Abstract
Purpose
To compare hyperfractionation versus standard fractionation for T2N0 vocal cord carcinoma in a randomized controlled trial.
Methods and Materials
Patients with T2 vocal cord cancer were stratified by substage (T2a vs. T2b) and randomly assigned to receive either hyperfractionation (HFX) to 79.2 Gy in 66 fractions of 1.2 Gy given twice a day, or standard fractionation (SFX) to 70 Gy in 35 fractions given once a day. The trial was designed to detect a 55% reduction in the local failure hazard rate with 80% statistical power.
Results
Between April 1996 and July 2003, 250 patients were enrolled. Of 239 patients analyzable for outcomes, 94% were male, 83% had KPS 90-100, and 62% had T2a tumor. Median follow-up for all surviving patients was 7.9 years (range: 0.6 – 13.1). The 5-year local control (LC) rate was 8 points higher (but not statistically significant: p=0.14) for HFX (78%) vs SFX (70%), corresponding to a 30% hazard rate reduction. Five-year disease-free survival (DFS) was 49% vs 40% (p=0.13) and overall survival (OS) 72% vs 63% (p=0.29). HFX had higher rates of acute skin, mucosal, and laryngeal toxicity. Grade 3-4 late effects were similar with 5-year cumulative incidence of 8.5% (3.4-13.6%) after SFX and 8.5% (3.4-13.5%) after HFX.
Conclusions
Five-year local control was modestly higher with HFX compared to SFX for T2 glottic carcinoma, but the difference was not statistically significant. These results are consistent with prior studies of hyperfractionation showing a benefit in local control. Substaging by T2a vs T2b carries prognostic value for DFS and OS. For cost and convenience reasons other altered fractionation schedules have been adopted in routine practice.
Keywords: laryngeal cancer, fractionation, local control, organ preservation, clinical trial
INTRODUCTION
Laryngeal carcinoma, approximately 23% of head and neck squamous cancer[1], may arise in any mucosal surface of the larynx, with the vocal cords (glottis) the most common subsite (75%). Treatment of early (T1-2) glottic cancers generally results in high rates of local control and larynx preservation. Retrospective series show approximately 70% 5-year local control for T2 glottic lesions treated with conventional fractionation[2].
Hyperfractionation (HFX), i.e., the use of doses per fraction lower than 1.8-2.0 Gy, was tested in clinical trials based on the hypothesized difference in fractionation sensitivity between late side-effects and some tumor types, which should allow escalation of the biologically equivalent tumor dose for a fixed equivalent dose for late effects. In locally advanced head and neck squamous cell carcinomas (HNSCC) two large phase III trials (European Organization for Research and Treatment of Cancer [EORTC] trial 22791[3], Radiation Therapy Oncology Group [RTOG] trial 9003 [4] demonstrated significant improvement in loco-regional tumor control without significant increase in late toxicity. RTOG launched a prospective randomized trial, RTOG 9512, in 1996 to test whether HFX improves local control for T2 glottic carcinoma relative to standard once a day fractionation (SFX). Secondary objectives were to estimate disease-free survival, overall survival and toxicity associated with each schedule.
METHODS
Previously untreated patients with biopsy-proven T2N0 glottic carcinoma signed a study-specific consent form and were randomly assigned to SFX or HFX, stratified by substage T2a vs T2b. All patients had modified AJCC Stage II tumor, performance status (KPS) ≥ 60, and no surgery except biopsy. Patients undergoing prior debulking or complete laser excision of the primary were ineligible. Cases were stratified by T-subcategory (T2a, extending above or below the vocal cord, or T2b, with impaired mobility) and randomized according to Zelen's principle[5] to HFX or SFX with a 1:1 allocation ratio.
Patients were evaluated during radiotherapy and 4 weeks later for acute toxicity. Tumor control and late effects were evaluated clinically by mirror exam or clinic endoscopy every 3 months through year 1, every 4 months through year 2, twice in year 3, then annually.
