Abstract
Particulate matter (PM) exposures have been linked to mortality, low birth weights, hospital admissions, and diseases associated with metabolic syndrome, including diabetes mellitus, cardiovascular disease, and obesity. In a previous in vitro and in vivo study, data demonstrated that PM10µm collected from Jeddah, Saudi Arabia (PMSA) altered expression of genes involved in lipid and cholesterol metabolism, as well as many other genes associated with metabolic disorders. PMSA contains a relatively high concentration of nickel (Ni), known to be linked to several metabolic disorders. In order to evaluate if Ni and PM exposures induce similar gene expression profiles, mice were exposed to 100µg/50µl PMSA (PM-100), 50µg/50µl nickel chloride (Ni-50), or 100µg/50µl nickel chloride (Ni-100) twice a week for 4 weeks and hepatic gene expression changes determined. Ultimately, 55 of the same genes were altered in all 3 exposures. However, where the two Ni groups differed markedly was in the regulation (up or down) of these genes. Ni-100 and PM-100 groups displayed similar regulations, whereby 104 of the 107 genes were similarly modulated. Many of the 107 genes involved in metabolic syndrome and include ALDH4A1, BCO2, CYP1A, CYP2U, TOP2A. In addition, the top affected pathways such as fatty acid α-oxidation, and lipid and carbohydrate metabolism, are involved in metabolic diseases. Most notably, the top diseased outcome affected by these changes in gene expression was cardiovascular disease. Given these data, it appears that Ni and PMSA exposures display similar gene expression profiles, modulating the expression of genes involved in metabolic disorders.
Keywords: gene expression, metabolic diseases, liver, cholesterol, particulate matter, nickel
Introduction
Particulate matter (PM) exposure has long been associated with many diseases classified as metabolic syndrome (MtS) diseases. Metabolic syndrome refers to 5 risk factors: obesity, hypertension, hyperglycemia, high triglycerides, and low high density lipoprotein (HDL). Subjects who have 3 or more of these risk factors are considered to display MtS. Epidemiological and molecular studies demonstrated PM exposure to be associated with MtS risk factors: hypertension (Pope et al, 2015), hyperglycemia (Fleisch et al, 2014; Li et al, 2014; Wang et al, 2014; Zheng et al, 2013), high triglycerides (Rizzo et al, 2014; Yeatts et al, 2007), obesity (Roberts et al, 2014), and low HDL (Li et al, 2013). In general, PM exposures are beginning to be taken more seriously after years of research demonstrated links between PM and increased hospital admissions (Colucci et al, 2006; Vigotti et al, 2007), chronic obstructive pulmonary disease (Gan et al, 2013), lung cancer (Yanagi et al, 2012), asthma (Karakatsani et al, 2012), migraine headaches (Chiu and Yang, 2015), uticaria (Kousha and Valacchi, 2015) and cardiovascular diseases (Brook et al, 2010; Mazzoli-Rocha et al, 2010; Nishiwaki et al, 2012; Beckerman et al, 2012; Chang et al, 2013; Chiu et al, 2013). PM was categorized to be the major component of air pollution producing the most deleterious effects on human health (Colucci et al, 2006; Samet and Krewski, 2007).
Jeddah, the second largest city in the Kingdom of Saudi Arabia, contains many factors that make it a site for heavy PM exposure. There are power plants, oil refineries, industrial companies, and over 1.4 million cars, not to mention heavy dust storms. All of these factors contribute to high risk potential for PM exposures (Khodeir et al, 2012). Incidences of diabetes mellitus have significantly increased in Saudi Arabia over the past decade. Al-Rubeaan et al (2014) found in a cohort of over 5000 subjects, abnormal glucose metabolism occurred in almost 35% of subjects. An investigation performed by Al-Nozha et al (2004) noted overall prevalence for diabetes mellitus in adults in Saudi Arabia was 23.7%. Saudi Arabia is already burdened with MtS-associated diseases, and given the progressive nature of this country, PM exposures are only likely to be increased and consequently MtS-associated disease frequencies will rise (Al-Malki et al, 2003; Madani et al, 2000)‥
Recently, the use of gene expression profiling has increased in an attempt to more comprehensively elucidate mechanisms underlying PM-mediated adverse health effects (Huang, 2013). To investigate this ongoing issue, our lab undertook studies to characterize the influence of PM collected from Saudi Arabia using both in vitro and in vivo exposures. PM from Saudi Arabia (PMSA) was collected at the University campus and is a mixture of coarse and fine PM, with a PM2.5/PM10 ratio of 0.33. In vitro exposure showed BEAS-2B (human bronchial epithelial cells) acutely treated with PMSA displayed dysregulation in pathways involving lipid and cholesterol metabolism (Sun et al, 2012). In vivo exposure revealed mice exposed to 100µg/50µl of PMSA for 24 hr displayed alterations in many genes involved in metabolic syndrome (Brocato et al, 2014).
