Abstract
Neurogenesis continues in the human subventricular zone and to a lesser extent in the hippocampal subgranular zone throughout life. Subventricular zone-derived neuroblasts migrate to the olfactory bulb where survivors become integrated as interneurons and are postulated to contribute to odor discrimination. Adult neurogenesis is dysregulated in many neurological, neurovascular and neurodegenerative diseases. Alcohol abuse can result in a neurodegenerative condition called alcohol-related brain damage. Alcohol-related brain damage manifests clinically as cognitive dysfunction and the loss of smell sensation (hyposmia) and pathologically as generalized white matter atrophy and focal neuronal loss. The exact mechanism linking chronic alcohol intoxication with alcohol-related brain damage remains largely unknown but rodent models suggest that decreased neurogenesis is an important component. We investigated this idea by comparing proliferative events in the subventricular zone and olfactory bulb of a well-characterized cohort of 15 chronic alcoholics and 16 age-matched controls. In contrast to the findings in animal models there was no difference in the number of proliferative cell nuclear antigen-positive cells in the subventricular zone of alcoholics (mean ± SD = 28.7 ± 20.0) and controls (27.6 ± 18.9, p = 1.0). There were also no differences in either the total (p = 0.89) or proliferative cells (p = 0.98) in the granular cell layer of the olfactory bulb. Our findings show that chronic alcohol consumption does not affect cell proliferation in the human SVZ or olfactory bulb. In fact only microglial proliferation could be demonstrated in the latter. Therefore neurogenic deficits are unlikely to contribute to hyposmia in chronic alcoholics.
Keywords: Chronic alcoholism, Human brain tissue, Proliferation, Adult neurogenesis
Introduction
Alcohol abuse is the world’s third leading cause of disease and disability (World Health Organisation, 2011). In the United States 6.7% of the population over 12 years of age were classified as heavy drinkers while 23% of the population had participated in binge drinking (Substance Abuse and Mental Health Services Administration, 2010). In Australia, 13% of all adults currently indulge in high-risk alcohol consumption (Australian Bureau of Statistics, 2006) and the societal cost is estimated to be $15 billion per annum (Collins and Lapsley, 2008).
One of the more serious consequences of chronic alcohol consumption is alcohol-related brain damage (ARBD). ARBD is characterized by impairment of cognitive functions (Green et al., 2010), including working memory deficits (Pfefferbaum et al., 2001) and smell sensation (hyposmia) (Rupp et al., 2003). On postmortem examination the brains of chronic alcoholic are mildly atrophic, largely due to white matter (WM) deficits (Harper et al., 1988; Pfefferbaum et al., 2009), but also focal neuronal loss (Harper et al., 1987). Previous work from our group has shown cortical neuronal loss from the prefrontal cortex of alcoholics, whereas other areas such as the primary motor cortex show no neuronal loss (Kril et al., 1997). Fundamental questions remain about how the chronic abuse of alcohol leads to ARBD, an area that is clouded by the concomitant effects of alcohol on other organs such as the liver, on nutrition, on risk-taking behavior potentially associated with head injury and the close association of alcoholism with other lifestyle choices such as smoking (Brust, 2010).
Adult mammalian neurogenesis, a recently identified paradigm in the adult human brain, occurs in the subgranular zone (SGZ) of the hippocampus and subventricular zone (SVZ) of the wall of the lateral ventricle (Eriksson et al., 1998). Neuroblasts derived from the SVZ migrate tangentially to the olfactory bulb (OB), via the rostral migratory stream (RMS), where most of the survivors become integrated into the granule cell layer (GCL) as interneurons (Altman, 1969; Doetsch and Alvarez-Buylla, 1996; Lois and Alvarez-Buylla, 1994). Animal studies suggest that these interneurons contribute to odor discrimination (Enwere et al., 2004; Sakamoto et al., 2011).
Neurogenesis is known to be dysregulated in neurological (epilepsy) (Liu et al., 2008), psychiatric (depression) (Lucassen et al., 2010), neurovascular (stroke) (Jin et al., 2006; Marti-Fabregas et al., 2010) and neurodegenerative diseases (reviewed by Curtis et al.(Curtis et al., 2007a) and Thompson et al. (Thompson et al., 2008)). The latter include Alzheimer’s (Crews et al., 2010), Huntington’s disease (HD) (Curtis et al., 2003, 2005) and possibly Parkinson’s disease (Hoglinger et al., 2004; van den Berge et al., 2011).
ARBD is also a neurodegenerative disease and animal models of both binge and chronic alcohol consumption have shown a decrease in hippocampal (SGZ) (He et al., 2005; Nixon and Crews, 2002; Taffe et al., 2010) and SVZ neurogenesis (Hansson et al., 2010), although a further study suggested that chronic alcohol exposure only decreased SGZ neurogenesis with no effect on the OB (Herrera et al., 2003).
Recent studies showing minimal neurogenesis in the adult human SGZ (Low et al., 2011; Lucassen et al., 2010) combined with work from our own laboratory showing no hippocampal neuron loss in chronic alcoholics (Harding et al., 1997) suggests that SGZ neurogenesis is unlikely to play a major role in ARBD.
In contrast there are relatively high levels of proliferation in the human SVZ (Low et al., 2011) and chronic alcohol consumption could well reduce adult born interneurons in the OB and contribute to the hyposmia associated with ARBD. Here we test this hypothesis by comparing proliferative events in the SVZ and OB of 15 chronic alcoholics and 16 controls.
Materials and methods
Case selection
Prior approval for this project was obtained from the Human Research Ethics Committee of the University of Sydney (HREC #13027). Tissue was obtained from the New South Wales Tissue Resource Centre (NSW TRC), a member of the Australian brain Bank Network and part-funded by the National Institute on Alcohol Abuse and Alcoholism (R24AA012725) to provide brain tissue for alcoholism research. The brain donor program of the NSW TRC is approved by the University of Sydney’s Human Research Ethics Committee (HREC #X11-0107) and their banking procedures have been previously described (Sheedy et al., 2008). NSW TRC supplied information on age, gender, cause of death, post-mortem interval (PMI), brain pH, alcohol consumption, smoking status, liver pathology and macro- and microscopic neuropathology. The latter included a detailed assessment for metabolic (hepatic) encephalopathy (HE)(Harris et al., 2008), scored as none, mild (1 or more Alzheimer Type II astrocytes (At2a) per 200× field), moderate (2–3 At2a per field) or severe (mostly At2a). Lifestyle factors, including alcohol consumption and a Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) diagnosis were based on next of kin questionnaires and the available medical records. Mean daily alcohol consumption rates were recorded as grams of ethanol per day based on the number of standard drinks using Australian National Health and Medical Research Council guidelines (http://www.nhmrc.gov.au/your-health/alcohol-guidelines). Lifetime consumption (kg) was calculated using mean daily consumption rate × number of years drinking × 365. Chronic alcoholics were defined as those individuals who had consumed greater than 80 g of alcohol (ethanol) per day for the majority of their adult life (usually >30 years) while controls had consumed less than 20 g of alcohol per day. Cigarette use was described in mean pack years where 1 mean pack year equals 1 packet of (20) cigarettes per day for one year.