SFX consisted of 2 Gy per fraction, once a day to a total dose of 70 Gy in 35 fractions in 7 weeks. Two-dimensional RT using 2 or 3 co-planar portals was used. Field reduction at 50 Gy was permitted to reduce arytenoid dose. HFX consisted of 1.2 Gy per fraction, twice a day with a minimum interval of 6 hours, to a total dose of 79.2 Gy in 66 fractions in 6.5 weeks. Field reduction at 60 Gy was permitted. Beam energies were 4-6 MV or Cobalt-60. Portal dimension was generally 6×6 cm centered over the thyroid cartilage. Regional lymph nodes were not intentionally included, although some level II and III neck nodes were in the portals. Dose was prescribed at mid-depth along central axis with the gross target volume receiving at least 95% of the prescribed dose. Thin (2-5mm) bolus was used over the anterior larynx in patients with lesions involving the anterior commissure or patients with thin soft tissue anterior to the thyroid cartilage.
The primary endpoint was local control at 5 years. The protocol specified a target sample size of 240 patients based on detecting a 55% reduction in local failure, corresponding to an improvement in 5-year local control from 70% to 85%, or a hazard ratio (HR) of 0.456. Statistical design parameters were: overall significance level of 0.05 for the entire study, statistical power of 0.80, and three 2-sided significance tests. Two of them were interim tests after accruing 50% and 100% of the required sample size for possible early reporting if the nominal p<0.001. The third (final) test, with nominal significance level 0.048, occurred after all patients had been potentially followed for a minimum of 2 years. The sample size was increased by 10% to guard against patients retrospectively reclassified as ineligible. Local control rates were estimated using the cumulative incidence method[7] to account for the competing risk of death without local failure. Patients were censored for locoregional control after 5 years. Disease-free and overall survival rates were estimated with the Kaplan-Meier method[8]. The Cox proportional hazards model[9] with T-subcategory as a covariate was used to estimate and test the HR between the HFX and the SFX arms. HR<1 indicates a reduction in failure rate after HFX.
Patients without clinical complete response (CR), i.e., persistent local disease, in the primary site were classified as having a local failure (LF) and the time of failure is backdated to study day 1. Patients with CR who subsequently experienced local recurrence were considered a failure on the date of reported relapse; otherwise patients were censored at their last follow-up visit. Patients were considered to be at risk for failure until they died. Since the study population had no nodal disease at entry, the only difference between a local and a locoregional failure occurred for patients who had nodal progression.
Second primaries in the head and neck region, as reported by the treating institution and not reviewed by the study chair, were not considered a local failure. One patient on the SFX arm, classified by the treating institution as dying from the index (study) cancer without specific documentation of disease progression, was not considered a local failure.
Disease-free survival (DFS) included local recurrence or persistent local disease, nodal failure, distant metastasis, second primary tumor of all sites, or death from any cause. The date of DFS failure was the first occurrence of any of these events; otherwise patients are censored at their last follow-up visit. Patient-reported voice quality was not measured.
RESULTS
Between 1996 and 2003, 87 institutions enrolled 250 patients with T2 glottic cancer. Of the 250 enrolled, 11 patients were excluded from all the treatment comparisons (4 – SFX: 2 – nodal disease; 2 – restaged as T3-4; 7 – HFX: 3 – nodal disease; 3 – restaged as T3-4; 1 – withdrawn consent) leaving 239 patients to be analyzed. The median (range) follow-up for all surviving patients was 7.9 years (0.6 – 13.1). Pretreatment characteristics are well balanced between the trial arms (Table 1).
Table 1.
Pretreatment Characteristics
| Once/day RT (n=119) | Twice/day RT (n=120) | |
|---|---|---|
| Age (years) | ||
| Median | 65 | 64.5 |
| Min - Max | 34 - 88 | 28 - 91 |
| Q1 - Q3 | 57 - 73 | 57 - 73 |
| Gender | ||
| Male | 110 ( 92.4%) | 114 ( 95.0%) |
| Female | 9 ( 7.6%) | 6 ( 5.0%) |
| Race | ||
| White | 91 ( 76.5%) | 104 ( 86.7%) |
| Hispanic | 3 ( 2.5%) | 2 ( 1.7%) |
| Black | 21 ( 17.6%) | 11 ( 9.2%) |
| Oriental | 2 ( 1.7%) | 1 ( 0.8%) |
| Native American | 1 ( 0.8%) | 1 ( 0.8%) |
| Other | 1 ( 0.8%) | 1 ( 0.8%) |
| KPS | ||
| 50 | 1 ( 0.8%) | 0 ( 0.0%) |
| 60 | 3 ( 2.5%) | 1 ( 0.8%) |
| 70 | 5 ( 4.2%) | 2 ( 1.7%) |
| 80 | 13 ( 10.9%) | 15 ( 12.5%) |
| 90 | 63 ( 52.9%) | 69 ( 57.5%) |
| 100 | 34 ( 28.6%) | 33 ( 27.5%) |
| Primary site | ||
| Glottic larynx, NOS | 17 ( 14.3%) | 25 ( 20.8%) |
| Vocal cords | 102 ( 85.7%) | 95 ( 79.2%) |
| T stage | ||
| T2a | 74 ( 62.2%) | 74 ( 61.7%) |
| T2b | 45 ( 37.8%) | 46 ( 38.3%) |
Q1 = first quartile; Q3 = third quartile.