Since genes involved in metabolic syndrome are primarily transcribed in the liver, a 4 week exposure was performed in mice using 100 µg PMSA or 50 or 100 µg of nickel chloride (NiCl). Both Ni and PM exposures are associated with MtS and Ni is present in a relatively high concentration in the PMSA. The aim of this study was to see if exposure to Ni or PMSA dysregulate pathways involved in MtS in a similar manner.
Materials and Methods
Animals
Specific pathogen-free 8–10 week-old male FVB/N mice weighing 23–25 g were purchased from Taconic Farms (German-town, NY). All animals were housed in an approved facility at NYUSOM and acclimated for 1–2 weeks under controlled temperature (22 ± 2°C) and relative humidity (30–50%) with a 12-hr light/dark cycle prior to use in any experiments. Mice were provided ad libitum access to standard lab chow and filtered water except during oropharyngeal exposures. At the time of exposures, mice were randomly assigned to each exposure group (n = 5). All protocols were approved by the NYU School of Medicine IACUC. One out of 5 mice died in the Ni-50, PM-100, and control groups and 2 out of 5 mice died in the Ni-100 group by the end of the 4 weeks. All deaths occurred during aspiration.
Oropharyngeal Aspirations (OPA)
Mice (n=5) were exposed via aspiration to 100 µg PM10 (3.92 mg/kg) collected from Jeddah, Saudi Arabia. The cumulative dose of PM received by the mice over the 4 week period was 39.36 mg/kg. The dose of PM2.5 received by each mouse was 1.29 mg/kg. The two other treatment groups received NiCl2 - 50 or 100 µg. Control mice (n=5) were exposed to an equivalent volume of sterile pyrogen free water. For each aspiration, mice were anesthetized in a closed container containing isoflurane (1–3% in oxygen) (Butler Schein, Dublin, OH), weighed, and aspirated with a volume of approximately 50 µl
Animal Processing Post-Exposure
Oropharyngeal aspirated animals were euthanized 24hr post-final via intraperitoneal (ip) injection of pentobarbital (150–200 mg/kg). At necropsy, blood was drawn from the vena cava and serum was isolated and stored at −20°C. Lungs, kidneys, spleen, and liver were collected and stored at −80°C for future analyses.
Particle Characterization
Details regarding the particle collection and extraction techniques, as well as, the components of PMSA were previously described (Khodeir et al, 2012; Sun et al, 2012). PMSA was analyzed by x-ray fluorescence for the concentration of 27 elements. Re-suspended soil and oil combustion contributed 82% of the mass and mixed industrial sources, traffic sources, and marine aerosols were also found to be present. The PMSA was heavily concentrated with silicon, calcium, sulfur, aluminum and iron. Other metals present include nickel, vanadium, arsenic, lead, cadmium, manganese, titanium and magnesium.
RNA extraction and microarray hybridization
Total RNA was extracted from lungs of control, Ni, and PMSA-exposed mice using Trizol (Invitrogen) and further purified using RNeasy Plus Micro Kit (Qiagen). To synthesize double-stranded cDNA (dsDNA), 100ng total RNA was used. cRNA was synthesized from dsDNA template, and subsequently used to produce sense single-stranded cDNA (ssDNA) with incorporated deoxyuridine triphosphate. The ssDNAs were fragmented, end-labeled, and hybridized to Affymetrix Mouse Gene 1.0 ST Array (Affymetrix). Hybridization and scanning of the arrays were performed using a standard procedure.
Microarray data analysis
Microarray data analysis was performed using GeneSpring v12.0 (Agilent Technologies). All microarray data is MIAME compliant and raw data were deposited in NCBIs Gene Expression Omnibus (GEO ID: GSE38172). The expression value of each probe set was determined after quantile normalization using RMA algorithm and baseline transformation to median levels of control samples. Differentially expressed genes were identified using one-way ANOVA (p<0.05). Functional annotation was analyzed with the Gene Ontology (GO) classification system using DAVID software (http://david.abcc.ncifcrf.gov/home.jsp). Gene network and pathway analysis was performed using Ingenuity Pathway Analysis (http://www.ingenuity.com).
Real time quantitative PCR
Total RNA extracted from control and treated lung tissue was converted to single stranded cDNA using Superscrip® III (Invitrogen). Quantitative real-time PCR analysis was performed using SYBR green PCR system (Applied Biosystems) on ABI prism 7900HT system (Applied Biosystems). Relative gene expression levels were normalized to ACTB expression. All PCR reactions were performed in duplicate.