Immunohistochemistry
Tissue blocks (20 mm × 15 mm × 3 mm) incorporating the wall of the lateral ventricle and corpus callosum at the level of the head of the caudate were obtained from fixed brain slices of 15 alcoholics and 16 controls. The 3 mm slices had been previously stored in 15% formalin for periods ranging from 4 to 11 years. Blocks of tissue were cryoprotected for two days in 30% sucrose in 0.1 M TBS (pH 7.4) with 0.1% (v/v) sodium azide, before embedding in OCT compound (Tissue-Tek, Torrance, CA, USA), freezing using CO2 and sectioning into thick (48 µm) sections using a freezing microtome (Leica microsystems, Wetzlar, Germany). Thick sections were also cut from the available olfactory bulbs (OB) of eight controls and five alcoholics. Antigen retrieval for PCNA immunohistochemistry was performed on free-floating thick sections incubated overnight at 60 °C in 10 mM sodium citrate (pH 8.5). Sections were then incubated in 50% ethanol containing 1% (v/v) hydrogen peroxide for 30 min, followed by 10% normal horse serum (NHS) in 0.1 M TBST containing 0.1% Triton X-100 for 30 min. The PCNA primary antibody (PC-10; sc-56, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 1:750 (or 1:1000 for some OB sections) was applied for 1 h at room temperature (RT), then overnight at 4 °C, followed by 1 h at RT. The sections were incubated in a biotinylated secondary antibody (1:200; Vector Laboratories Inc., Burlingham, CA,) for 1 h, an avidin/biotin/peroxidase complex (1:100; Vectastain Elite ABC, Universal) for 1 h and diaminobenzidine (DAB) in the presence of 5% hydrogen peroxide for 20 min. In addition 7 µm thick sections of the middle lateral ventricle wall (level of nucleus accumbens) were cut from a formalin-fixed paraffin-embedded (FFPE) block of one of the controls (64-year old male) where the tissue had been fixed for three weeks before embedding. The thin sections were immunostained for PCNA and a second proliferative marker Ki-67 (MIB1, M7240, Dako, Carpinteria, CA). Here a harsher antigen retrieval regime was used with sections in 10 mM Tris/1 mM EDTA (TE buffer; pH 9) heated to 120 °C for 30 min in a decloaking chamber (Biocare Medical, Concord, CA) and incubated with PCNA (1:750) or Ki-67 (1:100). Cresyl violet (CV) and luxol fast blue (LFB) staining of thick and thin sections from selected individuals was performed according to standard protocols to demonstrate the cytoarchitecture.
FFPE thin sections were also cut from an OB of an additional neurologically normal control (49-year old male). This OB had been fixed for eight weeks before embedding. Antigen retrieval was performed in 10 mM Tris 1 mM EDTA buffer pH 9.0 using a decloaking chamber set at 95 °C for 20 min. The neuronal density in the GCL was investigated using the pan-neuronal marker NeuN (rabbit polyclonal (ABN78), 1:200, Merck Millipore, Billerica, USA) as described above.
Immunofluorescent microscopy experiments were also performed on these OB FFPE thin sections. PCNA antibody was used in a cocktail with either beta III tubulin (rabbit polyclonal, 1:250, GR79976, Abcam, Cambridge, UK), GFAP (glial fibrillary acidic protein; rabbit polyclonal, 1:200, Z0334, Dako), Iba1 (ionized calcium-binding adapter molecule 1, rabbit polyclonal (019–19741) 1:150, Wako Chemicals USA Inc), Olig2 (rabbit polyclonal, 1:100, GR80025-1, Abcam), NG2 (a chondroitin sulfate proteoglycan, rabbit polyclonal, 1:200, AB5320, Merck Millipore) or NeuN (1:100) antibodies.
Sections were incubated with the secondary antibodies: AlexaFluor 488, goat anti-rabbit IgG, A-11008 and AlexaFluor 594, goat anti-mouse IgG, A-11005 (Invitrogen) at 1:200 dilution, for 30 min. Sections were then incubated in 0.1% sudan black B (B.D.H Laboratory Chemicals Group) in 70% ethanol for 4 min, and counterstained with DAPI (3 µM; Invitrogen, D306). Sections were viewed with a fluorescent microscope (Olympus AX-70) and images taken with an Olympus DP70 camera and imported using DP controller v2.2 software. Images were also obtained using confocal microscopy (Leica SPE-II microscope with Leica LAS AF software platform, Leica Microsystems, Wetzlar, Germany) at the Advanced Microscopy Facility, Bosch Research Institute, University of Sydney. Contrast and brightness were minimally altered using GNU Image Manipulation Program (GIMP) v2.8 and co-localization images were created using either DP Manager v2.2 or Leica LAS AF software (confocal images). Images were annotated in Photoshop CS5 (Adobe) and GIMP v2.8.
Quantification
The combination of rare events and poorly defined boundaries meant that standard stereological methods could not be applied here. For the SVZ, the research scientist was blinded to case status and an inter-rater reliability of less than 5% was initially established with an experienced neuropathologist. Manual counting was performed on an Olympus BH-2 (Olympus Optical Co. Japan) using an eyepiece graticule at 200× magnification. All PCNA-positive cells beneath a 0.5 mm long strip of the ependymal layer were quantified in the dorsal, middle and ventral regions of the rostral SVZ and the mean PCNA count calculated (adapted from Curtis et al., 2005). The ventral region was torn during preparation in approximately 50% of the sections and in these cases the mean PCNA count was determined from the dorsal and middle regions only.
Olfactory bulbs
The GCL was identified based on its medial location in the OB and its morphology of irregularly arranged (clumped) but densely packed micro-nuclear cells. Care was taken to avoid the anterior olfactory nuclei that can be present within the OB in humans (Smith et al., 1993). As pan-neuronal markers such as NeuN and MAP2 are poorly detected by immunostaining in medium to long-term fixed brain tissue (Lyck et al., 2008) total cells were counted on cresyl violet-stained thick sections using an eyepiece graticule at 1000× magnification (total area per field = 0.01 mm2). As a high neuron:glial ratio had been previously reported for the murine GCL (Parrish-Aungst et al., 2007) total cells in the GCL were used as a surrogate for neurons. PCNA-positive cells were counted at 200× magnification (total area per field = 0.25 mm2). All cells within a graticule were counted unless they were associated with capillaries and where possible, three areas were counted for each bulb (rostral, middle and caudal) and three counts of non-overlapping graticules were performed for each area. The mean cell counts per mm2 were converted to mm3 by dividing by the thickness of the sections (approximately 0.05 mm). Due to the rarity of events, counting in the OB was performed by two research scientists and PCNA-positive cells reached by consensus.
Statistical analysis
The software program JMP 8 (SAS Institute Inc, Cary, US) was used to perform all statistical analyses with a p-value < 0.05 accepted as the level of significance with no corrections made for multiple comparisons. Group differences were determined using analysis of variance or Chi2 analysis. A stepwise regression analysis was also used to investigate the relationship between SVZ PCNA-positive cells and potential confounders such as fixation period, liver disease, HE status and smoking pack years.
Results
Our aim was to determine whether chronic alcohol exposure has a deleterious effect on proliferation in the origin (SVZ) and destination (OB) of adult-born neurons in the human brain. A reduction in proliferation was expected given the decreased neurogenesis seen in animal models of chronic alcohol intoxication.
High alcohol, cigarette consumption and liver disease often co-exist in chronic alcoholics
There were no differences in gender, mean postmortem interval (PMI), brain pH, tissue fixation time, or brain weights between the cases and controls (Table 1). However, along with alcohol consumption, cigarette use and presence of severe liver disease was significantly higher in alcoholics. Most of the alcoholics in the study were current smokers (n = 14) and on average had smoked four-fold more cigarettes than controls. Moderate or severe liver pathology was seen in 8/15 alcoholics, compared to only moderate pathology in three controls while 7/15 alcoholics had neuropathological changes consistent with mild or moderate hepatic encephalopathy (HE) (Harris et al., 2008). The Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for alcohol dependence was met by 9/15 alcoholics, while the remainder met the less severe criteria for alcohol abuse (American Psychiatric Association, 2000).
Table 1.