KPS = Karnofsky Performance Status.
NOS = not otherwise specified.
Quality assurance (RT fields, dose, tumor coverage) and compliance (treatment delivery) was performed on all cases. All patients randomized to SFX received it. Of patients randomized to HFX, 96 (80%) received two daily fractions on all days while 24 (20%) received one or more days with a single radiation fraction (18 patients – 1 or 2 days; 4 patients – 3 to 5 days; and 2 patients - > 5 days). Five patients had major unacceptable deviations from protocol prescription, 2 in SFX and 3 in HFX, due to dose and/or field size and/or fractionation.
Tables 2 and 3 show acute and late toxicity. A higher incidence of acute grade 3+ toxicity, primarily laryngeal edema, mucosal, and skin reactions, was seen with HFX than SFX (33.3% vs. 22.7%; p=0.084) but there was no difference in 5-year cumulative incidence of late grade 3+ toxicity, 8.5% after SFX and 8.5% after HFX (including 3 cases requiring tracheostomy after SFX and 2 cases after HFX).
Table 2.
Number of Patients with a Toxicity by Type and Grade - Acute Radiotherapy Toxicity
| Once/day RT (n=119) Grade |
Twice/day RT (n=120) Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Hematologic | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Skin | 45 | 56 | 6 | 0 | 0 | 44 | 55 | 13 | 0 | 0 |
| Mucous membrane/stomatitis | 28 | 36 | 5 | 0 | 0 | 19 | 46 | 10 | 0 | 0 |
| Salivary gland | 34 | 15 | 0 | 0 | 0 | 36 | 18 | 1 | 0 | 0 |
| Pharynx/esophagus | 35 | 53 | 4 | 0 | 0 | 30 | 64 | 4 | 0 | 0 |
| Larynx | 32 | 54 | 14 | 1 | 0 | 29 | 54 | 20 | 1 | 0 |
| Subcutaneous tissue | 5 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| Spinal cord | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Other neurological | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Upper GI | 11 | 5 | 0 | 0 | 0 | 10 | 9 | 1 | 0 | 0 |
| Bone | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Joint | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 16 | 2 | 1 | 0 | 0 | 10 | 7 | 1 | 0 | 0 |
Acute toxicity
RTOG/EORTC Acute Toxicity Grading
Table 3.
Number of Patients with a Toxicity by Type and Grade - Late Radiotherapy Toxicity
| Once/day RT (n=118) Grade |
Twice/day RT (n=119) Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Hematologic | 18 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 |
| Skin | 41 | 5 | 0 | 1 | 0 | 41 | 14 | 1 | 1 | 0 |
| Mucous membrane/stomatitis | 30 | 7 | 2 | 0 | 0 | 22 | 7 | 2 | 1 | 0 |
| Salivary gland | 42 | 7 | 0 | 0 | 0 | 34 | 11 | 1 | 0 | 0 |
| Pharynx/esophagus | 40 | 9 | 2 | 1 | 0 | 30 | 15 | 3 | 0 | 0 |
| Larynx | 51 | 28 | 5 | 4 | 0 | 54 | 33 | 3 | 3 | 0 |
| Subcutaneous tissue | 25 | 4 | 1 | 0 | 0 | 25 | 10 | 0 | 0 | 0 |
| Spinal cord | 16 | 2 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 |
| Other neurological | 14 | 1 | 0 | 0 | 0 | 16 | 1 | 0 | 0 | 0 |
| Upper GI | 18 | 0 | 1 | 0 | 0 | 13 | 1 | 0 | 0 | 0 |
| Bone | 16 | 0 | 0 | 0 | 0 | 13 | 1 | 0 | 0 | 0 |
| Joint | 16 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 |