Results
Exposure to PMSA or Ni on gene expression of liver cells in vivo
Gene expression changes were investigated in livers of FVB/N mice exposed for 4 weeks via aspiration to Ni at 50 µg nickel chloride (Ni-50) or 100 µg nickel chloride (Ni-100), or 100 µg PM collected from Saudi Arabia (PM-100). Fold change analysis (one-way ANOVA, alpha level only) identified 1,054 dysregulated genes by Ni-50; 476 were down-regulated and 578 were up-regulated (Tables 1–3). Ni-100 altered the expression of 2,701 genes; 1,298 were down-regulated and 1,403 were up-regulated. PM-100 affected expression of 716 genes; 476 were down-regulated and 578 were up-regulated. The fold change of the altered genes was greater than 1.1. Tables 1–3 show the top 10 up- and down-regulated genes for each exposure group.
Table 1.
Top ten up- and down-regulated genes for mice exposed to 50 µg of nickel chloride.
| Gene Name | Gene Symbol | Fold Change |
|---|---|---|
|
Cytochrome P450, family 4, subfamily a, polypeptide 14 |
Cyp4a14 | 2.83 |
| Acyl-CoA thioesterase 3 | Acot3 | 2.14 |
|
Inhibitor of DNA binding 1 |
Id1 | 1.92 |
| Vanin 1 | Vnn1 | 1.84 |
|
Small nucleolar RNA, C/D box 14C |
Snord14c | 1.79 |
|
Suppressor of cytokine signaling 2 |
Socs2 | 1.78 |
|
Cytochrome P450, family 4, subfamily a, polypeptide 31 |
Cyp4a31 | 1.77 |
|
Small nucleolar RNA, C/D box 14E |
Snord14e | 1.76 |
|
Cytochrome P450, family 4, subfamily a, polypeptide 10 |
Cyp4a10 | 1.71 |
| Heat shock protein 1 | Hsph1 | 1.66 |
| Metallothionein 2 | Mt2 | −2.54 |
| Orosomucoid 2 | Orm2 | −2.42 |
|
Cytochrome P450, family 2, subfamily b, polypeptide 10 |
Cyp2b10 | −1.69 |
| Orosomucoid 3 | Orm3 | −1.59 |
|
Leucine-rich repeats and transmembrane domains 1 |
Lrtm1 | −1.53 |
| Early growth response 1 | Egr1 | −1.52 |
| Hemochromatosis type 2 | Hfe2 | −1.51 |
| Lipocalin 2 | Lcn2 | −1.50 |
|
Acyl-CoA synthetase short-chain family member 2 |
Acss2 | −1.45 |
| Carboxylesterase 2C | Ces2C | −1.45 |
Table 3.
Top ten up- and down-regulated genes for mice exposed to 100 µg of particulate matter from Saudi Arabia
| Gene Name | Gene Symbol | Fold Change |
|---|---|---|
|
Cytochrome P450, family 4, subfamily a, polypeptide 14 |
Cyp4a14 | 2.34 |
|
Cytochrome P450, family 4, subfamily a, polypeptide 10 |
Cyp4a10 | 1.85 |
|
Cysteine-rich with EGF-like domains 2 |
Creld2 | 1.71 |
| Heat shock protein 1 | Hsph1 | 1.65 |
|
Serine peptidase inhibitor, clade A, member 7 |
Serpina7 | 1.63 |
| G0/G1 switch gene 2 | G0s2 | 1.58 |
|
Cysteine sulfinic acid decarboxylase |
Csad | 1.49 |
| Acyl-CoA thioesterase 2 | Acot2 | 1.49 |
|
Glutathione S-transferase, alpha 1 |
Gsta1 | 1.48 |
| Jun proto-oncogene | Jun | 1.47 |
| Orosomucoid 1 | Orm1 | −2.42 |
| Orosomucoid 3 | Orm3 | −1.89 |
| Metallothionein 2 | Mt2 | −1.80 |
|
Zinc finger and BTB domain containing 16 |
Zbtb16 | −1.54 |
|