Demographic and clinical factors.
| Characteristics (±SD) | Alcoholics (n = 15) |
Controls (n = 16) |
p-value |
|---|---|---|---|
| Mean age (years) | 55.2 (8.8) | 54.0 (9.2) | 0.71 |
| Gender M/F | 13/2 | 10/6 | 0.12a |
| Mean PMI | 28.5 (9.8) | 31.5 (16.0) | 0.52 |
| Mean brain weight (g) | 1398 (148) | 1388 (149) | 0.96 |
| Mean brain pH | 6.5 (0.29) | 6.6 (0.27) | 0.96 |
| Mean fixation period (months) | 20.4 (10.7) | 26.4 (27.3) | 0.43 |
| Severity of liver pathologyb (0, 1, 2 and 3) | 4, 3, 6 and 2 | 5, 6, 3 and 0c | 0.18a |
| DSM-IV diagnosis: alcohol abuse and dependenced | 6 and 9 | ||
| Hepatic encephalopathology (HE) score (none, mild, moderate, severe) | 8, 5, 2 and 0 | n.d. | |
| Mean daily alcohol consumption (g/day)d | 133.7 (67.3) | 6.2 (6.1) | <0.0001 |
| Mean peak daily alcohol consumption (g/day)d | 218.4 (94.4) | 19.3 (12.6) | <0.0001 |
| Mean lifetime alcohol consumption (kg) | 1591 (709) | 69 (73.2) | <0.0001 |
| Smoking ever/never (current)d | 14/1 (14) | 7/7 (5)c | 0.007a |
| Mean smoking pack yearsd | 33.7 (21.7) | 9.3 (17.3) | 0.003 |
Significant p-values are in bold type (p < 0.05).
n.d. = not done.
Chi-square test.
Liver pathology: 0 = nil or congestion, 1 = mild steatosis, 2 = severe steatosis/mild fibrosis; 3 = cirrhosis or severe inflammation (acute).
Missing data.
Estimated from medical history and next of kin questionnaires.
Proliferation is not decreased in the subventricular zone of chronic alcoholics
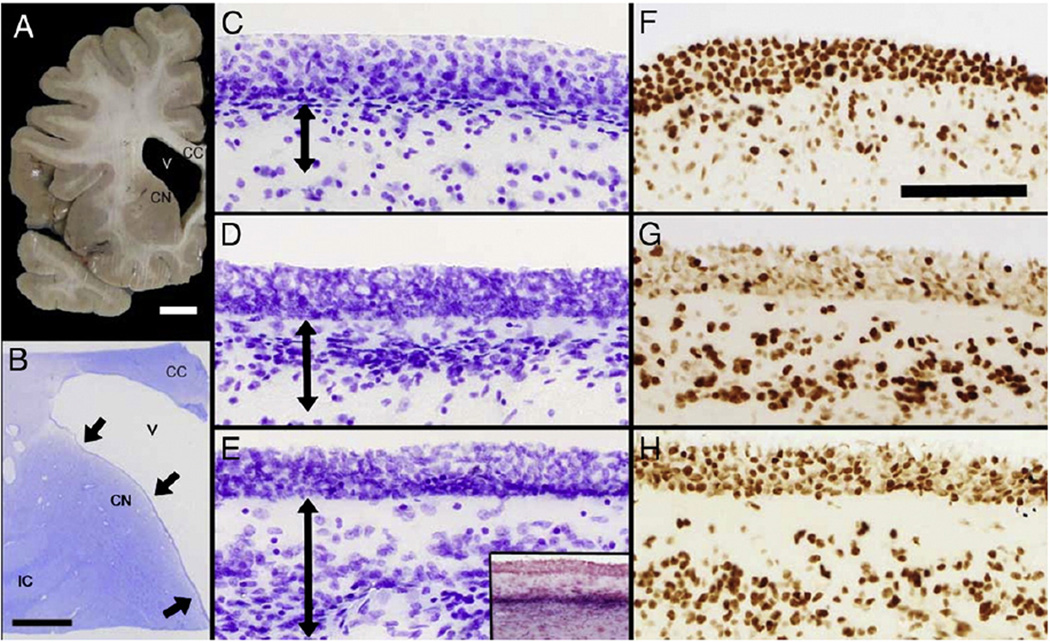
This study focused on the rostral wall of the lateral ventricle adjacent to the head of the caudate nucleus (Figs. 1A and B). The cytoarchitecture of the SVZ varied along the dorso-ventral axis, with a hypocellular layer immediately below the ependymal layer (EP), that becomes more prominent in the wider ventral region (over the head of the caudate) (Figs. 1C – E). Strikingly, the EP appears as a multicellular layer in mounted thick (48 µM) sections due to the flattening of the ependymal surface. A thin band of myelin separates the SVZ from the underlying parenchyma (Fig. 1E (inset)).
Fig. 1.
The anatomy and PCNA immunostaining of the adult human subventricular zone. This series of micrographs depicts the rostral region of the lateral aspect of the lateral ventricle wall in neurologically normal brains. (A) A photograph of a hemi-coronal section showing the corpus callosum (CC), the lateral ventricle (V) and the head of the caudate nucleus (CN). Scale bar = 1 cm. (B) A photomicrograph of a cresyl violet-stained thick section (48 µm) of the rostral lateral ventricle wall. The internal capsule (IC) is now more discernable than in (A) while black arrows mark the three sampling sites used in this study. Scale bar = 5 mm. (C–E) Cresyl violet-stained sections show the cytoarchitecture of the dorsal (C), middle (D) and ventral (E) regions of the lateral ventricle wall. Double-ended black arrows indicate the extent of the subventricular zone (SVZ) in each region. The SVZ itself and the hypocellular layer adjacent to the overlying ependymal layer (EP) increase in width in a dorso-ventral direction. In thick sections the EP appears to contain several layers, but this is a preparation artifact. (E inset) Luxol fast blue staining (with Fast Red counterstain) reveals the myelin layer (intense purple) that demarcates the SVZ from the underlying parenchyma. (F–H) Photomicrographs of PCNA immunostaining of the dorsal (F), middle (G) and ventral regions (H) of the ventricle wall. PCNA-positive cells in the SVZ are increased in a dorso-ventral direction whereas staining of the supposedly post-mitotic EP was more variable. Scale bars (C–H) = 100 µm.
Our expectation was that SVZ proliferation would decrease with prolonged exposure to alcohol. To test this hypothesis the PCNA-positive cells beneath a 0.5 mm long strip of the EP were quantified in three sites (dorsal, middle and ventral) and the mean regional counts compared between the two groups. There was no difference in mean PCNA-positive cells in the SVZ of alcoholics (n = 14, mean ± SD = 28.7 ± 20.0) and controls (n = 16, 27.6 ± 18.9, p = 1.0). There was also no difference when the three SVZ regions were compared individually: dorsal (22.4 ± 17.3 versus 24.8 ± 23.0, p = 0.75), middle (32.3 ± 26.8 versus 25.2 ± 17.6, p = 0.41) and ventral (42.5 ± 28.3 versus 33.8 ± 25.3, p = 0.53) (Figs. 1F – H). One caveat here is that counts were obtained from the ventral region of only 15 individuals (6 alcoholics). In the other individuals the mean counts were based on just the dorsal and middle regions, while one alcoholic was excluded as only their dorsal SVZ region could be quantified.
Our results are similar to those reported by Curtis et al. who found means of 25.3, 21.3 and 41.8 PCNA-positive cells in the dorsal, middle and ventral part of their controls (n = 3) (Curtis et al., 2005). As our counting strategy was slightly different, the Curtis et al. figures need to be adjusted by a factor of 0.8 giving equivalent mean counts of 31.6, 26.6 and 52.3 for their dorsal, middle and ventral regions, respectively.
There was also no correlation between PCNA-positive cells in the SVZ and either mean daily (r2 = 0.01, p = 0.58) or lifetime alcohol consumption (r2 = 0.006, p = 0.68).
There are no changes in the total number of cells or proliferative events in the granule cell layer of chronic alcoholics
Most surviving neuroblasts from the mammalian SVZ become integrated into the GCL as interneurons (Altman, 1969; Doetsch and Alvarez-Buylla, 1996; Lois and Alvarez-Buylla, 1994). The murine GCL has a cell density of approximately 410,000 cells/mm3 and a high neuron:glia ratio (Parrish-Aungst et al., 2007). It has been estimated that half the interneurons are turned over annually (Imayoshi et al., 2008) and at any one time there is likely to be between 7 and 11,000 new neurons/mm3 in the murine OB (Mouret et al., 2009a). There have been no studies quantifying proliferative events in the human GCL although a recent study measuring 14C levels suggested that turnover in the adult OB was low and restricted to non-neuronal cells (Bergmann et al., 2012). It is also possible that GCL interneurons, like those in the frontal association cortex, are susceptible to the direct neurotoxic effects of alcohol (Kril et al., 1997). In this case we might expect a decrease in GCL cells in excess of that explained by a reduced turnover of new neurons.
