 2002), and later to pattern the otic vesicle (Hammond and Whitfield, 2011; Kwak et al., 2002; Lecaudey et al., 2007). Our misexpression studies do not address these later functions because transgene activity is only transient. This likely explains why even maximal trasngene activity was not sufficient to stably induce morphological development of ectopic otic vesicles. Serial heat shock can sometimes prolong the effects of Fgf misexpression (Sweet et al., 2011), but in the current study maximal Fgf misexpression induced such robust expression of spry4, and presumably other feedback inhibitors, that secondary heat shocks were relatively ineffective (our unpublished observations). However, co-misexpression of hs:fgf8 with either hs:pax8 or hs:pax2a frequently led to formation of ectopic otic vesicles expressing a full array of otic markers. Although endogenous pax2/8 genes are induced ectopically by Fgf, activation of hs:pax2/8 transgenes avoids the lag-time normally required for this response. We speculate that such co-activation triggers a self-reinforcing feedback loop that stabilizes otic development. Consistent with this notion, co-activation of hs:fgf8 and hs:pax2a leads to greater ectopic expression of fgf24 in anterior preplacodal ectoderm, which likely prolongs Fgf signaling and helps maintain pax2a expression. Additionally, Pax2/8 function might protect otic cells from potential inhibitory effects of Fgf over-expression. In the endogenous otic field, for example, Fgf over-expression no longer cell-autonomously inhibits otic fate once expression of pax8 and pax2a have been established. A similar protective mechanism might explain why co-misexpression of Pax2/8 with Fgf stabilizes formation of ectopic otic placodes in anterior preplacodal ectoderm.
The mechanism by which Fgf over-expression cell-autonomously inhibits otic development remains unclear. Because Fgf acts as a DV morphogen during gastrulation, it is possible that excess Fgf inhibits otic fate by specifying more dorsal ectodermal fates. However, we detect no enhanced expression of characteristic markers of neural crest (foxd3) or neural plate (sox19 or krox20) (data not shown). Another common role for Fgf is maintenance of stem cell pluripotency (Lanner and Rossant, 2010), raising the possibility that excess Fgf exerts a general block to differentiation. If so, it is not clear why inhibition is limited to cells that directly express Fgf, since immediate neighbors presumably also experience high Fgf signaling. Some Fgf ligands, including Fgf3, can regulate mitosis and differentiation by being imported directly into the nucleus without prior secretion (Kiefer et al., 1994; Kiefer and Dickson, 1995). However, no similar activity has been reported for Fgf8, raising the possibility that a more general mechanism mediates cell-autonomous inhibition by multiple Fgf ligands. This remains an important unresolved question.
Foxi1 and otic competence
Foxi1 function is complex. It is initially expressed throughout nonneural ectoderm in response to Bmp (Hans et al., 2004; Solomon et al., 2003) and serves as a general competence factor for all preplacodal ectoderm (Kwon et al., 2010). In a distinct subsequent process, foxi1 downregulates in most of its original domain but upregulates in otic and epibranchial precursors as they begin to experience elevated Fgf signaling. In this latter process, foxi1 is thought to act as a spatially localized competence factor required for the otic/epibranchial response to Fgf (Hans et al., 2004; Solomon et al., 2003; Solomon et al., 2004). However, we find that Fgf misexpression is sufficient to upregulate foxi1 throughout ectoderm surrounding the head. Because foxi1 is Fgf-responsive, the foxi1 domain might not be the principal means of spatially restricting otic/epibranchial competence; rather it is the availability of sufficiently high levels of Fgf. In this context it would be more appropriate to consider foxi1 an essential early mediator of Fgf during otic induction rather than an independent competence factor that localizes the response to Fgf. Additional evidence for this view comes from analysis of foxi1 mutants and morphants. Although loss of foxi1 ablates otic expression of pax8, a small domain of pax2a still forms in response to Fgf (Hans et al., 2004; Solomon et al., 2004). However, not even maximal Fgf over-expression can enlarge this domain in the absence of foxi1. Thus, foxi1 is not required to position the endogenous domain of otic competence, but instead foxi1 is required downstream of Fgf to expand otic development beyond this restricted domain. Interestingly, it has been noted that upregulation of foxi1 in the otic/epibranchial domain does not require Fgf (Solomon et al., 2004). However, this appears to reflect partial redundancy between the Fgf and Pdgf pathways since blocking both pathways with pharmaceutical inhibitors severely impairs development of preplacodal ectoderm blocks local upregulation of foxi1 (Kwon et al. 2010; and our unpublished observations).
Pax8 appears to be a key mediator of Foxi1 in expanding the initial domain of otic development. Knockdown of pax8 substantially reduces the size of the otic placode (Ikegena et al., 2011; Mackeret et al., 2005), and co-misexpression of Pax8 and Fgf8 dramatically expands otic development in the absence of foxi1 (Fig. 10L). On the other hand, Pax8 plus Fgf8 were unable to expand otic development in the absence of foxi1, indicating that other Foxi1-target genes also contribute to this function.
Highlights.
Fgf misexpression can inhibit or promote otic development, depending on context.
Optimal Fgf expands expression of early otic markers into anterior cranial ectoderm.
Misexpression of Pax2a/8 expands some otic markers but cannot bypass Fgf or Foxi1.