Cytochrome P450, family 39, subfamily a, polypeptide 1 |
Cyp39a1 | −1.45 |
| Early growth response 1 | Egr1 | −1.44 |
|
Solute carrier family 8 (sodium/lithium/ calcium exchanger), member B1 |
Slc24a6 | −1.39 |
|
Solute carrier family 22 (organic anion transporter), member 7 |
Slc22a7 | −1.38 |
| Interleukin 1 receptor, type I | Il1r1 | −1.37 |
| PRA1 domain family 2 | Praf2 | −1.35 |
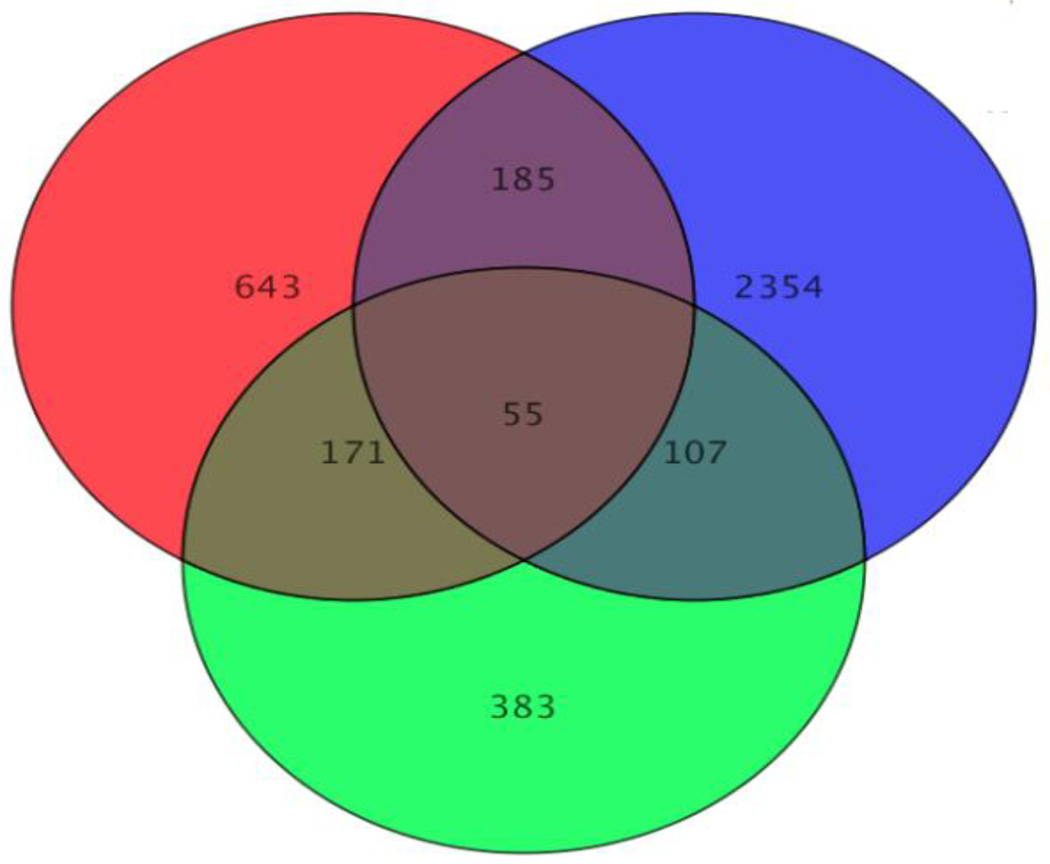
Figure 1 is a Venn diagram illustrating the overlap of dysregulated genes amongst the 3 exposure groups. There were 55 of the same genes altered in all 3 exposures. Between Ni exposures, regulation of similar genes varied greatly. Only 34 out of the 55 genes were altered in the same direction, up or down. Surprisingly, PM-100 and Ni-100 altered the expression of the 55 genes in a similar fashion; 52 out of the 55 genes were regulated in the same direction. The similarity in altered gene expression was further investigated between Ni-100 and PM-100 by examining all 107 genes that were changed by both exposures. Data demonstrated that 104 out of 107 altered genes were regulated in the same direction. Tables 4 (down-regulated) and 5 (up-regulated) list the top 10 genes affected by both Ni and PM.
Figure 1. Venn Diagram.
Red (50 µg Ni) (Ni-50); Blur (100 µg Ni) (Ni-100); Green (100 µg PM) (PM-100). Ni-50 dysregulated 1,054 genes; Ni-100 dysregulated 2,701 genes; PM-100 dysregulated 716 genes. There were 55 similar genes altered by all 3 exposure groups. The two doses of nickel only changed 34 out of the 55 genes in the same direction, while the PM-100 and the Ni-100 changed 52 out of the 55 genes in the same direction. Ni-100 and PM-100 affected 107 similar genes, 104 of which were modulated in the same direction.
1Table 4.
Down-regulated genes similar for mice exposed to 100 µg of particulate matter from Saudi Arabia or 100 µg of nickel chloride.
| Gene Name | Gene Symbol |
Regulation |
|---|---|---|
|
Serine/arginine-rich splicing factor 3 |
Srsf3 | Down |
|
Cytochrome P450, family 2, subfamily u, polypeptide 1 |
Cyp2U1 | Down |
|
ATP synthase mitochondrial F1 complex assembly factor 1 |
Atpaf1 | Down |
| Interleukin 6 receptor, alpha | Il6ra | Down |
| MutS homolog 2 | Msh2 | Down |
|
ATP synthase mitochondrial F1 complex assembly factor 1 |
Atpaf1 | Down |
|
Mitogen-activated protein kinase associated protein 1 |
Mapkap1 | Down |
| Stem-loop binding protein | Slbp | Down |
| Asparagine-linked glycosylation 14 | Alg14 | Down |
|
Protein tyrosine phosphatase, receptor type, A |
Ptpra | Down |
| Polymerase (RNA) I polypeptide D | Polr1d | Down |
|
Protein inhibitor of activated STAT 3 |
Pias3 | Down |
| Transmembrane protein 184a | Tmem184a | Down |
|
Cysteine sulfinic acid decarboxylase |
Csad | Down |
| Synaptotagmin | Syt1 | Down |
This is not a complete list of genes down-regulated by nickel and particulate matter.