Given the variety of interneuron phenotypes in the GCL combined with a lack of reliable pan-neuronal markers for long-term formalin-fixed human brain tissue (Lyck et al., 2008) we used total cell counts as a surrogate for the GCL interneurons. Unfortunately only a subset of OBs was available for study here but we found no difference in granule cell numbers between alcoholics (n = 5, 267,618 ± 62,692 cells/mm3) and controls (n = 8, 272,007 ± 53,325 cells/mm3, p = 0.89) (Figs. 2A and B). There was also no decrease in PCNA-positive cells in alcoholics (147.6 ± 76.9 versus 148.9 ± 109.9 cells/mm3 in controls, p = 0.98) (Fig. 2C). In contrast to the murine GCL (11,000 new neurons or 3% of total granule cells), PCNA-positive cells appear to be rare in the human OB, representing less than 0.005% of total granule cells in the controls (Figs. 1E and F). Lastly, neither total GCL cells nor PCNA-positive GCL cells were correlated with mean daily or lifetime alcohol consumption.
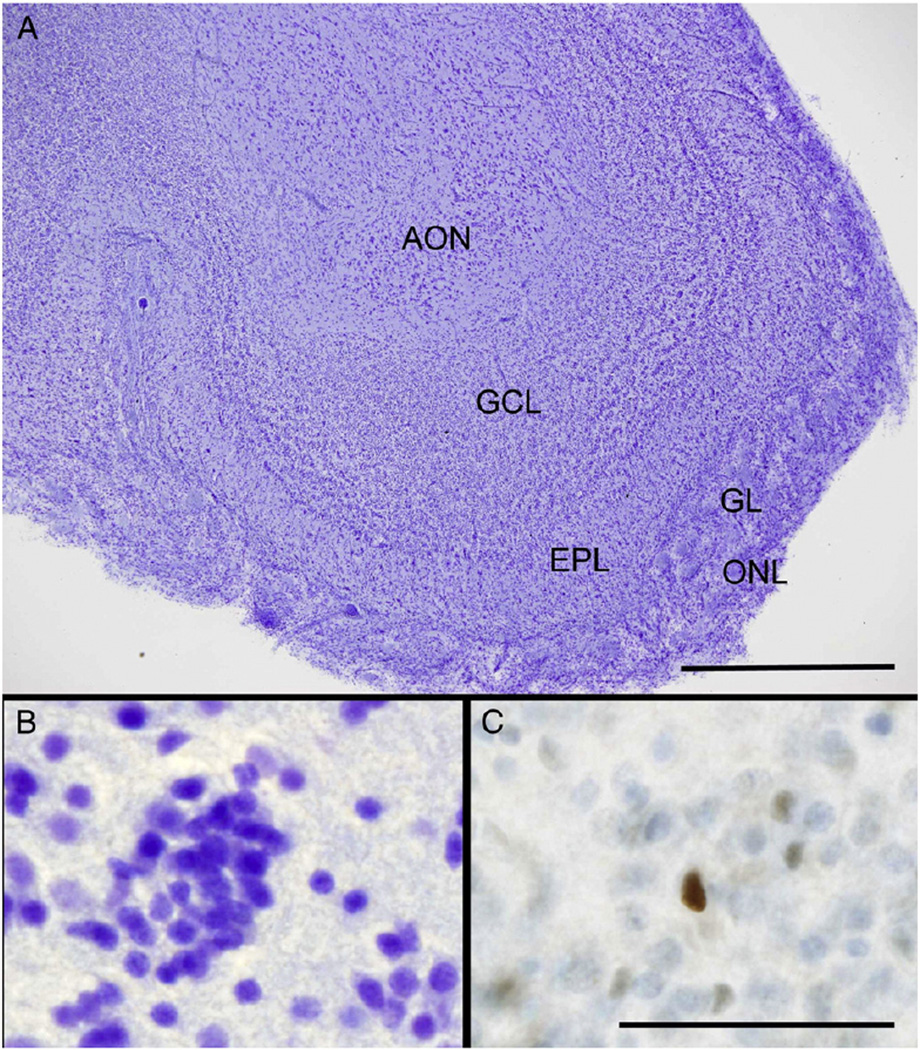
Fig. 2.
The anatomy and PCNA immunostaining of the adult human olfactory bulb. (A) A low power micrograph of a human OB shows its laminated structure. From the periphery, the OB is made up of the olfactory nerve layer (ONL), the glomerular layer (GL), the external plexiform layer (EPL) and the central granule cell layer (GCL). In this OB, the GCL surrounds a bulbar component of the diffuse anterior olfactory nucleus (AON). The mitral cell layer lies between the EPL and GCL. Scale bar = 0.5 mm. (B) A high power view of the GCL showing the typical clumping pattern of the micronuclear interneurons, the predominant cell type in this layer. (C) A single PCNA-positive cell is demonstrated within the GCL. Scale bars (B–C) = 50 µm.
Potential confounders have no apparent impact on proliferation in the adult human brain
Chronic alcoholics commonly possess the neurotoxic combination of both heavy alcohol and tobacco use combined with liver disease and all three could potentially affect neurogenesis. In terms of post-mortem effects, fixation time can decrease the availability of epitopes including PCNA to immunodetection (Hall et al., 1990). We performed a multivariate analysis to determine whether any of these factors were associated with PCNA immunopositivity in the SVZ, along with age, gender, brain weight, brain pH, PMI, and total lifetime alcohol consumption. There were no significant associations, with age having the highest relative effect (p = 0.18). A Pearson correlation analysis of age and SVZ-PCNA showed a positive trend (r2 = 0.12, p = 0.06).
We also performed stratified analyses within the alcoholic group but found no significant differences in SVZ-PCNA positivity between HE alcoholics (n = 6) and non-HE alcoholics (n = 8, p = 0.98), or cirrhotic alcoholics (n = 2) and non-cirrhotic alcoholics (n = 12, p = 0.76).
Smoking abuse essentially acts as a surrogate for alcohol consumption in this study with 94% of alcoholics compared to 50% of controls current or past smokers and a significant correlation between lifetime alcohol intake and mean pack years (r2 = 0.23, p = 0.008). As expected there was no relationship between smoking history (ever/never, n = 21, 26.7 ± 17.6 versus n = 8, 35.4 ± 21.9, p = 0.22) or mean pack years (r2 = 0.0001, p = 0.98) and SVZ PCNA-positive cells.
PCNA staining in the human lateral ventricle wall is more prolific than seen with another common proliferation marker, Ki-67
It is now known that PCNA has additional roles outside of the G1/S phases of the cell cycle including DNA repair suggesting that a proportion of SVZ events could represent post-mitotic PCNA expression. Other proliferative markers have been used in SVZ studies including Ki-67 (Macas et al., 2006; Sanai et al., 2011), MCM2 and Musashi-1 (Low et al., 2011) and we sought to use Ki-67 staining to corroborate our PCNA findings in the SVZ.
In our experience Ki-67 (Fig. 3A) and PCNA (Fig. 3D) produce similar patterns of staining in (FFPE) thin sections of high-grade brain tumors such as a glioblastoma multiforme (GBM; WHO, grade 4). However Ki-67 required a much more severe antigen retrieval regimen. In provisional work here we investigated Ki-67 staining in several free-floating thick sections, but these were extensively damaged by harsh antigen retrieval and were unsuitable for staining. As an alternative, we stained 7 µm FFPE sections of the lateral ventricle wall at a more posterior region (adjacent to the nucleus accumbens) from a one of the controls (64-year old male) for both PCNA and Ki-67. The density of PCNA positive cells in the SVZ was consistent with the results for thick sections (Figs. 3B and C), while only one to two Ki-67 positive cells were seen over the entire SVZ (Figs. 3E and F).
