Abstract
Purpose of review
To understand chronic inflammatory diseases such as atherosclerosis, we require in-depth knowledge on immune cell differentiation, function of specific immune cell subsets and endothelial cell-mediated extravasation. In this review we summarize a number of very recent observations on the pivotal function of NR4A nuclear receptors in immunity and atherosclerosis.
Recent findings
NR4A nuclear receptors are involved in negative selection of thymocytes, Treg differentiation and the development of Ly6C− monocytes has recently been shown to be dependent on the subfamily member Nur77. Nur77 and Nurr1 attenuate atherosclerosis in mice whereas NOR1 aggravates vascular lesion formation.
Summary
These exciting, novel insights on the function of NR4A nuclear receptors in immunity, vascular cells and atherosclerosis, will initiate a plethora of studies to understand the underlying molecular mechanisms, which will accumulate in the identification of novel targets to modulate chronic inflammatory disease.
Keywords: T cells, monocytes/macrophages, atherosclerosis, nuclear receptors
Introduction on NR4A nuclear receptors
The NR4A subfamily belongs to the nuclear hormone receptor superfamily and is comprised of three members; Nur77 (NR4A1, also referred to as NGFI-B or TR3), Nur-related factor-1 (Nurr1, NR4A2) and the Neuron-Derived Orphan Receptor 1 (NOR1, NR4A3, MINOR). [1*,2] Like other nuclear receptors, the NR4A receptors bind DNA with a highly structured and homologous (~94% within the subfamily) two-zinc finger DNA binding domain (DBD). The N-terminal transactivation domains (TAD) diverge in amino-acid sequence and the structure of these domains is intrinsically disordered (Figure 1). X-ray crystallography studies revealed that the C-terminal ligand binding domains (LBD) are composed, like most nuclear receptors, of 12 helices. However, the NR4A receptors lack a classical hydrophobic ligand binding pocket in the LBD as a result of polar bulky side chains in the region usually occupied by agonists. [3,4] This may explain why, so far, no traditional ligands have been identified for the NR4A nuclear receptors.
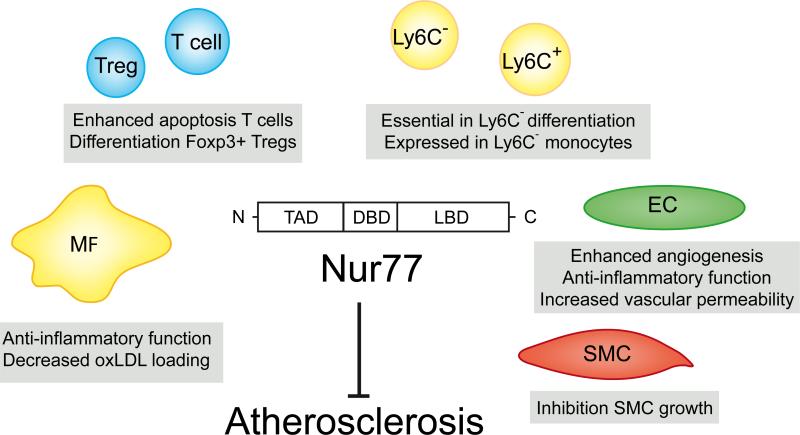
Figure 1. Nur77 in immune and vascular cells.
Nur77 function has been studied in T-cells, monocytes, macrophages, endothelial cells and smooth muscle cells and the outcome of these analyses is indicated in boxes at the specific cell types. For Nurr1 and NOR1 less information is available, but overall Nurr1 appears to have similar functions in these cell types as Nur77, whereas NOR-1 mostly mediates opposite effects.
All three NR4A receptors bind as monomers to response elements of a limited number of known direct target genes. In addition, Nur77 and Nurr1 form in contrast to NOR1, homodimers or heterodimerize with retinoid X receptor. NR4A receptors influence gene transcription also through transrepression; for example, Nur77 exhibits a direct, inhibitory interaction with the p65 subunit of NFκB.