1Table 5.
Altered genes similar for mice exposed to 100 µg of particulate matter from Saudi Arabia or 100 µg of nickel chloride.
| Gene Name | Gene Symbol |
Regulation |
|---|---|---|
| Polo-like kinase 1 | Plk1 | Up |
|
Spondin 2, extracellular matrix protein |
Spon2 | Up |
|
Retinol saturase (all trans retinol 13,14 reductase) |
Retstat | Up |
| M-phase phosphoprotein 9 | Mphosph9 | Up |
|
Vascular endothelial growth factor B |
Vegfb | Up |
| Kinesin family member 20A | Kif20a | Up |
| Proline rich 23A | Prr23a | Up |
| Scleraxis | Scx | Up |
| Serine incorporator 2 | Serinc2 | Up |
| Stathmin 1 | Stmn1 | Up |
| Olfactory receptor 485 | Olfr485 | Up |
|
Glutamate receptor, ionotropic, AMPA1 (alpha 1) |
Gria1 | Up |
| M-phase phosphoprotein 9 | Mphosph9 | Up |
|
Neutral cholesterol ester hydrolase 1 |
Nceh1 | Up |
| Neuralized homolog 1b | Neurl1B | Up |
This is not a complete list of genes up-regulated by nickel and particulate matter.
Metabolic syndrome (MtS) - associated genes
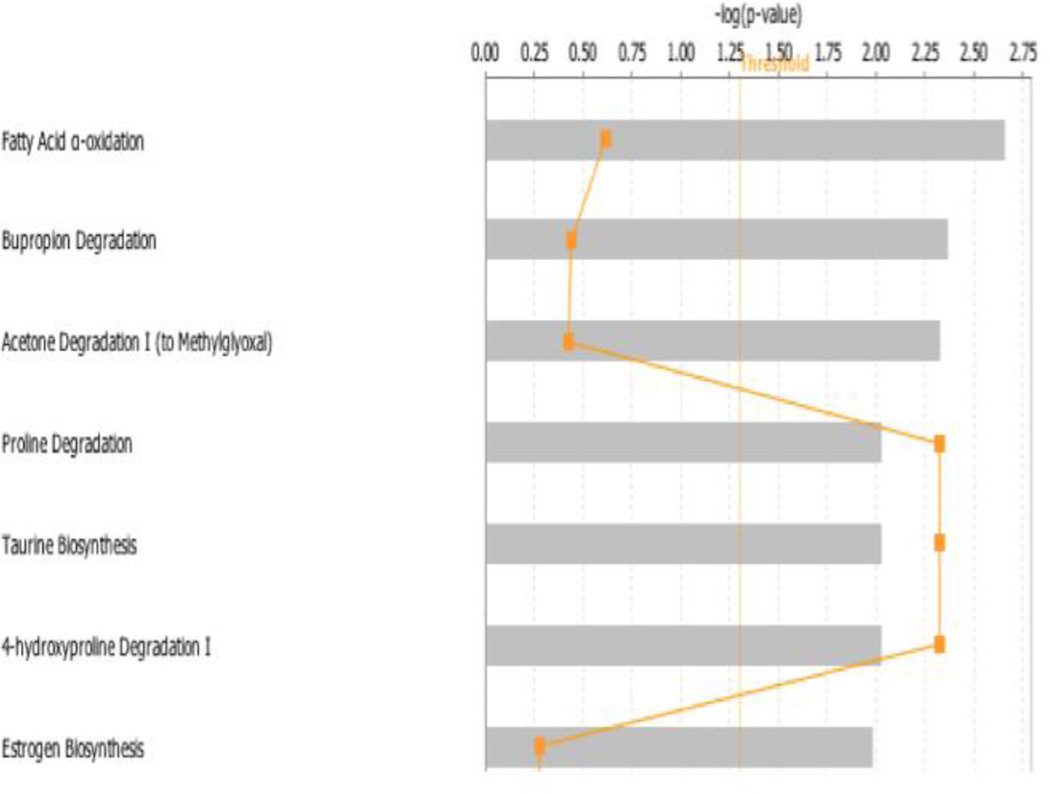
To investigate the biological function of the 107 genes differentially modulated by Ni-100 and PM-100 exposures, the list of changed genes were uploaded into the Ingenuity Pathway Analysis (IPA) tool. Many of the dysregulated genes altered in the same direction by Ni-100 and PM-100 were involved in pathways associated with MtS. The top canonical pathways (Figure 2) ranked by p-value were fatty acid α-oxidation, bupropion degradation, and acetone degradation. ALDH4A1 (-1.13) and BCO2 (-1.15) were the two major genes deregulated in the fatty acid α-oxidation pathway that play a role in metabolic syndrome (Amengual et al, 2011; Pang et al, 2014; Yoon et al, 2004). CYP1A2 (-1.12) and CYP2UI (-1.18) were both down-regulated in the bupropion and acetone degradation pathways and both genes encode enzymes that are involved in lipid metabolism (Chuang et al, 2004; Shertzer et al, 2004).