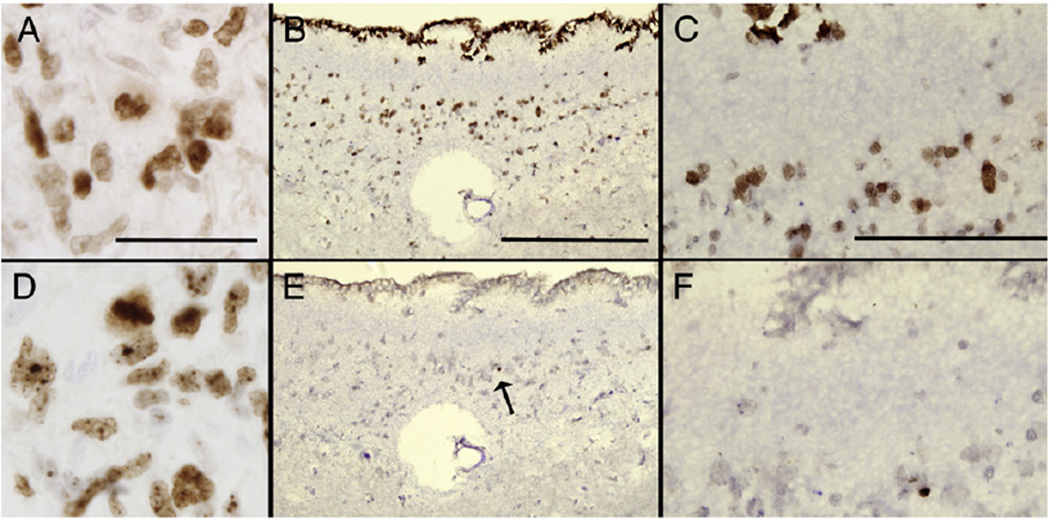
Fig. 3.
A comparison of Ki-67 and PCNA immunostaining in the adult human brain. These photomicrographs are of thin (7 µm) paraffin-embedded sections of a glioblastoma multiforme (GBM; WHO, grade 4) (A and D) and a more posterior (caudal) section of the lateral ventricle wall (adjacent to the nucleus accumbens) from a 64-year old male control (B, C, E, F). High power images of the GBM show equivalent amounts of (A) PCNA and (D) Ki-67 staining with a mitotic figure in the latter. Scale bars (A and D) = 50 µm. Low power images show the disparity between (B) PCNA and (E) Ki-67 staining in the lateral ventricle wall. A black arrow in (E) shows a single Ki-67 positive cell. In thin sections the ependymal layer is now seen as a single-layer cuboidal epithelium that is PCNA (B), but not Ki-67-positive. (E) Scale bars (B and E) = 200 µm. High power views of (C) PCNA and (F) Ki-67 immunostaining of the SVZ. Scale bars (C and F) = 100 µm.
Cell phenotypes in the human olfactory bulb
Given the additional flexibility of short-term fixed 7 µm FFPE sections for immunohistochemistry we explored the cellular makeup of the GCL in an additional neurologically normal individual (49-year old male). This OB had been fixed for eight weeks prior to paraffin embedding. Unlike the long-term fixed thick sections the OB and GCL in particular were immunopositive for the pan-neuronal marker, NeuN (Fig. 4). This allowed us to calculate a GCL neuron:glia ratio of approximately 3:1 (3.06 ± 0.73), a figure higher than that previously reported in mice (1.6:1)(Parrish-Aungst et al., 2007).
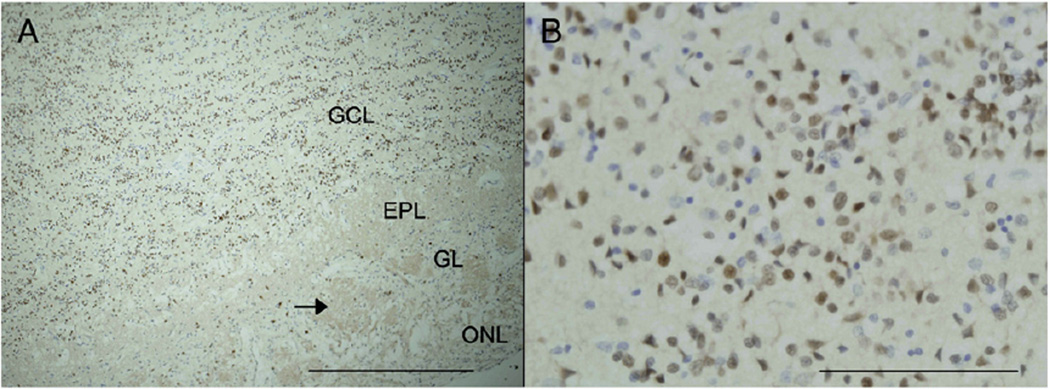
Fig. 4.
NeuN staining of the olfactory bulb. Positive immunostaining for the pan-neuronal marker NeuN was observed in short-term FFPE thin sections of the olfactory bulb from an additional neurologically normal individual (49-year old male). A low power micrograph from a section counterstained with haematoxylin (A) demonstrates the typical architecture of the human olfactory bulb including the densely packed granule cell layer (GCL). Other regions include the peripheral olfactory nerve layer (ONL), the glomerular layer (GL) with its distinctive non-cellular and oval-shaped glomeruli (black arrow) and the external plexiform layer (EPL) adjacent to the GCL. Scale bar = 500 µm. The high power micrograph (B) shows that the majority of the cells in the GCL are NeuN-positive interneurons. Scale bar = 100 µm.
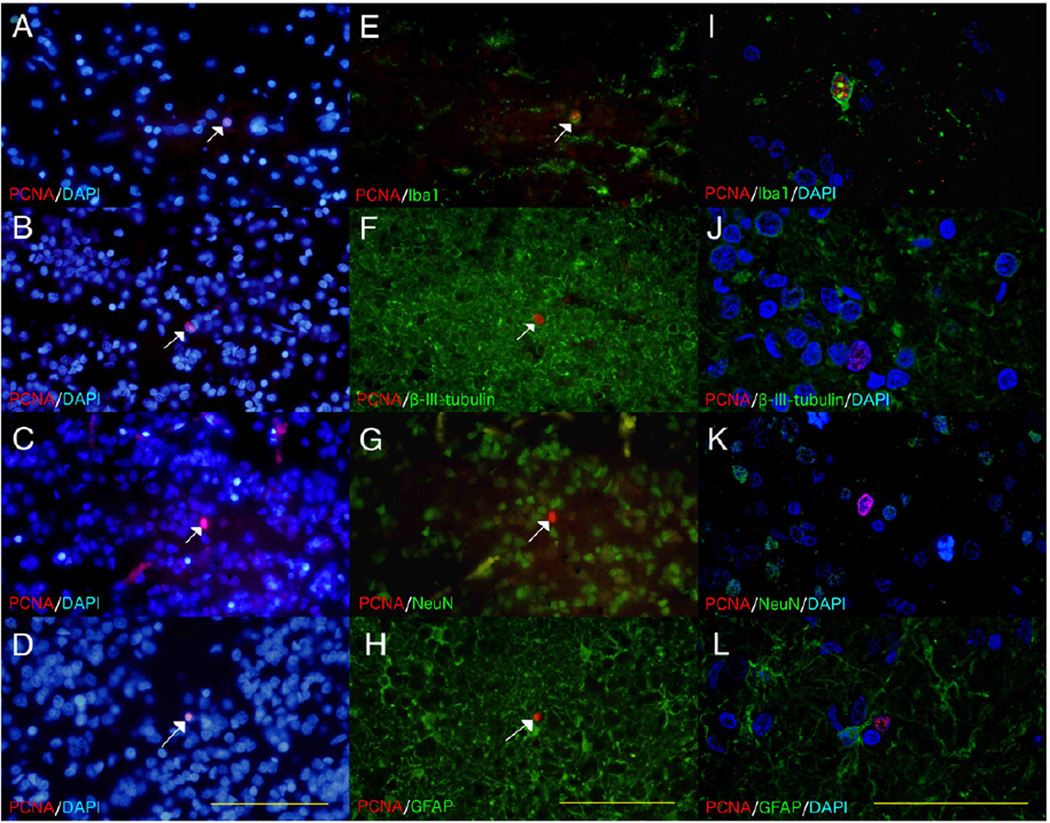
We then carried out immunofluorescent microscopy to determine the likely phenotype of the PCNA-positive cells in the GCL. Widefield microscopy allowed us to demonstrate co-localization with the cytoplasmic microglial marker, Iba1 (Fig. 5E)) and exclude co-localization with the nuclear mature neuronal marker, NeuN (Fig. 5G) However given complex and intertwining nature of neuronal and astroglial processes it was not possible with widefield microscopy to exclude co-localization between PCNA and the cytoplasmic markers for immature neurons (beta III tubulin (Fig. 5F)), and astrocytes (GFAP (Fig. 5H)). The subsequent use of confocal microscopy demonstrated that PCNA only co-localized with Iba1 (Fig. 5I) and not with the other markers (Figs. 5(J–L).