| Other | 20 | 5 | 3 | 1 | 0 | 22 | 8 | 1 | 0 | 0 |
Late Toxicity
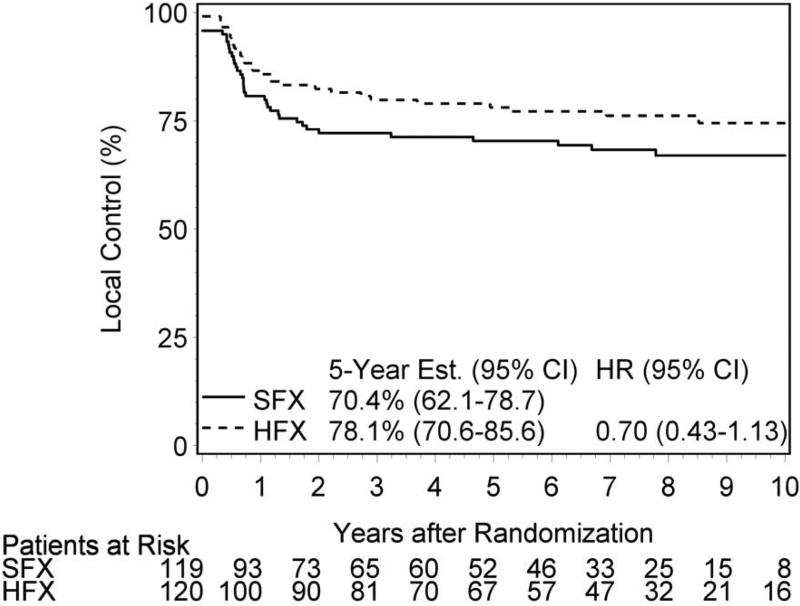
Figure 1 and Table 4 present local control, disease free survival and overall survival outcomes according to trial arm. Sixty-seven patients experienced local failure by 5-years (35 – SFX and 26 – HFX). Local control was 70% vs 78% for SFX and HFX (HR 0.70; p=0.14). In addition, ten patients had isolated nodal relapse as site of first failure (4 – SFX and 6 – HFX). Locoregional control was 67% vs 73% (HR 0.77; p=0.26). Of the 117 deaths, 25% were attributed to the study cancer, 20% to second primary cancers, 40% unrelated to cancer or treatment (co-morbid conditions) and 15% unknown.
Figure 1.
Local Control
Table 4.
Treatment Outcomes at 5 years
| Once/day RT (n=119) | Twice/day RT (n=120) | ||
|---|---|---|---|
| Local Control | 70% | 78% | p=0.14; HR 0.70 |
| Local-regional control | 67% | 73% | p=0.26; HR 0.77 |
| Disease-free survival | 40% | 49% | P=0.13; HR 0.79 |
| Overall survival | 63% | 72% | p=0.29; HR 0.82 |
An analysis of outcome by subcategory T2b versus T2a was conducted with stratification for trial arm. The hazard rate for local control was higher in T2b compared with T2a disease (5-year: T2b 70.0% vs. T2a 76.8%) HR 1.51 (0.93-2.44; p=0.10). Outcome was significantly worse in T2b disease for loco-regional control (5-year: T2b 63.3% vs. T2a 74.1%) (HR 1.65 (1.05-2.59); p=0.03), disease-free survival (5-year: T2b 31.4% vs. T2a 52.4%) (HR 1.62 (1.19-2.22); p=0.002) and overall survival (5-year: T2b 50.0% vs. T2a 77.5%) (2.06 (1.43-2.97); p=0.0001).
There were no signficiant differences in outcome by substage for local control for HFX vs SFX (HR: T2a 0.58 (0.30-1.12), p=0.11; T2b 0.87 (0.42-1.78), p=0.70; p-value for interaction 0.42), locoregional control (HR: T2a 0.61 (0.33-1.14), p=0.12; T2b 1.00 (0.52-1.93), p=0.99; p-value for interaction 0.28), disease-free survival (HR: T2a 0.74 (0.49-1.11), p=0.15; T2b 0.86 (0.54-1.38). p=0.54; p-value for interaction 0.63), or overall survival (HR: T2a 0.65 (0.39-1.08), p=0.10; T2b 1.06 (0.62-1.79), p=0.84; p-value for interaction 0.19)
DISCUSSION
RTOG 9512 is the only prospective trial of HFX conducted specifically in T2N0 glottic cancer. Results show an 8 point difference in local control (78% vs 70%) at 5 years favoring HFX (p=0.14), corresponding to a 30% reduction in the hazard rate (HR=0.70), as well as a trend for improved disease-free survival. There were no significant differences in outcomes between SFX and HFX by substage (T2a vs T2b). Acute toxicity was slightly higher with HFX, but very acceptable; there was no difference in severe late effects. Long term voice quality was not measured, but >85% of patients had only mild to moderate hoarseness or edema by toxicity critieria. This was achieved with conventional two-dimensional radiotherapy techniques. Current IMRT techniques might reduce might enhance outcomes further but data have not been reported in early glottic cancers.