NR4A receptors are typical early response genes of which the expression is rapidly induced (within 30 minutes) by a diverse range of stimuli including peptide hormones, inflammatory cytokines, mitogens, oxidized lipoproteins, and physical stimulation (for example, shear stress and mechanical stretch). Posttranslational modifications of NR4A family members is especially important functionally in light of the fact that their ligand binding pockets appear to be closed.
All three NR4As are known to be expressed in multiple cell types that are of crucial importance in immunity and in chronic inflammation of the vessel wall resulting in atherosclerosis. Exciting novel insights were gained recently on the function of NR4A nuclear receptors in monocytes, macrophages and T cells, as well as in atherosclerosis, of which an overview will be presented in this review.
NR4A nuclear receptors in myeloid cells
NR4A family members display important intrinsic roles in myeloid progenitor cell differentiation. Nur77 and NOR1 are potent suppressors of acute myeloid leukemia. NOR1/Nur77-double knockout mice develop acute myeloid leukemia with abnormal expansion of myeloid progenitor cells.[5] Studies of NOR1 and Nur77 in hypoallelic (NOR1+/−
Nur77−/− and NOR1−/− Nur77+/−) mice have shown that they recapitulate the pathological features of myelodysplastic–myeloproliferative neoplasms, with enhanced proliferation and excessive apoptosis of hematopoietic stem cells and myeloid progenitors.[6] Notably, Nurr1 has been shown to have a regulatory role in maintaining quiescence of hematopoietic stem cells.[7] This work demonstrates an important role for members of the NR4A family of nuclear receptors in myeloid cell differentiation in bone marrow.
Monocytes are a heterogeneous population of circulating cells that differentiate in the bone marrow from myeloid progenitors, and at least two distinct blood monocyte subsets exist in mice and humans. In mice, Ly6C+ (CD11b+CD115+MHC2−CCR2+) monocytes are inflammatory and migrate to injured or infected sites, and Ly6C− (CD11b+CD115+ MHC2− CX3CR1hi) monocytes patrol the resting vasculature and participate in the resolution of inflammation.[8] Nur77 is most highly expressed in Ly6C− monocytes, and these patrolling monocytes are absent in Nur77−/− mice.[9**] Nur77−/− mice have normal numbers of myeloid progenitor cells indicating that the defect occurs during later stages of monocyte development.[9**] The few Ly6C− monocytes remaining in the bone marrow of Nur77−/− mice are arrested in S-phase of the cell cycle and undergo apoptosis, implying that Nur77 functions as a master regulator of the differentiation and survival of the Ly6C− monocyte subset. There is also high Nur77 expression, and likely a common function, in the homologous human CD14dimCD16+ population of patrolling monocytes.[10] Studies by Carlin et al. show that these Nur77-dependent patrolling monocytes are “intravascular housekeepers” that scavenge microparticles and remove cellular debris from the microvasculature.[12**] However, the extent to which patrolling monocytes directly participate in the resolution of inflammation and the specific populations of macrophages/myeloid effector cells derived from patrolling monocytes are currently unknown.
In the absence of Nur77 expression there is increased NFκB activity in monocytes [9**], and peritoneal macrophages[11**], likely due to a reduction in IκBα expression. It is possible that in the absence of Nur77 inhibition of the NFκB pathway is reduced thus skewing myeloid cells towards an inflammatory phenotype. A similar role of Nurr1 in the inhibition of pro-inflammatory neurotoxic mediators via NFκB inhibition in both microglia and astrocytes has also been described.[13]
Taken together, NR4A receptor-mediated suppression of inflammatory factors in macrophages may at least in part be explained by repression of the NFκB signaling pathway. Nevertheless, further research is needed to determine specific molecular mechanisms and target genes by which NR4A family members can suppress inflammatory mediators in myeloid cells.