Fig. 5.
Co-localization studies in the granular cell layer. Immunofluorescence was used to determine the phenotype of the PCNA-positive cells in FFPE thin sections of the granule cell layer (GCL) from an additional neurologically normal individual (49-year old male). All sections were stained with the nuclear marker DAPI, although DAPI is not shown in images E–H for ease of viewing. The PCNA/DAPI (A–D) and corresponding PCNA/cell specific marker (E–H) widefield micrographs show rare PCNA-positive nuclei (white arrows). These widefield images show co-localization (yellow) between PCNA and the cytoplasmic microglial marker, Iba1 (E) but no co-localization with the nuclear mature neuronal marker, NeuN (G). There was also no obvious co-localization (yellow) between PCNA and the cytoplasmic markers for immature neurons, beta III tubulin (F), and astrocytes, GFAP (H). Confocal micrographs from areas adjacent to those used in the widefield images show co-localization of PCNA and Iba1 (I) but not beta III tubulin (J), NeuN (K) or GFAP (L). Scale bars = 100 µm (A–H) and 50 µm (I–L).
Discussion
Our results suggest that chronic alcoholism does not affect cell proliferation in the adult human SVZ or OB. Our findings are in direct contrast to the deleterious effects of prolonged alcohol exposure on SVZ neurogenesis in rats (Hansson et al., 2010) and a number of studies in the rodent hippocampal SGZ (reviewed by Crews and Nixon, 2009). They are more consistent with an additional study in rats with prolonged exposure to alcohol (6 weeks) that reported decreased neurogenesis in the SGZ but not in the OB (Herrera et al., 2003).
We also report no difference in total granule cell numbers suggesting that interneurons in the GCL, that make up the majority of the cell population, are not susceptible to the direct neurotoxic effects of alcohol or other potential neurodegenerative effects such as hepatotoxicity associated with chronic alcoholism (Temmel et al., 2005). Although immunostaining with the pan-neuronal marker, NeuN was restricted to FFPE sections of a single neurologically normal individual, the neuron: glia ratio of around 3:1 seems to support our decision to use total GCL cells as a surrogate for the interneurons. This ratio does however exceed the 1.6:1 described for the murine GCL (Parrish-Aungst et al., 2007) and further work is required to accurately quantify the human GCL cytoarchitecture.
Animal models strongly suggest that the GCL and particularly adult-born interneurons play an active role in odor discrimination (Enwere et al., 2004; Moreno et al., 2009; Mouret et al., 2009b; Sakamoto et al., 2011). From our work, the lack of group differences combined with the rarity of proliferative cells and their likely microglial phenotype collectively argues against a role for adult neurogenesis in the pathogenesis of hyposmia of chronic alcoholics (Rupp et al., 2003).
As our cell counts in the OB were restricted to the GCL it is possible that other neuronal populations, such as the mitral cells, are susceptible to the neurotoxic effects of alcohol. Having said this, most pathology in ARBD is actually associated with white matter atrophy with neuronal loss only being reported in a few isolated regions such as the prefrontal cortex (Kril et al., 1997). It is perhaps not surprising that there were no relationships between GCL total cells and lifetime alcohol consumption.
Our OB results are consistent with a previous suggestion that the olfactory cortex is a more likely site for the abnormalities associated with odor processing in chronic alcoholics (Maurage et al., 2011). However we are not aware of any pathological studies that have examined cortical regions involved in olfactory processing such as the insula or the piriform and orbitofrontal cortices. In contrast, neuronal loss has been reported in the amygdala in chronic alcoholics (Alvarez et al., 1989), a region receiving direct input from the OB. Amygdaloid atrophy in alcoholics has also been demonstrated with magnetic resonance imaging study (Makris et al., 2008).
Outside of ARBD, patients with neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) show deficits in both SVZ neurogenesis (Curtis et al., 2007a; Thompson et al., 2008) and smell sensation. Although potential relationships between SVZ neurogenesis and hyposmia may occur, typical AD (primarily tau)(Tsuboi et al., 2003) and Lewy body pathology (Hubbard et al., 2007; Ubeda-Banon et al., 2010) in the OB and to a variable extent throughout the entire olfactory system, would appear to be a more obvious pathological substrate. Interestingly in Huntington’s disease (HD), despite the significant increase in SVZ proliferation (Curtis et al., 2003), both patients (Moberg et al., 1987) and asymptomatic mutation carriers (Larsson et al., 2006) still experience deficits in olfactory function.
Our study was unusual for pathological studies of human brain autopsy tissue in that it relied on non-stereological counting methods. These were utilized because of the combination of ill-defined boundaries of the SVZ and GCL and the rarity of the events being demonstrated. If stereological methods were applied, most of the frames sampled in the GCL, in particular, would contain no cells of interest making the accuracy of the method poor while we did not have the whole anatomical structure to measure from in all cases. Our approach is supported by other studies that have used non-stereological counting to estimate rare proliferating cells in neural tissue (Benes and Lange, 2001; Devesa et al., 2011)
The primary aim of our study was to explore whether proliferation in the SVZ and OB is affected by chronic alcohol abuse. However, we also examined one of the largest groups of neurologically normal individuals studied to date and it seems pertinent to discuss our findings in the general context of adult neurogenesis. This relatively recent finding in the human brain has generated great excitement regarding its potential therapeutic manipulation for neuropsychiatric conditions including ARBD (Crews and Nixon, 2009). Adult neurogenesis in the SGZ was originally demonstrated using the exogenous marker BrdU (5-bromo-2′-deoxyuridine) in combination with mature neuronal markers including NeuN (Eriksson et al., 1998). Eriksson et al. also showed BrdU-positive cells in the SVZ although neurogenesis was only demonstrated subsequently by co-localization between beta III tubulin and PCNA (Curtis et al., 2003). Adult born neurons were then identified in the human OB (Bedard and Parent, 2004) before Curtis et al. (2007b) linked the two structures by demonstrating an active RMS.
In particular the discovery of ongoing neurogenesis in the adult SGZ has created great excitement and research endeavor. Especially because of the morbidity associated with neurological disorders that manifest with symptoms of hippocampal neuron loss such as depression and memory deficits (Thompson et al., 2008). These include ARBD (Crews and Nixon, 2009) while decreased hippocampal neurogenesis has also been postulated to underlie addiction vulnerability including to alcohol and disorders such as schizophrenia that are highly co-morbid with substance abuse (Chambers, 2012).