Figure 2. Top Canonical Pathways.
Top 7 canonical pathways identified by IPA from genes changed more than 1.1-fold (p≤0.05). Bars represent −log (p-value) for significance; orange lines represent the ratio of changed genes to the total number of genes in the specific pathway.
Network analysis of the altered genes revealed that 13 significant networks were affected by the change in expression. Several networks involved in MtS were affected including “carbohydrate metabolism, lipid metabolism, small molecule biochemistry” with 11 focus molecules and a significance score of 20 (the negative log of the p-value). Other interesting networks include “cell cycle, cellular movement, cellular assembly and organization” (score: 50); “infectious disease, cellular compromise, gastrointestinal disease” (score: 30); “behavior, nervous system development and function, tissue development” (score: 27).
The most prominent disease affected by the altered genes was cardiovascular disease with 13 affected molecules including TOP2A, VEGFB, and IL6R. Other affected diseases that are worth mentioning include, developmental disorders, skeletal and muscle disorders, organismal injury and abnormalities, and connective tissue disorders. TSA and FoxM1 were found to be upstream regulators predicted to be activated. Both of these molecules are upstream regulators of TOP2A, CCNA2, and PLK1.
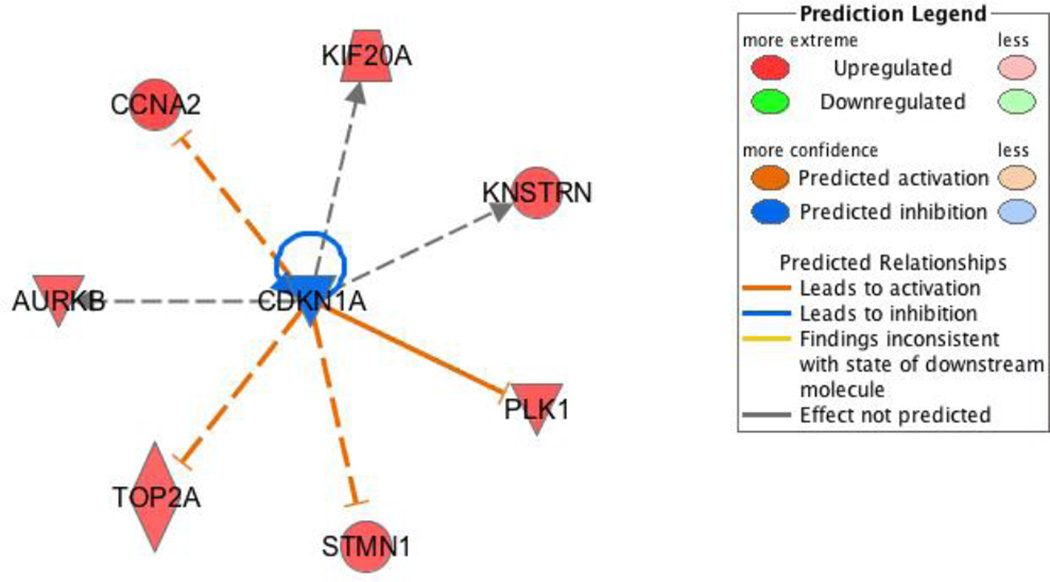
CDKN1A, which encodes p21, a very potent cyclin-dependent kinase inhibitor (Broude et al, 2007), was identified as an upstream regulator of many of MtS-associated genes that were increased by Ni-100 and PM-100. CDKN1A was predicted to be inhibited and this led to up-regulation of TOP2A, PLK1, CCNA2, and others (Figure 3). Other upstream regulators worth noting are Rb and E2F. Rb sequesters E2F in the nucleus and prevents it from acting as a transcription factor to promote cell cycle progression from G1 to S-phase (Hiebert,1993; Hiebert et al,1992 ). These proteins increased gene expression of the same genes as p21, which was expected since p21 inhibition of CDK2 and CDK4/6 leads to dephosphorylation and activation of Rb (Broude et al, 2007).
