Summary
Two recent studies validate the LMNA-NTRK1 fusion as an oncogenic driver and therapeutic target of TRK inhibitors. The LMNA-NTRK1 fusion occurs at low frequency across multiple tumor types. The studies highlight the increasing need to develop molecular biomarker-based clinical trials across cancer subtypes.
A cornerstone of modern precision cancer therapeutics is the identification of an oncogenic driver mutation within an individual patient’s tumor (1). In non-small cell lung cancer (NSCLC), for example, driver alterations include activating point mutations, in-frame deletions, amplifications, or gene rearrangements in several crucial kinases and these genetic targets provide specific predictive biomarkers of the likely clinical response to the cognate oncoprotein-targeted drug (1). Exploiting such molecular biomarkers, such as oncogenic EGFR and EML4-ALK gene rearrangements in NSCLC and oncogenic BRAF in melanoma and NSCLC, to individualize and improve cancer therapy by dividing and conquering the specific molecular subsets of cancer is a general paradigm for progress in the field (2–4).
A myriad of genetic targets and targeted therapies has emerged in the last several years, heralding an exciting era for potentially rapid progress (1). Commensurate with this substantial opportunity, there remain significant challenges. Foremost of these challenges is that genetic targets both within and across cancer subtypes must be identified in patients efficiently and reliably. Many of these molecular cancer subgroups represent relatively small numbers of patients within a given histologic cancer subtype. Thus, in the molecular era it is becomingly increasingly important to recognize and reliably credential the growing number of clinical biomarkers that can potentially predict therapeutic response across tumors of different histologic backgrounds. Further, doing so at the outset of clinical drug development allows timely and synchronous evaluation of the clinical relevance of the biomarkers and the efficacy of the matched targeted therapies. To meet this need, so-called basket trials are being developed to investigate the effects of targeted agents in a molecularly-defined subpopulation across multiple anatomical and histological subtypes. One such example where a unique oncogenic alteration is distributed across multiple tumor types at relatively low frequency involves gene fusions of the tropomyosin-related kinase (TRK) family.
Two new articles in Cancer Discovery highlight the clinical utility of targeting TRK fusions with small molecule TRK inhibitors using both preclinical and clinical analysis in soft-tissue sarcoma (STS) and colorectal cancer (5,6). The first important study by Doebele and colleagues reports on the preclinical and clinical efficacy of a selective TRK inhibitor, LOXO-101. The authors highlight the rapid clinical and radiographic response of a single patient with metastatic undifferentiated STS who was initially enrolled in a phase I dose-escalation study with LOXO-101 (NCT02122913). The patient was not required to have a TRK fusion upon enrollment. However, upon genomic profiling during standard of care (SOC) neo-adjuvant therapy, the patient’s tumor was found to harbor a fusion involving the lamin A/C (LMNA) and NTRK1 (gene that encodes TRKA) genes, LMNA-NTRK1. The patient subsequently underwent a limb-sparing surgery for definitive local control of the primary tumor, but was noted to have progressive metastatic pulmonary disease refractory to doxorubicin-based chemotherapy. She was enrolled on the LOXO-101 clinical trial and achieved rapid clinical, radiographic, and serological responses to the 100 mg twice daily dosing. The clinical data as well as preclinical in vitro and in vivo studies convincingly showed that NTRK gene fusions are actionable oncogenic targets of TRK inhibitor therapy across different histologic cancer subtypes, validating prior work (7,8). This study nicely highlights the value of conducting cross-cancer comparisons of the function and targeting of a particular oncogenic target.