However more recent studies have challenged the prolificacy of neurogenic events in both the normal adult human SGZ (Low et al., 2011; Lucassen et al., 2010) and the SVZ, RMS and OB (Bergmann et al., 2012; Sanai et al., 2011; Wang et al., 2011). Similarly the dysregulation of SGZ neurogenesis in pathological conditions such as AD (Crews et al., 2010) and SVZ neurogenesis in PD (Hoglinger et al., 2004) have been questioned (Boekhoorn et al., 2006; van den Berge et al., 2010). In particular, Boekhoorn et al. found no evidence of neurogenesis in AD patients or controls, suggesting that Ki-67 immunopositive cells in the AD SGZ were most likely to be newborn astrocytes. These inconsistent findings are likely to reflect, amongst other factors, the relative prolificacy of adult neurogenesis between humans and animal models, and the choice of proliferative markers (Boekhoorn et al., 2006) and these factors are discussed further below.
Our findings with PCNA suggest that neuroblasts continue to be produced in the adult human SVZ but few, if any, reach the GCL. Given the technical limitations of immunohistochemical techniques in long-term fixated tissue we had to rely on a single short-termed fixed OB to demonstrate co-localization between proliferative and cell-specific markers. Notwithstanding our limited observations we could only show PCNA co-positivity with the microglial marker, Iba1, with no evidence of either neuronal or astroglial proliferation. While microglial activation is seen in ARBD (He and Crews, 2008) it is not clear whether there is also proliferation. We cannot comment on microglial activation but we saw no difference between alcoholics and controls in PCNA cells that would support altered microglial proliferation in the olfactory bulb.
We also cannot rule out the possibility that some PCNA-positive cells were oligodendrocyte precursor cells, as our nuclear oligodendrocytic lineage marker Olig2 antibody stained all cell nuclei while the specific oligodendrocyte precursor cell marker, NG2, appears to be detectable in frozen human brain tissue only (Ahmed et al., 2012).
The lack of adult-born neurons in the OB examined here does contrast with the work of Bedard et al. who showed co-localization between Ki-67 and the early neuronal marker, NeuroD (Bedard and Parent, 2004). Bedard and colleagues also showed co-localization between beta III tubulin and another immature neuron marker, doublecortin, but not with either Ki-67 or PCNA. However our findings of microgliogenesis are consistent with a recent study that utilized nuclear bomb test-derived 14C in genomic DNA and found evidence of non-neuronal, but not neuronal cell turnover, in the adult human OB (Bergmann et al., 2012). Interestingly the study by Bergmann and colleagues included subjects with substance (alcohol) abuse (30%) of which 3/9 had a diagnosis of alcohol dependence.
PCNA is highly sensitive for proliferative events in both neoplastic and physiologically proliferative tissues (Hall et al., 1990) and shows equivalent immunoreactivity in the SVZ with another proliferative marker MCM2 (Low et al., 2011). In contrast proliferative markers such as Ki-67 (Macas et al., 2006; Sanai et al., 2011) and phosphohistone H3 (pHH3) (van den Berge et al., 2011) reveal only ‘trace’ amounts of positive cells in the adult human SVZ. Given the current controversy about the true extent of SVZ neurogenesis in the adult human brain (Arellano and Rakic, 2011) we had hoped to add a degree of clarification to this issue. However we were only able to demonstrate that these two markers do indeed behave quite differently in fixed human brain tissue. Our lack of success with Ki-67 reinforced to us that the relative sensitivity of these proliferative markers is a complex issue that must take into account the fixation method, fixation period as well as the biological half-life of the target epitopes. PCNA in mitotically active cells is most abundant during the G1 and S phase of the cell cycle (Kurki et al., 1988) but it has a relatively long half-life (estimated to be 20 h (Bravo and Macdonald-Bravo, 1987)). In comparison Ki-67 is expressed across all active phases of the cell cycle but has a short half-life (approximately 1 h (Bruno and Darzynkiewicz, 1992)). While the relative antigen retrieval regimes required here suggest that formalin fixation of brain tissue results in greater cloaking of the MIB-1 antigen (Ki-67)(Shi et al., 1997) compared to PCNA (clone PC10). We are unsure if Ki-67 retrieval was optimal but we were able to demonstrate similar levels of staining as PCNA in adjacent FFPE thin sections of a GBM. We do note that Boekhoorn et al. (2006) and Wharton et al., (2005) have been able to demonstrate Ki-67-positive glial cells in the hippocampi of AD patients and aged-matched controls.
However PCNA, unlike Ki-67, is also expressed in post-mitotic cells under conditions such as DNA repair (Stoimenov and Helleday, 2009) and therefore it could potentially overestimate proliferative events in the SVZ. We are continuing to explore the extent of proliferation in the adult human SVZ but at present we can only state that the true number of mitotic events lies somewhere between those reported here and previously with PCNA staining (Curtis et al., 2003, 2005; Low et al., 2011; van den Berge et al., 2011) and the trace levels seen with Ki-67 (Macas et al., 2006; Sanai et al., 2011).
ARBD is unique among the neurodegenerative diseases in that abstinence from alcohol leads to a degree of functional recovery and work in animal models suggests that this involves neurogenesis (Crews et al., 2005). Our study did not include individuals who had abstained from alcohol prior to their death so we were unable to directly test this hypothesis. However given that chronic alcohol consumption doesn’t actually reduce proliferation it seems unlikely that recovery could be attributed to a compensatory increase in neurogenesis.
Lastly, in terms of comparative physiology, there are substantive behavioral changes when adult SVZ neurogenesis is deliberately interrupted in macrosmatic species such as mice (Sakamoto et al., 2011). It also appears that SVZ neurogenesis in rats and mice is more sensitive to alcohol than in microsmatic humans, with both fetal exposure (Akers et al., 2011) and chronic intoxication of adults (Hansson et al., 2010) leading to long lasting deficits. Similarly chronic alcoholism decreases SGZ neurogenesis in rodents and this has been linked to symptoms of depression and learning and memory deficits in alcoholics themselves (Nixon, 2006). While we cannot comment directly on the human SGZ we note that Low et al. not only found very little proliferation in the human SGZ but that a history of depression did not affect SGZ precursor cell numbers (Low et al., 2011).
Overall the contrasting responses to neurotoxic substances such as alcohol combined with the disparate prolificacy of adult-born neurons in the human OB (Bergmann et al., 2012) and SGZ (Boekhoorn et al., 2006; Low et al., 2011; Lucassen et al., 2010) suggests that neurogenic niches of laboratory animals behave quite differently to their human counterparts under both physiological and pathological conditions.
Conclusion
In summary chronic alcohol intoxication does not affect cell proliferation in the SVZ or OB of the adult human brain. In fact only microglial proliferation could be demonstrated in the latter. Although our findings contrast with those from animal models of alcohol intoxication, it appears unlikely that aberrant neurogenesis or microgliogenesis in the OB contributes to hyposmia in chronic alcoholics.
Acknowledgments
The authors would firstly like to thank the donors and their families for their kind gift that has allowed this research to be undertaken. The New South Wales Tissue Resource Centre (NSW TRC), for providing tissue samples and Dr. Michael Rodriguez and Mr. Patrick Moody for their discussions and assistance with Ki-67 staining in human brain tissue. We would like to acknowledge Ms. Sanaz Maleki and Dr. Louise Cole (Core Facilities Manager, Bosch Institute Advanced Microscopy Facility, The University of Sydney) for her support and assistance with microscopy. The NSW TRC is part of the NSW Brain Bank Network and Australian Brain Bank Network and is supported by the University of Sydney, National Health and Medical Research Council (NHMRC), Schizophrenia Research Institute and the National Institutes of Alcoholism and Alcohol Abuse (NIAAA) and NSW Department of Health. This work was supported by the NIAAA (NIH (NIAAA) R24AA012725) and the NHMRC (grant #401551).