NR4A nuclear receptors in T cells
NR4A family members play a significant role in T lymphocyte development and activation. Nur77 is induced by T-cell receptor (TCR) signaling in T-cell hybridomas and thymocytes and has been described to induce apoptosis of CD4+CD8+ (DP) thymocytes bearing α/β TCRs with a high affinity for self-antigens in the thymus. Negative selection is a key process in enforcing central tolerance. There are many questions that surround the mechanism of negative selection, but it is currently held that apoptosis initiated by either Bim or Nur77 is critical for negative selection.[14,15] A unique BAC transgenic Nur77GFP mouse demonstrated that antigen receptor signaling was a major inducer of GFP in lymphocytes. Furthermore, Moran et al.[16**] found that GFP was not induced by TLR (toll-like receptor) ligands or other inflammatory stimuli, did not require co-stimulation, and was dependent on MHC for induction and maintenance in T-cells. Using this model, Moran et al. were able to show that regulatory T-cells (Treg) and invariant NKT cells perceived stronger TCR signals than conventional T-cells during development.[16**]
In thymocytes, NOR1 protein is induced to a very high level upon TCR stimulation and has similar kinetics to Nur77. Constitutive expression of NOR1 in thymocytes leads to massive apoptosis and up-regulation of CD25, suggesting a functional redundancy between Nur77 and NOR1 gene products. In contrast, Nurr1 is undetectable in stimulated thymocytes, however it has been shown that Nurr1 has a strong ability to induce Foxp3 and repress inflammatory cytokines, including IFNγ thus regulating the Th1/Treg balance.[17**] Recently, Sekiya et al.[17**] analyzed mice lacking one or more NR4A proteins in T-cells. They found that mice lacking individual NR4A transcription factors (either Nur77, Nurr1, or NOR1) did not have an overtly altered phenotype. However, combined deletion of two of the three family members Nur77 and NOR1, was associated with a considerably greater abundance of thymic and peripheral Treg cells, whereas triple deletion of all three members results in a deleterious effect on Treg development. Sekiya et al. also showed that NR4A-deficient T-cells produce more IL-4 and IFNγ in vitro with the inflammation observed in vivo being driven by Th2 cells.
Thus, these recent studies have shed light on the roles that these transcription factors play in immunological homeostasis. The NR4A receptors function in lymphocytes to maintain homeostasis and tolerance by inducing apoptosis in high-affinity, self-reactive T-cells, inducing Foxp3 expression in Treg cells, and by limiting cytokine production by non-Treg cells.
NR4A nuclear receptors in endothelial cells and smooth muscle cells
NR4A nuclear receptors are expressed in endothelial cells downstream of VEGF and consequently were proposed to be involved in angiogenesis.[18] Angiopoietin-1 (Ang-1), another important angiogenic factor, also enhances Nur77 expression, though via different signalling pathways.[19*,20**] Whereas VEGF is known to promote NFκB activity and thus leukocyte adhesion to endothelial cells, Ang-1 mediated Nur77 expression results in increased levels of the NFκB inhibitor IκBα and thus delimits the inflammatory response in endothelial cells.[19*,21]
All three NR4A receptors potentiate endothelial cell survival in vitro. Nur77 has been demonstrated to inhibit the inflammatory response of these cells as illustrated by reduced expression of cellular adhesion molecules ICAM-1 and VCAM-1, whereas NOR1 was shown to enhance the expression of these molecules and increase monocyte adhesion to endothelial cells.[21,22]. The pro-angiogenic potential of Nur77 has been described in great detail by Zeng and Dvorak.[20**,23,24] Local overexpression of Nur77, as well as studies in Nur77-deficient mice, revealed a strong pro-angiogenic function for this nuclear receptor. Nur77 has been described to also modulate vascular permeability by increasing endothelial nitric-oxide synthase expression and down-regulation of cell junction proteins such as Claudin-5 and VE-Cadherin.[25] Of interest, adult Nur77-deficient mice have normal blood vessels, indicating that Nur77 is not essential during development of the vascular system, but rather has a crucial function in endothelial cells under pathological conditions.