The rationale for hyperfractionation for our trial was drawn from two large fractionation studies enrolling mostly non-larynx cases, EORTC 22791 and RTOG 90-03 [3,4](Horiot; Beitler in press). Direct comparison with those trials is problematic: the former was limited to oropharynx, and the latter was in advanced disease. Nonetheless, the hazard ratio in RTOG 9512 at 0.70 compares favorably with these trials (HR=0.68; HR=0.81, respectively) as well as the meta-analysis estimates for hyperfractionation by Bourhis et al.[10] at 0.77. Since the design and conduct of this trial, other fractionation schedules have been tested and implemented in early stage glottic cancer.
Accelerated fractionation using 225 cGy per fraction (slight hypo-fractionation) also appears effective at increasing local control with acceptable toxicity. Yamazaki et al. showed a significant benefit from accelerated fractionation in a randomized trial of 180 T1 glottic carcinomas using 2.25 Gy per fraction to 63-66Gy (LC: 92 vs 77%; p=0.004) [11](Yamazaki 2006). Moon et al [12](Moon 2013) also compared 225 cGy fractions to 63-67.5 Gy for T1-2 glottic cancers in a 156 patient randomized trial. Local-progression-free survival was 11 points higher (89% vs 78%) in the hypofractionated arm, but the study, like our trial was under-powered to show significance (p=0.213). Hliniak randomized 395 patients with T1-T3, N0 glottic and supraglottic cancers to 66 Gy in 33 fractions over 45 days or accelerated treatment of 66Gy in 38 days by delivering BID on Thursdays. There was no benefit in overall locoregional control (p=0.37), but in the subset of glottic cancers (292 patients) LRC was higher (p=0.04) [13](Hliniak 2002). These studies, including ours, also highlight the challenge of conducting randomized trials in H&N subsites with small patient numbers. DAHANCA 6 and 7 randomized 908 larynx (690 glottic; 218 supraglottic) cancers to 66 Gy at 2 gy per fraction accelerated (in 5.5 weeks) vs conventional (6.5 weeks). Five-year larynx preservation was 80% vs 68% (p=0.007) [14](Overgaard 2003).
Retrospective analysis at several centers have found 225 cGy fractions over 25-28 treatments produce excellent outcomes. Chera reviewed 585 T1-2 patients, found overall treatment time to be a significant factor and better outcomes with 225 cGy fractions [15](Chera 2010). Le found fraction size, total dose and overall time impacted control of 83 T2 glottic cancers [16](Le 1997). Garden found approximately 80% local control in 228 T2 glottic cancers with both hyperfractionation and hypofractionation [17](Garden 2003). Severe late effects in these series are low (2-8 %) despite a slightly larger fraction size, due to the small volume irradiated and slightly lower total dose. Reports from these centers indicate 225cGy per fraction is their preferred treatment for T2 glottic cancers.
Outcomes with concurrent chemotherapy in early stage glottic cancer have not been reported. However, due to less than optimal outcomes in T2b tumors, concurrent chemotherapy may be considered [15](Chera 2010). Surgical techniques for early larynx cancers have also evolved with greater use of lazer resection with good tumor control and acceptable voice quality [18](Canis 2013)
The possible prognostic importance of impaired cord mobility in T2 glottic cancer[19] was evaluated by McCoul and Har-El[20] who pooled data from 21 reports and found a statistically better outcome in T2a disease. Our data confirm a prognostic difference based on substage, including a trend for better local control in T2a vs T2b disease, and significant differences in locoregional control, disease-free survival, and overall survival, but no differential effect by fractionation. We encourage the American Joint Committee on Cancer to consider T2a vs. T2b substaging of glottic carcinoma for inclusion in the Cancer Staging Manual.
