Sedentary lifestyle, a key risk factor for multiple adverse health outcomes, is rapidly becoming a worldwide phenomenon. Studies have shown that physical exercise significantly reduces metabolic risks associated with obesity, type 2 diabetes, and cardiovascular-related diseases as it improves insulin sensitivity in major insulin-target tissues, predominantly skeletal muscle (1–4). The mechanisms underlying the beneficial effects of exercise on skeletal muscle insulin sensitivity are not fully understood and have been the focus of numerous research efforts in the field.
The AMP-activated protein kinase (AMPK) is a master regulator of fuel metabolism and insulin action in muscle (5–7). Acute and long-term exercise training potently stimulate the kinase activity of AMPK in skeletal muscle of humans and rodents (8). The extent of AMPK activation depends on the intensity and duration of the exercise and is strongly associated with increased insulin sensitivity (9–10). Evidence from AMPK knockout mouse models showed that suppression of AMPK activity in skeletal muscle resulted in impaired muscle contraction and voluntary running capacity, suggesting a necessity for AMPK signaling in muscle physiological function (11–12). Importantly, activation of AMPK was proposed as a regulatory mechanism that underlies exercise-induced glucose uptake in muscle, thereby leading to increased systemic insulin sensitivity (13–14). Yet, the downstream signaling pathways by which exercise-induced AMPK regulates the metabolic actions of insulin have not been elucidated.
Autophagy is an evolutionarily conserved lysosomal catabolic pathway that plays a key role in skeletal muscle metabolism (15). Under normal physiological conditions, autophagy operates at basal level and maintains cellular homeostasis by recycling defective proteins and malfunctioned organelles. However, during the course of physical exercise the autophagic process is strongly induced. Stimulation of autophagy serves to sustain the high-energy demands of the muscles during exercise by promoting the degradation of macromolecules and subcellular organelles by autophagosomes and lysosomes. This releases useful substrates such as amino acids that can be reused as an energy source. Importantly, a recent study found that autophagy regulates muscle glucose homeostasis and increases insulin sensitivity in response to physical exercise (16). It thus appears that autophagy could be a central player of exercise-induced insulin sensitivity in skeletal muscle. However, whether the enhanced cellular catabolism orchestrated by autophagy during physical exercise contributes to exercise-induced insulin sensitivity is an intriguing unanswered question.
A potential link between exercise and autophagy has been suggested in the context of AMPK action (17–18). One of the first observations for the involvement of AMPK in the autophagic action emerged from a study in the yeast Saccharomyces cerevisiae in which mutation of the AMPK ortholog SNF1 gene led to defective fuel metabolism. Genetic screening analysis revealed that the ATG1 and ATG13 genes, both of which are involved in the autophagy signaling cascade, may be regulated by SNF1 (19). Follow-up studies showing that inhibition of AMPK activity by compound C or overexpression of dominant negative AMPK completely inhibits autophagy lead to the conclusion that AMPK functions as an upstream activator of autophagy in mammalian cells (20). Further study identified the ATG1/ULK1 as an AMPK substrate. Indeed, AMPK directly interacts and phosphorylates ATG1/ULK1, leading to ATG1/ULK1 activation. As a result, downstream signaling of autophagy is activated (19). These findings raise the question of whether autophagy mediates some of the metabolic functions of AMPK in response to physical activity.
The study led by Liu and colleagues investigated the role of AMPK in the signaling mechanism underlying the effects of exercise on muscle autophagy and insulin sensitivity (21). The authors demonstrated that a single-bout exercise is sufficient to initiate autophagy in skeletal muscles and this was highly associated with AMPK activation. By studying muscle-specific AMPK alpha2 deficient mice, the authors also found that deficiency of AMPK alpha2 impaired stimulation of autophagy in skeletal muscle during physical exercise, suggesting that AMPK activation is necessary for exercise-induced autophagic response in skeletal muscle. Furthermore, the current study revealed that activation of autophagy increased glucose uptake in C2C12 myotubes whereas inhibition of autophagy suppressed it. On the basis of these findings, the authors concluded that 1. AMPK alpha 2 mediates exercise’s effects to stimulate muscle autophagy and that muscle autophagy is involved in the regulation of glucose metabolism and insulin sensitivity in insulin-target cells/tissues. These observations are in agreement with a previous report by He and colleagues, showing that muscle autophagy contributes to the beneficial metabolic effect of exercise (22). He at al. found that autophagy, induced by exercise, involves disruption of the BCL2–beclin-1 complex, an important mediator of autophagy signaling. They created a knock-in mouse carrying mutations in the BCL2 phosphorylation sites, which prevent stimulus-induced disruption of the BCL2–beclin-1 complex. It is thus hypothesized that the ability of exercise to induce autophagy is impaired in metabolically active organs such as skeletal muscle. As expected, they found that long-term exercise failed to overcome high-fat diet-induced glucose intolerance in in the BCL2 mutant mice, further highlighting an essential role for autophagy in the beneficial metabolic effect of exercise in insulin-resistant state.