The function of NR4A nuclear receptors in smooth muscle cells has been studied extensively over the years both in vitro and in multiple animal models. [for recent reviews, see [1,2] ] All three NR4A receptors are expressed in response to numerous factors that modulate smooth muscle cell proliferation, however, the outcome on cell function is different. Nur77 and Nurr1 inhibit smooth muscle cell proliferation, whereas NOR1 has a mitogenic function in these cells.[26,27,2] A possible explanation for the disparity in function between NR4A receptors may involve the fact that NOR1 only acts as a monomer, whereas Nur77 and Nurr1 can both homodimerize and form heterodimers with RXR, which may affect downstream gene expression. To come to a better understanding of the exact function, target genes need to be identified for each NR4A subfamily member in these specific cell types and continued investigation should include analysis of the interactome of the NR4A proteins.
NR4A nuclear receptors in atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the arterial vessel wall that is initiated through local activation of endothelial cells and subsequent lipid accumulation and extravasation of inflammatory cells into the vessel wall. Specific subsets of monocytes and T-cells are crucial at the onset of atherosclerotic lesion formation, whereas activated smooth muscle cells are an additional source of inflammatory factors and add mass and stability during lesion progression.[28] Three laboratories have recently determined independently the impact of Nur77-deficient bone marrow on atherosclerosis in LDLR−/− mice after bone marrow transplantation and two of them observed increased atherosclerosis with higher macrophage content[29**,11**], whereas one group reported no differences in lesion formation compared to wild-type bone marrow transplanted mice[30*]. The discrepancy in disease outcome in apparently comparable bone marrow transplantation experiments may be explained by subtle but undoubtedly non-trivial differences in experimental setup, such as pathogen-status, irradiation regime, recovery after transplantation and variable composition and duration of the atherogenic diet. Significantly, Hanna et al,[11**] also analyzed ApoE/Nur77-double knockout mice and obtained similar results: deficiency of Nur77 leads to increased atherosclerosis. Of note, the latter mice lack Nur77 throughout the body and since Nur77 has been described to also affect endothelial cells, smooth muscle cells and different subsets of T-cells this model is far more complex.
One may expect that the in vitro experiments in these studies with cultured primary macrophages would result in more consistent observations, however, completely different cell populations were studied. Obviously, resident peritoneal macrophages and bone marrow-derived macrophages as used by Hanna/Hamers et al.[29**,11**] are relatively quiescent, whereas peritoneal macrophages obtained after thioglycollate stimulation or from ApoE−/− mice after 11 weeks of diet may be considered as activated macrophages. Both Hamers- and Hanna et al.[29**,11**] reported that Nur77-deficiency associates with macrophage polarization towards the pro-inflammatory phenotype, exhibiting increased IL-12, IFNy, iNOS expression, and nitric oxide synthesis. The underlying mechanism of the pro-inflammatory response was proposed to involve increased toll-like receptor expression and enhanced NFkB activity[11**] and a most significant increase in SDF1α expression[29**]. One should realize that all macrophages studied were originally derived from distinct monocyte (precursor) populations, with hardly any Ly6C− monocytes present in Nur77-deficient mice, which most likely affects the characteristics of the fully differentiated macrophages. Therefore, Hanna et al.[11**] studied macrophages derived from Ly6C+ sorted monocytes, which is a relevant approach to assess the function of Nur77 specifically in this macrophage subset. These experiments revealed the inhibition of oxidized LDL accumulation by Nur77.
Chao et al.[30*] also performed bone marrow transplantations with NOR1-deficient fetal liver cells in LDLR−/− mice and observed no change in atherosclerosis, which is again in contrast with published data demonstrating that ApoE/NOR1-double knockout mice develop more lesion than ApoE−/− mice[22]. In the latter study, however, especially the pro-inflammatory function of NOR1 in endothelial cells was studied and no data were presented on NOR1 deficiency in macrophages.