Figure 3. Upstream Regulator: CDKN1A.
IPA identified many upstream regulators predicted to be active or inactive based on the gene expression profile. CDKN1A, also known as p21, was predicted to be strongly inhibited based on the surrounding genes, all of which are activated. All of these genes besides one (KNSTRN) that work to inhibit CDKN1A are involved in metabolic syndrome. The figure legend describes predicted relationships of all the genes to the upstream regulator.
PM modulated the expression of several MtS-associated genes that was not altered by Ni. STAT5A (-1.17) was down-regulated and this gene encodes a protein involved in lipid storage (Teglund et al, 1998) and metabolism of fatty acids (Schirra et al, 2008). APO4 (-1.33) encodes a protein involved in the metabolism of cholesterol (Duverger et al, 1991) and increases biosynthesis of fatty acids (Goldberg et al, 1990).
Gene expression validation
To validate the gene expression changes observed in the microarray analysis, total RNA was extracted from livers of PM or Ni- exposed mice and purified. Several candidate genes were selected for quantitative real time PCR (RT-qPCR). Gene fold changes were compared to those obtained from microarrays. ALDH4a4, CYP1a2, TOP2A, BCO2, CYP2U1, PLK1, and AURKB were selected as candidate genes to validate the microarray. All of these genes were altered in the same direction in both Ni-100 and PM-100 exposed mice. Table 6 summarizes the average fold change of the selected genes from the microarray analysis, as well as corresponding RT-qPCR results.
Table 6.
RT-qPCR validation.
| Gene Symbol | Microarray (PM-100) |
RT-qPCR1 (PM-100) |
Microarray (Ni-100) |
RT-qPCR1 (Ni-100) |
|---|---|---|---|---|
| ALDH4a1 | −1.13 | −1.22 | −1.16 | −1.38 |
| CYP1a2 | −1.12 | −1.19 | −1.14 | −1.67 |
| TOP2A | 1.12 | 1.07 | 1.21 | 1.18 |
| BCO2 | −1.15 | −1.34 | −1.25 | −1.22 |
| CYP2U1 | −1.18 | −1.84 | −1.41 | −1.61 |
| PLK1 | 1.20 | 2.65 | 1.29 | 1.72 |
| AURKB | 1.17 | 1.33 | 1.25 | 1.21 |
PM-100: 100 µg Particulate Matter from Jeddah, Saudi Arabia; Ni-100: 100 µg of nickel chloride
Data is presented as a mean of duplicates.
Discussion
Metabolic syndrome- associated diseases are on the rise in Saudi Arabia
Cardiovascular disease and diabetes, two chronic diseases that result from the risk factors of MtS, are on the rise in the Kingdom of Saudi Arabia. Obesity has also become a major health concern in Saudi Arabia. A study by (Al-Othaimeen AI, 2007) found that 38% of male and 28% female of the 19,598 Saudi Arabian citizens tested were overweight. In addition, a number of other studies highlighted the obesity problem in Saudi Arabia (Al-Malki et al, 2003; Madani et al, 2000). Studies evaluating locations with high levels of PM exposure demonstrated that PM increases the amount of hospital admissions and promotes cardiovascular disease and diabetes. Recently, (Khodeir et al, 2012) conducted a multi-week, multiple site sampling campaign to determine the source apportionment and elemental composition of PM10 in Jeddah. The major source factors for PM10 were soil re-suspensions, oil combustion, mixed industrial sources, traffic sources, and marine aerosols. Components of the PM10 from Jeddah were characterized by Khodeir et al (2012). Jeddah consistently exceeds the 80 µg/m3 PM established by the Presidency of Meteorology and Environment in Saudi Arabia.
Top Canonical Pathways
IPA and DAVID were used to analyze the 107 genes similarly altered by both Ni-100 and PM-100 and the top canonical pathways involved in MtS are presented in Figure 2. The top canonical pathway was fatty acid oxidation where BCO2 and ALDH4A1 were both down-regulated. Dysregulation in fatty acid oxidation pathways are involved in insulin sensitivity (Randle et al, 1994) and obesity (Furukawa and Araki, 2013; Furukawa et al, 2004). A detailed review by Wakil and Abu-Elheiga (2009) on fatty acids and MtS discuss the key roles that fatty acids play as cellular signaling molecules and how their dysfunctions form the etiology of MtS. The gene encoding ALDH4A1 (aldehyde dehydrogenase family 4A1) leads to production of proline and deficiency of this enzyme results in the metabolic disease hyperprolinemia (Kamoun et al, 1998; Onenli-Mungan et al, 2004). Several reports supported the notion that down-regulation of ALDH4A1 might increase cell damage due to generation of reactive oxygen species (ROS). ALDH4A1 blocks Nrf2, which is a transcription factor, that activates genes involved in fatty acid oxidation (FAO) (Pang et al, 2014). Thus down-regulation in expression of ALDH4A1 may potentially increase FAO. An investigation by Yoon et al (2004) suggested that p53 might play a protective role against cell damage induced by generation of intracellular ROS, through transcriptional activation of ALDH4A1. ALDH4associated effects on p53 may be mediated by enzymatic interaction with MDM2 (Nicholson et al, 2014). Thus a down-regulation of ALDH4 might potentially lead to enhanced cell damage induced by ROS.