References
- Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL. Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol. 2012 doi: 10.1111/j.1750-3639.2012.00637.x. http://dx.doi.org/10.1111/j.1750-3639.2012.00637.x (Article first published online: 23 OCT 2012) [DOI] [PMC free article] [PubMed]
- Akers KG, Kushner SA, Leslie AT, Clarke L, van der Kooy D, Lerch JP, Frankland PW. Fetal alcohol exposure leads to abnormal olfactory bulb development and impaired odor discrimination in adult mice. Mol. Brain. 2011;4:29. doi: 10.1186/1756-6606-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Alvarez I, Gonzalo LM, Llor J. Effects of chronic alcoholism on the amygdaloid complex. A study in human and rats. Histol. Histopathol. 1989;4:183–192. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 2000. Text Revision. [Google Scholar]
- Arellano JI, Rakic P. Neuroscience: gone with the wean. Nature. 2011;478:333–334. doi: 10.1038/478333a. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics. Alcohol Consumption in Australia: A Snapshot, 2004-05. Canberra: Australian Burea of Statistics; 2006. [Google Scholar]
- Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 2004;151:159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, Kutschera W, Johnson L, Landen M, Druid H, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol. Dis. 2006;24:1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J. Cell Biol. 1987;105:1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992;25:31–40. doi: 10.1111/j.1365-2184.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- Brust JC. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int. J. Environ. Res. Public Health. 2010;7:1540–1557. doi: 10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.12.005. http://dx.doi.org/10.1016/j.drugalcdep.2012.12.005 (pii: S0376-8716(12)00481-4) (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Collins DJ, Lapsley HM. The Costs of Tobacco, Alcohol and Illicit Drug Abuse to Australian Society in 2004/05. National Drug Strategy Monograph Series. 2008 [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, et al. Alcoholic neurobiology: changes in dependence and recovery. Alcohol. Clin. Exp. Res. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, Hansen L, Masliah E. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J. Neurosci. 2010;30:12252–12262. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M, Connor B, Faull RL. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RL. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington’s disease human brain. Neuroscience. 2005;132:777–788. doi: 10.1016/j.neuroscience.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Faull RL, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat. Rev. Neurosci. 2007a;8:712–723. doi: 10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007b;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Devesa P, Reimunde P, Gallego R, Devesa J, Arce VM. Growth hormone (GH) treatment may cooperate with locally-produced GH in increasing the proliferative response of hippocampal progenitors to kainate-induced injury. Brain Inj. 2011;25:503–510. doi: 10.3109/02699052.2011.559611. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol. Clin. Exp. Res. 2010;34:443–450. doi: 10.1111/j.1530-0277.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R, et al. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J. Pathol. 1990;162:285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int. J. Neuropsychopharmacol. 2010;13:583–593. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus. 1997;7:78–87. doi: 10.1002/(SICI)1098-1063(1997)7:1<78::AID-HIPO8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? Br. Med. J. (Clin. Res. Ed.) 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Brain shrinkage in alcoholics is not caused by changes in hydration: a pathological study. J. Neurol. Neurosurg. Psychiatry. 1988;51:124–127. doi: 10.1136/jnnp.51.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Chimelli L, Kril J, Ray D. Greenfield’s Neuropathology. United Kingdom: Hodder Arnold; 2008. Nutritional Deficiencies, Metabolic Disorders and Toxins Affecting the Nervous System; pp. 675–731. [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur. J. Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J. Anat. 2007;211:117–124. doi: 10.1111/j.1469-7580.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kurki P, Ogata K, Tan EM. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J. Immunol. Methods. 1988;109:49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- Larsson M, Lundin A, Robins Wahlin TB. Olfactory functions in asymptomatic carriers of the Huntington disease mutation. J. Clin. Exp. Neuropsychol. 2006;28:1373–1380. doi: 10.1080/13803390500473746. [DOI] [PubMed] [Google Scholar]
- Liu YW, Curtis MA, Gibbons HM, Mee EW, Bergin PS, Teoh HH, Connor B, Dragunow M, Faull RL. Doublecortin expression in the normal and epileptic adult human brain. Eur. J. Neurosci. 2008;28:2254–2265. doi: 10.1111/j.1460-9568.2008.06518.x. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Low VF, Dragunow M, Tippett LJ, Faull RL, Curtis MA. No change in progenitor cell proliferation in the hippocampus in Huntington’s disease. Neuroscience. 2011 Dec 29;199:577–588. doi: 10.1016/j.neuroscience.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Lyck L, Dalmau I, Chemnitz J, Finsen B, Schroder HD. Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex. J. Histochem. Cytochem. 2008;56:201–221. doi: 10.1369/jhc.7A7187.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J. Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, Martinez-Ramirez S, Jimenez-Xarrie E, Marin R, Marti-Vilalta JL, Garcia-Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. [Google Scholar]
- Maurage P, Callot C, Chang B, Philippot P, Rombaux P, de Timary P. Olfactory impairment is correlated with confabulation in alcoholism: towards a multi-modal testing of orbitofrontal cortex. PLoS One. 2011;6:e23190. doi: 10.1371/journal.pone.0023190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg PJ, Pearlson GD, Speedie LJ, Lipsey JR, Strauss ME, Folstein SE. Olfactory recognition: differential impairments in early and late Huntington’s and Alzheimer’s diseases. J. Clin. Exp. Neuropsychol. 1987;9:650–664. doi: 10.1080/01688638708405208. [DOI] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J. Neurosci. 2009a;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret A, Murray K, Lledo PM. Centrifugal drive onto local inhibitory inter-neurons of the olfactory bulb. Ann. N. Y. Acad. Sci. 2009b;1170:239–254. doi: 10.1111/j.1749-6632.2009.03913.x. [DOI] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J. Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J. Comp. Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. NeuroImage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp CI, Kurz M, Kemmler G, Mair D, Hausmann A, Hinterhuber H, Fleischhacker WW. Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol. Clin. Exp. Res. 2003;27:432–439. doi: 10.1097/01.ALC.0000057945.57330.2C. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8479–8484. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011 Sep 28;478(7369):382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian Brain Bank: a critical investment with a high return! Cell Tissue Bank. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry: past, present, and future. J. Histochem. Cytochem. 1997;45:327–343. doi: 10.1177/002215549704500301. [DOI] [PubMed] [Google Scholar]
- Smith RL, Baker H, Greer CA. Immunohistochemical analyses of the human olfactory bulb. J. Comp. Neurol. 1993;333:519–530. doi: 10.1002/cne.903330405. [DOI] [PubMed] [Google Scholar]
- Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem. Soc. Trans. 2009;37:605–613. doi: 10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2010. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: NSDUH Series H-41. Substance Abuse and Mental Health Services Administration; 2011. [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmel AF, Pabinger S, Quint C, Munda P, Ferenci P, Hummel T. Dysfunction of the liver affects the sense of smell. Wien. Klin. Wochenschr. 2005;117:26–30. doi: 10.1007/s00508-004-0303-x. [DOI] [PubMed] [Google Scholar]
- Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ. Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes Brain Behav. 2008;7(Suppl. 1):28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol. Appl. Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Ubeda-Banon I, Saiz-Sanchez D, de la Rosa-Prieto C, Argandona-Palacios L, Garcia-Munozguren S, Martinez-Marcos A. alpha-Synucleinopathy in the human olfactory system in Parkinson’s disease: involvement of calcium-binding protein-and substance P-positive cells. Acta Neuropathol. 2010;119:723–735. doi: 10.1007/s00401-010-0687-9. [DOI] [PubMed] [Google Scholar]
- van den Berge SA, Middeldorp J, Zhang CE, Curtis MA, Leonard BW, Mastroeni D, Voorn P, van de Berg WD, Huitinga I, Hol EM. Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-delta. Aging Cell. 2010;9:313–326. doi: 10.1111/j.1474-9726.2010.00556.x. [DOI] [PubMed] [Google Scholar]
- van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, et al. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134:3249–3263. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu YY, Zhao CH, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011 Nov;21(11):1534–1550. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton SB, Williams GH, Stoeber K, Gelsthorpe CH, Baxter L, Johnson AL, Ince PG. Expression of Ki67, PCNA and the chromosome replication licensing protein Mcm2 in glial cells of the ageing human hippocampus increases with the burden of Alzheimer-type pathology. Neurosci. Lett. 2005;383:33–38. doi: 10.1016/j.neulet.2005.04.019. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. Global status report on alcohol and health. Geneva, Switzerland: Department of Mental Health and Substance Abuse (MSD) of the World Health Organization (WHO); 2011. [Google Scholar]