Conclusions
In conclusion, the NR4A nuclear receptor family members have recently emerged as major regulators of both immune and vascular cell function. All 3 family members appear to regulate hematopoietic stem cell proliferation. Nur77 has a crucial function in monocyte subset differentiation, is essential in development of patrolling Ly6C− monocytes and has a crucial anti-inflammatory function in early activation of macrophages. Nur77 is also a major regulator of early lymphocyte development in thymus. Both Nur77 and NOR1 play important roles in modulating smooth muscle cell proliferation and endothelial activation. All 3 NR4A members function to inhibit NFκB signaling to regulate inflammatory responses. Future studies to identify additional signaling pathways regulated by these nuclear receptors are warranted. To further our knowledge on NR4A nuclear receptors in atherosclerosis and other chronic inflammatory diseases, we need to rely on tissue-specific gene targeting strategies. Conditional knockout mice for each of these nuclear receptors will be useful to determine the impact of selective deletion of the nuclear receptors in myeloid cells, vascular cells, and lymphocytes on the development, progression, and possible regression of atherosclerosis.
Acknowledgments
Supported by the Dutch Heart Foundation, The Hague; grant #2008B037 and project P1.02 NEXTREAM of the research program of the BioMedical Materials institute, co-funded by the Dutch Ministry of Economic Affairs (all to C.d.V.); the American Heart Association (to R.N.H.) and National Institutes of Health HL118765 (to C.C.H.).
References and recommended reading
- 1*.van Tiel CM, de Vries CJ. NR4All in the vessel wall. J Steroid Biochem Mol Biol. 2012;130:186–193. doi: 10.1016/j.jsbmb.2011.01.010. [This review provides details on the data available in literature on NR4A receptors in smooth muscle cells and vascular disease] [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Bruemmer D. NR4A Orphan Nuclear Receptors in Cardiovascular Biology. Drug Discov Today Dis Mech. 2009;6:e43–e48. doi: 10.1016/j.ddmec.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker KD, Shewchuk LM, Kozlova T, et al. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell. 2003;113:731–742. doi: 10.1016/s0092-8674(03)00420-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Benoit G, Liu J, et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 5.Mullican SE, Zhang S, Konopleva M, et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730–735. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez-Herrick AM, Mullican SE, Sheehan AM, et al. Reduced NR4A gene dosage leads to mixed myelodysplastic/myeloproliferative neoplasms in mice. Blood. 2011;117:2681–2690. doi: 10.1182/blood-2010-02-267906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirin O, Lukov GL, Mao R, et al. The orphan nuclear receptor Nurr1 restricts the proliferation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1213–1219. doi: 10.1038/ncb2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Hanna RN, Carlin LM, Hubbeling HG, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C-monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [This study describes for the first time that Nur77−/− mice lack a subset of monocytes, the Ly6C-monocytes. This observation was a major break through in the field, espcecially because Nur77−/− mice were already published in 1995 (Sience 269:532-5) by Milbrandt and co-workers, who at that time reported the absence of a T-cell phenotype in these mice] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Hanna RN, Shaked I, Hubbeling HG, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [This study is one of the first studies on the role of Nur77 in bone marrow-derived cells in atherosclerosis, demonstrating that Nur77 has an anti-inflammatory function. These data identify Nur77 as a novel target for intevention in chronic inflammatory disease] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [A detailed analysis is performed involving in vivo imaging to assess the function of Ly6C(low) monocytes in maintenance of endothelial cell function. This is crucial to preserve a healthy and functional vessel wall] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saijo K, Winner B, Carson CT, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasser A, Puthalakath H, O'Reilly LA, et al. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 15.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Moran AE, Holzapfel KL, Xing Y, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [This novel reporter mouse expresses GFP from the Nur77 locus and is an excellent indicator of Nur77 tissue and cell-type specific expression and thus indicates potential locations of Nur77 activity] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Sekiya T, Kashiwagi I, Yoshida R, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat Immunol. 2013;14:230–237. doi: 10.1038/ni.2520. [T-cell specific deletion in mice of each of the NR4A receptors and combinations therefore provides crucial insight on redundancy of the nuclear receptors in Foxp3+ Treg differentiations] [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Jia H, Holmes DI, et al. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler Thromb Vasc Biol. 2003;23:2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 19*.Ismail H, Mofarrahi M, Echavarria R, et al. Angiopoietin-1 and vascular endothelial growth factor regulation of leukocyte adhesion to endothelial cells: role of nuclear receptor-77. Arterioscler Thromb Vasc Biol. 2012;32:1707–1716. doi: 10.1161/ATVBAHA.112.251546. [To understand the distinct outcome between VEGF and Ang-1 mediated angiogenesis involving Nur77 this study provides molecular insight] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Qin L, Zhao D, Xu J, et al. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood. 2013;121:2154–2164. doi: 10.1182/blood-2012-07-443903. [In this study the role of Nur77 in vascular permeability is demonstrated in multiple mouse models. This is of relevance in atherosclerosis, because Nur77 inhibits the inflammatory response of endothelial cells and at the same time promotes permeability] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You B, Jiang YY, Chen S, et al. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Howatt DA, Gizard F, et al. Deficiency of the NR4A orphan nuclear receptor NOR1 decreases monocyte adhesion and atherosclerosis. Circ Res. 2010;107:501–511. doi: 10.1161/CIRCRESAHA.110.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng H, Qin L, Zhao D, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D, Desai S, Zeng H. VEGF stimulates PKD-mediated CREB-dependent orphan nuclear receptor Nurr1 expression: role in VEGF-induced angiogenesis. Int J Cancer. 2011;128:2602–2612. doi: 10.1002/ijc.25600. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Qin L, Bourbon PM, et al. Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilizing endothelial junctions. Proc Natl Acad Sci USA. 2011;108:12066–12071. doi: 10.1073/pnas.1018438108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonta PI, Pols TW, van Tiel CM, et al. Nuclear receptor Nurr1 is expressed in and is associated with human restenosis and inhibits vascular lesion formation in mice involving inhibition of smooth muscle cell proliferation and inflammation. Circulation. 2010;121:2023–2032. doi: 10.1161/CIRCULATIONAHA.109.885673. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Calvo R, Guadall A, Calvayrac O, et al. Over-expression of Neuron-derived Orphan Receptor-1 (NOR-1) exacerbates neointimal hyperplasia after vascular injury. Hum Mol Genet. 2013;22:1949–1959. doi: 10.1093/hmg/ddt042. [DOI] [PubMed] [Google Scholar]
- 28.Zernecke A, Weber C. Improving the treatment of atherosclerosis by linking anti-inflammatory and lipid modulating strategies. Heart. 2012;98:1600–1606. doi: 10.1136/heartjnl-2012-301761. [DOI] [PubMed] [Google Scholar]
- 29**.Hamers AA, Vos M, Rassam F, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [This study is one of the first studies on the role of Nur77 in bone marrow-derived cells in atherosclerosis, demonstrating that Nur77 has an anti-inflammatory function. These data identify Nur77 as a novel target for intevention in chronic inflammatory disease] [DOI] [PubMed] [Google Scholar]
- 30*.Chao LC, Soto E, Hong C, et al. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J Lipid Res. 2013;54:806–815. doi: 10.1194/jlr.M034157. [This article shows that both Nur77 and NOR-1 deficiency in bone marrow does not affect atherosclerosis in mice, which is in contrast with earlier studies [11, 22, 29]] [DOI] [PMC free article] [PubMed] [Google Scholar]