The fact that activation of AMPK mediates exercise-induced autophagy and insulin sensitivity brings into question whether other modulators such as Sestrins are involved in the regulation of AMPK activation. Sestrins, a family of evolutionarily conserved stress-inducible proteins with anti-oxidant properties, were initially identified by screening for new p53 activated genes (23). Emerging data from several laboratories suggested a new role for Sestrins in the activation of the AMPK pathway, although the mechanism involved is unclear yet. Interestingly, Sestrins also suppressed the mTOR signaling cascade through AMPK dependent (24) or independent pathway (25). In response to metabolic stresses, including nutrient depletion, Sestrins were found to promote autophagy in mammalian cells (26). In fact, according to Liu et al, Sestrins is involved in the regulatory mechanism underlying exercise-induced AMPK activity in skeletal muscle (18). Support for this comes from the findings that exercise leads to a significant increase in the expression of Sestrins and the physical interaction between Sestrins and AMPK in skeletal muscles. These observations propose a new hypothesis that Sestrins are novel mediators of exercise-induced autophagy signaling in muscle, which is highly linked with insulin sensitivity. Overall, Liu et al integrate exercise, muscle AMPK activation, autophagy and insulin-action into a unified signaling pathway, exercise→AMPK/Sestrins→autophagy→insulin sensitivity (Figure 1).
Figure 1. A potential mechanism by which exercise increases insulin sensitivity via the AMPK/Sestrin-autophagy signaling pathway.
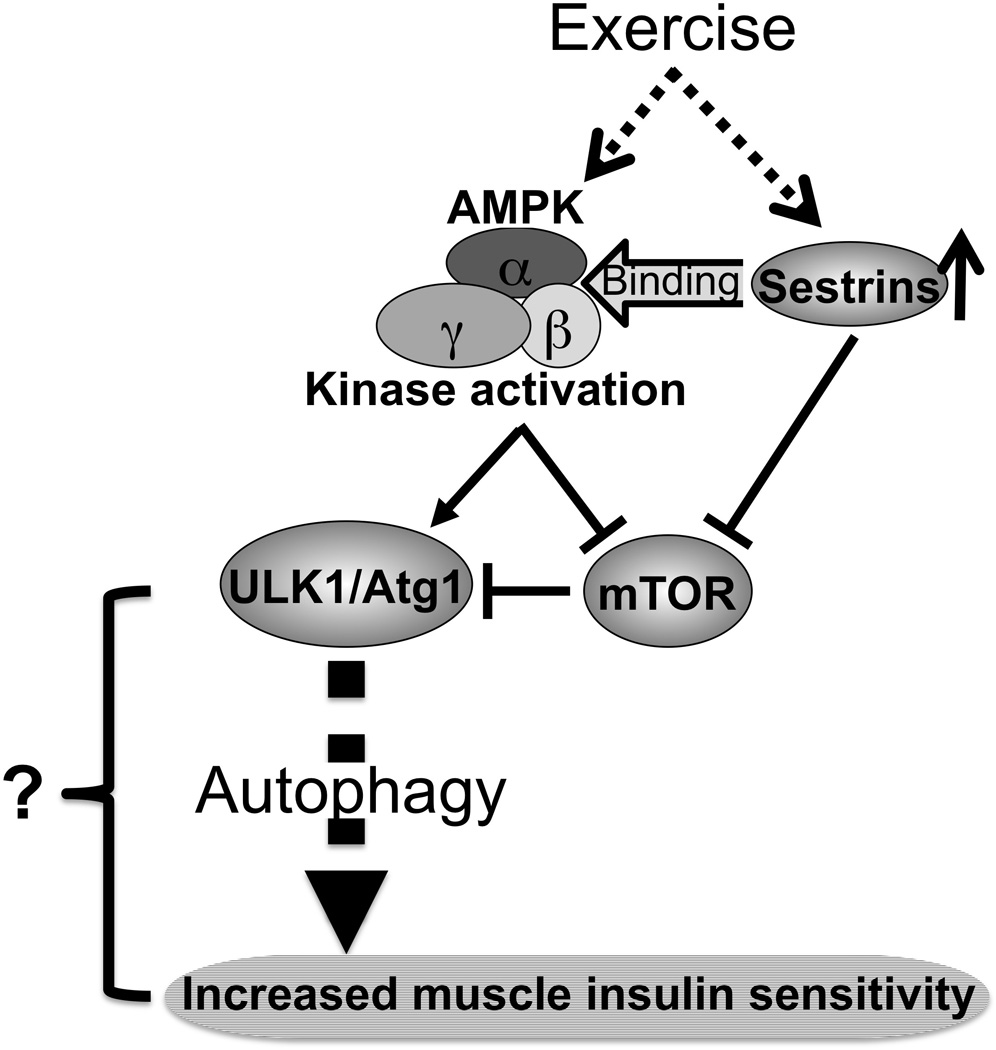
Exercise stimulates the kinase activity of AMPK and the expression of Sestrins in muscle, leading to increased molecular interaction between Sestrins and AMPK. Subsequently, AMPK rapidly stimulates ULK1/Atg1 activation either by suppressing mTOR signaling which results in dis-inhibition of ULK1/Atg1, or by phosphorylating ULK1/Atg1 directly. AMPK-mediated activation of ULK1/Atg1 induces autophagy via yet unidentified mechanisms. As a result, muscle insulin sensitivity is increased.