Comisexpression of Pax2a/8 with Fgf8 potentiates formation of ectopic otic vesicles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adám A, Bártfai R, Lele Z, Krone PH, Orbán L. Heat-inducible expression of a reporter gene detected by transient assay in zebrafish. Exp Cell Res. 2000;256:282–290. doi: 10.1006/excr.2000.4805. [DOI] [PubMed] [Google Scholar]
- Adamska M, Herbrand H, Adamski M, Krüger M, Braun T, Bober E. FGFs control the patterning of the inner ear but are not able to induce the full ear program. Mech Dev. 2001;109:303–313. doi: 10.1016/s0925-4773(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Bhat N, Riley BB. Integrin-α5 coordinates assembly of posterior cranial placodes in zebrafish and enhances Fgf-dependent regulation of otic/epibranchial cells. PLoS ONE. 2011;6(12):327778. doi: 10.1371/journal.pone.0027778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Frutos E, Vendrell V, Alvarez Y, Zelarayan LC, López-Hernández I, Ros M, Schimmang T. Tissue-specific requirements for Fgf8 during early inner ear development. Mech Dev. 2009;126:873–881. doi: 10.1016/j.mod.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Whitfield TT. Fgf and Hh signalling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle. Development. 2011;138:3977–3987. doi: 10.1242/dev.066639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Liu D, Westerfield M. Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development. 2004;131:5091–5102. doi: 10.1242/dev.01346. [DOI] [PubMed] [Google Scholar]
- Hans S, Christison J, Liu D, Westerfield M. Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol. 2007;7:5. doi: 10.1186/1471-213X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenega T, Urban JM, Gebhart N, Hatta K, Kawakami K, Ono F. Formation of the spinal network in zebrafish determined by domain-specific Pax genes. J Neurol. 2011;519:1562–1579. doi: 10.1002/cne.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett T, Yan YL. Double fluorescent in situ hybridization to zebrafish embryos. Trends Genet. 1996;12:387–389. doi: 10.1016/s0168-9525(96)90091-8. [DOI] [PubMed] [Google Scholar]
- Kiefer P, Dickson C. Nucleolar association of Fibroblast Growth Factor 3 via specific sequence motifs has inhibitory effects on cell growth. Mol Cell Biol. 1995;15:4364–4374. doi: 10.1128/mcb.15.8.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer P, Acland P, Pappin D, Peters G, Dickson C. Competition between nuclear localization and secretory signals determines the subcellular fate of a single CUG-initiated form of FGF3. EMBO J. 1994;13:4126–4136. doi: 10.1002/j.1460-2075.1994.tb06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kwak SJ, Phillips BT, Heck R, Riley BB. An expanded domain of fgf3 expression in the hindbrain of zebrafish valentino mutants results in mis-patterning of the otic vesicle. Development. 2002;129:5279–5287. doi: 10.1242/dev.129.22.5279. [DOI] [PubMed] [Google Scholar]
- Kwon H, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of Early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6(9):e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1968. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Ladher RK, O'Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- Lecaudey V, Ulloa E, Anselme I, Stedman A, Schneider-Maunoury S, Pujades C. Role of hindbrain in patterning of the otic vesicle: A study of the zebrafish vhnf1 mutant. Dev Biol. 2007;303:134–143. doi: 10.1016/j.ydbio.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Léger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Slack JMW. Postgastrulation effects of fibroblast growth factor on Xenopus development. Dev Dyn. 1998;212:75–85. doi: 10.1002/(SICI)1097-0177(199805)212:1<75::AID-AJA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mackereth MD, Kwak SJ, Fritz A, Riley BB. Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development. 2005;132:371–382. doi: 10.1242/dev.01587. [DOI] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Martin K, Groves A. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–887. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- McCabe K, Bronner-Fraser M. Molecular and tissue interactions governing induction of cranial ectodermal placodes. Dev Biol. 2009;332:189–195. doi: 10.1016/j.ydbio.2009.05.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonsa ES, Riley BB. Genetic analysis of tissue interactions required for otic placode induction in zebrafish. Dev Biol. 1999;206:100–112. doi: 10.1006/dbio.1998.9134. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–623. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–571. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51:463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Padanad MS, Riley BB. Pax2/8 proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, sox3 and fgf24. Dev Biol. 2011;351:90–98. doi: 10.1016/j.ydbio.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Büsslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235:351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Storch EM, Lekven AC, Riley BB. A direct role for Fgf but not Wnt in otic placode induction. Development. 2004;131:923–931. doi: 10.1242/dev.00978. [DOI] [PubMed] [Google Scholar]
- Schimmang T. Expression and functions of Fgf ligands during early otic development. Int J Dev Biol. 2007;51:473–481. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kwak SJ, Fritz A. Genetic interactions underlying otic placode induction and formation. Dev Dyn. 2004;230:419–433. doi: 10.1002/dvdy.20067. [DOI] [PubMed] [Google Scholar]
- Sun SK, Dee CT, VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol. 2011;358:113–121. doi: 10.1016/j.ydbio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell V, Carnicero E, Giraldez F, Alonso MT, Schimmang T. Induction of inner ear fate by FGF3. Development. 2000;127:2011–2019. doi: 10.1242/dev.127.10.2011. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roester T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of hte zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]