CONCLUSION
RTOG 9512 tested an HFX schedule in T2 glottic tumors similar to schedules tested in more locally advanced disease in RTOG 9003 and EORTC 22791. Although the difference between treatment arms did not reach statistical significance, the trial outcome is consistent with gains observed for hyperfractionation in more locally advanced disease, enhancing local control by 8 points. This was achieved with low acute and late toxicity. Other effective and more convenient fractionation schedules in the management of early glottic cancer include hypofractionation at 225cGy per fraction. Outcomes in patients with T2b tumors remain suboptimal and may benefit from concurrent chemotherapy.
SUMMARY.
250 patients with T2 vocal cord cancer were randomly assigned to hyperfractionation (HFX) or standard fractionation (SFX). Five-year local control was modestly, but not significantly, higher with HFX (78% vs 70%; p=0.14). Results are consistent with prior studies of hyperfractionation showing a benefit in local control. Substaging by T2a vs T2b carries prognostic value for DFS and OS. For cost and convenience reasons other altered fractionation schedules (e.g., 225/fraction) have been adopted in routine practice.
Acknowledgments
Supported by: NCI grant # U10CA021661
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Amdur RJ, Morris CG, et al. T1-t2n0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029–4036. doi: 10.1200/JCO.2001.19.20.4029. [DOI] [PubMed] [Google Scholar]
- 3.Horiot JC, Le Fur R, N'Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: Final analysis of a randomized trial of the eortc cooperative group of radiotherapy. Radiotherapy and Oncology. 1992;25:231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 4.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of rtog 90-03: A randomized trial of altered fractionation radiation for locally advanced head and neck cancer. International Journal of Radiation Oncology Biology Physics. 2014 doi: 10.1016/j.ijrobp.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 6.Cox JD, Stetz J Pajak TF. Toxicity criteria of the radiation therapy oncology group (rtog) and the european organization for research and treatment of cancer (eortc). International Journal of Radiation Oncology Biology Physics. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley and Sons; New York: 1980. [Google Scholar]
- 8.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Society C. 1958;53:457–481. [Google Scholar]
- 9.Cox DR. Regression models and life-tables (with discussion). Journal of the Royal Statistical Society B. 1972;34:178–220. [Google Scholar]
- 10.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (t1n0m0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Moon SH, Cho KH, Chung EJ, et al. A prospective randomized trial comparing hypofractionation with conventional fractionation radiotherapy for t1-2 glottic squamous cell carcinomas: Results of a korean radiation oncology group (krog-0201) study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013 doi: 10.1016/j.radonc.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Hliniak A, Gwiazdowska B, Szutkowski Z, et al. A multicentre randomized/controlled trial of a conventional versus modestly accelerated radiotherapy in the laryngeal cancer: Influence of a 1 week shortening overall time. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2002;62:1–10. doi: 10.1016/s0167-8140(01)00494-7. [DOI] [PubMed] [Google Scholar]
- 14.Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: Dahanca 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 15.Chera BS, Amdur RJ, Morris CG, et al. T1n0 to t2n0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:461–466. doi: 10.1016/j.ijrobp.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 16.Le QT, Fu KK, Kroll S, et al. Influence of fraction size, total dose, and overall time on local control of t1-t2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:115–126. doi: 10.1016/s0360-3016(97)00284-8. [DOI] [PubMed] [Google Scholar]
- 17.Garden AS, Forster K, Wong PF, et al. Results of radiotherapy for t2n0 glottic carcinoma: Does the “2” stand for twice-daily treatment? Int J Radiat Oncol Biol Phys. 2003;55:322–328. doi: 10.1016/s0360-3016(02)03938-x. [DOI] [PubMed] [Google Scholar]
- 18.Canis M, Martin A, Ihler F, et al. Transoral laser microsurgery in treatment of pt2 and pt3 glottic laryngeal squamous cell carcinoma - results of 391 patients. Head Neck. 2013 doi: 10.1002/hed.23389. [DOI] [PubMed] [Google Scholar]
- 19.Ang KK, Peters LJ. Vocal cord cancer: 2b worse than not 2b? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1990;18:365–366. doi: 10.1016/0167-8140(90)90117-f. [DOI] [PubMed] [Google Scholar]
- 20.McCoul ED, Har-El G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in t2 glottic carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135:479–486. doi: 10.1001/archoto.2009.47. [DOI] [PubMed] [Google Scholar]