Highlights.
Exercise increases the kinase activity of AMPK in skeletal muscle.
Exercise induces the expression of Sestrins in skeletal muscle.
The molecular interaction between APMK and Sestrins is increased by physical exercise.
AMPK activation is necessary for exercise-induced autophagic response in skeletal muscle.
A new signaling pathway, exercise→AMPK/Sestrins→autophagy→insulin sensitivity, is proposed.
The current study raises a number of unanswered questions in the context of AMPK biology, in conjunction with physical exercise.
Outstanding questions;
Does autophagy require AMPK-induced glucose uptake and exercise-induced insulin sensitivity?
What are the molecular mechanisms by which AMPK or Sestrins regulates autophagy?
What are the mechanisms underlying the autophagy-induced insulin sensitivity? Which proximal components of autophagy signaling are involved in this regulation?
Does exercise-induced interaction between Sestrins and AMPK regulate autophagy activation?
Does the AMPK/Sestrine complex affect specificity of AMPK-dependent phosphorylation that controls autophagy signaling during physical exercise?
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01DK083567 to Y.B.K).
List of abbreviation
- AMPK
AMP-activated protein kinase
- ATG1/13
autophagy related 1/13
- ULK1
unc-51 like autophagy activating kinase 1
- BCL2
B-cell lymphoma 2
- mTOR
mammalian target of rapamycin
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Blair SN, Kohl HW, Barlow CE, et al. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 2.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 3.Church TS, LaMonte MJ, Barlow CE, et al. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 4.Fenicchia LM, Kanaley JA, Azevedo JL, et al. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism. 2004;53:284–289. doi: 10.1016/j.metabol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Kahn BB, Alquier T, Carling D, et al. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pimentel GD, Ropelle ER, Rocha GZ, et al. The role of neuronal AMPK as a mediator of nutritional regulation of food intake and energy homeostasis. Metabolism. 2013;62:171–178. doi: 10.1016/j.metabol.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinberg GR, Jørgensen SB. The AMP-activated protein kinase: role in regulation of skeletal muscle metabolism and insulin sensitivity. Mini Rev Med Chem. 2007;7:519–526. doi: 10.2174/138955707780619662. [DOI] [PubMed] [Google Scholar]
- 10.Nakano M, Hamada T, Hayashi T, et al. α2 isoform-specific activation of 5'adenosine monophosphate-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-β-D-ribonucleoside at a physiological level activates glucose transport and increases glucose transporter 4 in mouse skeletal muscle. Metabolism. 2006;55:300–308. doi: 10.1016/j.metabol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill HM, Maarbjerg SJ, Crane JD, et al. AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A. 2011;108:16092–16097. doi: 10.1073/pnas.1105062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantier L, Fentz J, Mounier R, et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 2014;28:3211–3224. doi: 10.1096/fj.14-250449. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill HM. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab J. 2013;37:1–21. doi: 10.4093/dmj.2013.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshinaka K, Kawasaki E, Hokari F, et al. Effect of acute high-intensity intermittent swimming on post-exercise insulin responsiveness in epitrochlearis muscle of fed rats. Metabolism. 2009;58:246–253. doi: 10.1016/j.metabol.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam BT, Siu PM. Autophagic cellular responses to physical exercise in skeletal muscle. Sports Med. 2014;44:625–640. doi: 10.1007/s40279-013-0140-z. [DOI] [PubMed] [Google Scholar]
- 17.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Wilson WA, Fujino MA, et al. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol. 2001;21:5742–5752. doi: 10.1128/MCB.21.17.5742-5752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meley D, Bauvy C, Houben-Weerts JH, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Niu Y, Yuan H, et al. AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism. doi: 10.1016/j.metabol.2015.01.015. (this editorial related paper) [DOI] [PubMed] [Google Scholar]
- 22.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budanov AV, Sablina AA, Feinstein E, et al. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 24.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmigiani A, Nourbakhsh A, Ding B, et al. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014;9:1281–1291. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiuri MC, Malik SA, Morselli E, et al. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle. 2009;8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]