In the second exciting study, Russo and colleagues report on a metastatic colorectal cancer patient with the LMNA-NTRK1 fusion who similarly achieved a remarkable clinical and radiographic response to entrectinib (RXDX-101), a multikinase inhibitor targeting TRK, ALK, and ROS1. Following entrectinib response, the patient developed therapeutic resistance and disease progression. LMNA-NTRK1 status was monitored by circulating tumor DNA (ctDNA) analysis throughout entrectinib treatment, revealing the emergence of two novel NTRK1 kinase domain mutations (G595R and G667C) that were absent from ctDNA collected at the time of drug initiation. Longitudinal serological monitoring of NTRK1 mutant alleles revealed that ctDNA levels paralleled initial tumor response and then resistance to entrectinib. In concordance with their clinical observation, the authors revealed using both xenopatient and cell line based models that the two mutant NTRK1 (G595R and G667C) alleles emerged under drug selection and promoted entrectinib resistance, likely via steric hindrance that abrogates or reduces entrectinib binding in the catalytic pocket. Importantly, the G595R secondary on-site TRKA mutation caused cross-resistance to other TRK inhibitors, including LOXO-101.
Both of these impressive studies validate the LMNA-NTRK1 fusion as an oncogenic driver and bone fide therapeutic target of clinically available TRK inhibitors in STS and colorectal cancer. Moreover, the combined work highlights the emerging utility of targeting low frequency genomic alterations across multiple cancer subtypes as well as of complementary roles of blood- and tissue-based molecular diagnostics assays. These studies further add to our collective discussion centered upon two important questions in targeted therapy clinical trial design: (1) should specific molecular alterations supersede anatomical or histological classification? (2) how should clinicians monitor therapeutic response and resistance to targeted therapies (serial tissue biopsy and re-biopsy, ctDNA, circulating tumor cells (CTCs) analysis, or a combination of strategies)?
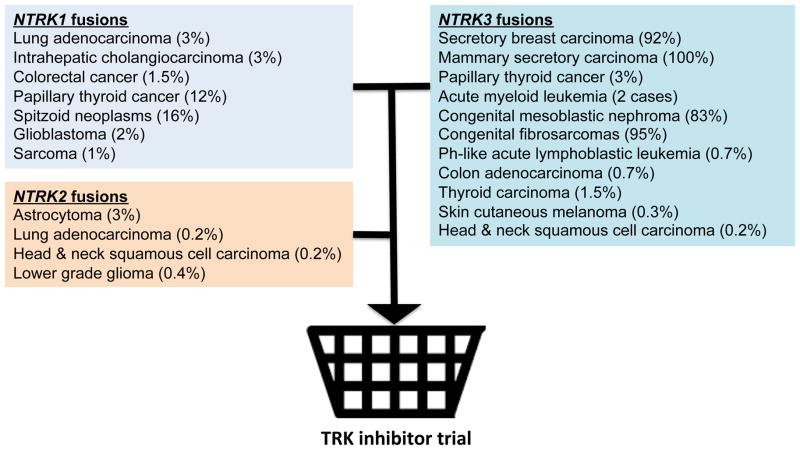
Comprehensive genomic profiling efforts have identified NTRK family fusions in numerous tumor types (Figure 1) (9). Intriguingly, while TRK fusions can sporadically occur at high frequency in relatively rare tumor types, such as mammary secretory carcinoma (100%) and congenital fibrosarcoma (90–100%), their frequency is much lower in more common cancers including lung adenocarcinoma (~3%), colorectal cancer (1.5%), and sarcoma (~1%) (9). A traditional clinical trial design, whereby patients are randomly assigned to a SOC regimen or SOC plus an experimental agent in a molecularly unselected patient population would have likely precluded the two profiled LMNA-NTRK1 fusion positive patients from obtaining clinical benefit from TRK inhibitor therapy. The utility of prospective molecular profiling to identify patient subsets that share common genomic alterations irrespective of tumor histology is the impetus behind basket trial methodological design. Using biomarker-driven clinical trials, clinicians can now investigate the effects of available targeted agents against rare, low frequency genetic alterations that potentially drive tumorigenesis across multiple cancer histologies. This is distinct from the recently published Custom Trial that focused on relatively common oncogenic drivers (EGFR, KRAS, BRAF) in common thoracic malignancies. The development of next generation basket trials should not be anatomically or histologically restricted, but instead include disease-defining molecular alterations, such as LMNA-NTRK1 fusions, to efficiently capture a unique molecular subpopulation that can benefit from TRK inhibitor therapy (Figure 1). A rare target should not be an orphan target in the molecular era of targeted therapy clinical development.
Figure 1.
Genetic profiling and basket trial concept for TRK fusion positive cancers. Shown are various cancer subtypes with NTRK oncogenic fusion genes and their prevalence, as well as the basket clinical trial concept.
In colorectal cancers that developed resistance to entrectinib, Russo and colleagues identified on-site mutations within the kinase domain (KD) of NTRK1 that were necessary for the resistant phenotype. Intriguingly, the authors were able to optimize a ctDNA-based platform to monitor NTRK1 mutant levels in response to entrectininb treatment. Importantly, the presence of mutant alleles in the patient’s plasma preceded radiographic progression on the order of months, suggesting that ctDNA is a sensitive assay to detect residual disease and disease recurrence in patients. While blood-based genotyping is clinically compelling and less invasive than traditional radiographically-directed needle biopsies of recurrent or metastatic tumors, it is currently limited to the analysis of known mutations (such as in NTRK1 in this case). Thus, monitoring of mutant specific ctDNA inherently ignores the complex biological diversity that is present in many cancers (10). In order to capture these additional mechanisms of resistance (ie. bypass tracks, off-target genetic mutations, and epigenetic changes), a more comprehensive analysis using ctDNA technology perhaps in parallel with tissue biopsies and other blood-based genotyping methods such as CTC analysis will be required.
Together, these two studies highlight the biological dependence on the oncogenic TRK fusion protein, LMNA-NTRK1, in tumor cells. Although novel TRK inhibitors, including LOXO-101 and entrectinib, can induce tumor regression in different cancer subtypes, these responses may be transient, as demonstrated in the Russo study. The mechanisms that drive TRK inhibitor resistance and disease recurrence seem to parallel other cancers with genetic targets, based on these early studies. We speculate that additional off-target resistance events will eventually be characterized through further patient-based analysis. These studies also reinforce the concept of biomarker-based clinical trial design to improve patient outcomes through precision therapeutics. It should be noted that both patients profiled in these two studies had metastatic drug-resistant disease that would conventionally be treated with additional cytotoxic agents, at the considerable cost of added toxicity. The impact of treatment with a novel targeted, non-cytotoxic agent (illuminated by genetic profiling) to improve symptomatology and maintain quality of life in addition to inducing tumor response should not be underestimated. These important studies add to a growing literature that should encourage the continued development of basket trials and serial molecular analyses to capture the presence and therapy-induced evolution of rare genetic alterations across multiple cancer subtypes. This approach will allow the efficient and rapid dissection of the specific molecular and tissue lineage-based determinants of both response and resistance to targeted agents against relatively uncommon, but nevertheless important, oncogenic drivers to accelerate improvement in clinical outcomes.
Acknowledgments
The authors acknowledge support from the A.P. Giannini Foundation (RAO) and the NIH Director’s New Innovator Award, Searle Scholars, and Pew-Stewart Scholars Programs (TGB).
Footnotes
Conflicts of interest: The authors declare no conflicts of interests.
References
- 1.Politi K, Herbst RS. Lung cancer in the era of precision medicine. Clin Cancer Res. 2015;21:2213–20. doi: 10.1158/1078-0432.CCR-14-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang Y-J, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doebele RC, Davis LE, Vaishnavi A, Le AT, Estrada-Bernal A, Keysar S, et al. An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101. Cancer Discovery. 2015;5:1049–57. doi: 10.1158/2159-8290.CD-15-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo M, Misale S, Wei G, Siravegna G, Crisafulli G, Lazzari L, et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal cancer. Cancer Discovery. 2015 doi: 10.1158/2159-8290.CD-15-0940. [DOI] [PubMed] [Google Scholar]
- 7.Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, Ercan D, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19:1469–72. doi: 10.1038/nm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardini E, Bosotti R, Borgia AL, De Ponti C, Somaschini A, Cammarota R, et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol Oncol. 2014;8:1495–507. doi: 10.1016/j.molonc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discovery. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–64. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]