BCO2 (beta-carotene oxygenase 2), which was also down-regulated in the fatty acid oxidation pathway, oxidizes beta-carotene for biosynthesis of vitamin A (Kiefer et al, 2001). Beta-carotene metabolizing enzymes are expressed in adipocytes and shown to reduce adipocyte size and body fat % in mice by regulating PPARγ activity (Amengual et al, 2011). Ni and PM-induced down-regulation of BCO2 may contribute to obesity.
Most of the other canonical pathways given by IPA were involved in MtS. The second most influenced pathway according to p-value was bupropion degradation. Bupropion is better known as Wellbutrin, a type of antidepressant that is also used to treat obesity. Dysregulation of the catabolic pathways involved in this drug’s metabolism is likely to interfere with the effects of the drug. There is now a phase III clinical trial for a sustained release form of bupropion called Naproxene (Apovian et al, 2013). Studies found that the drug is a new approach to tackling the problem of obesity and may soon be a unique and valuable therapeutic option (Apovian et al, 2013; Padwal, 2009; Wadden et al, 2011).
Other canonical pathways (in order of significance) included, acetone degradation, proline degradation, and taurine biosynthesis. Acetones are produced by decarboxylation of ketone bodies. Inability to degrade acetone might result in ketoacidosis and promote diabetes (Kamel and Halperin, 2015). Interfering with taurine biosynthesis might promote liver disease and hypertension (Zhang et al., 2014). Supplementation with taurine was found to reduce oxidative stress and promote liver health in a murine model.
Upstream Regulators
IPA found many upstream regulators that were predicted to be inhibited or activated based on the altered genes and the direction the genes changed. The most interesting of these regulators was CDKN1A (Figure 3). CDKN1A, also known as p21 is involved in the cell cycle by inhibiting cyclin dependent kinases which acts to terminate the cell cycle at the G1-S phase transition. IPA predicted p21 to be markedly inhibited based on the gene changes. All genes leading to p21 inhibition were up-regulated; PLK1, TOP2A, KIF20, CCNA2, AURKB, and STMN1. Moreover, many of these genes were also identified in MtS.
Up-regulation of PLK1 (polo-like kinase 1) is associated with hepatocellular carcinoma. Hypomethylation of the PLK1 gene is associated with hepatocellular carcinoma (Stefanska et al, 2011). According to clinicaltrials.gov, levofloxacin, a TOP2A inhibitor, is in phase IV clinical trials for the treatment of obesity in humans. Therefore, an up-regulation of TOP2A (DNA topoisomerase 2 A) as produced by Ni and PM treatment would promote obesity. KIF20A (kinesin family member 20 A) was found to be regulated by CLOCK in mouse livers. CLOCK is also involved in lipid metabolism (Oishi et al, 2005). Gnocchi et al (2015) indicated how the circadian clock and lipid metabolism are interconnected and further understanding of this relationship is pertinent in combatting metabolic diseases .
CCNA2 (cyclin that regulates CDK2) promotes the G1/S and G2/M cell cycle transitions. Up-regulation enhances transitions through the cell cycle and increase proliferation. CCNA2 expression is associated with hepatocellular carcinoma (Satow et al, 2010). STMN1 (stathmin1) promotes disassembly of microtubules by destabilizing microtubules and up-regulation was shown to enhance expression of VEGF (vascular endothelial growth factor) (Tamura et al, 2013).
Two other upstream regulators worth discussing are TSA (trichostatin-A) and FOXM1 (forkhead box M1). Both of these molecules were predicted to be activated based on gene expression. Surprisingly, the same set of genes used to predict p21 inhibition was used to predict activation of these upstream molecules; PLK1, TOP2A, KIF20A, CCNA2, STMN1, AURKB. TSA is a group 1 and II HDAC (histone deacetylase) inhibitor but does not inhibit sirtuins (group 3). Tian et al (2014) demonstrated the importance of acetylation in breast cancer. Acetylation-defective mutants of Pparγ1 were associated with reduced lipid synthesis in ErbB2 overexpressing breast cancer cells. Activation of FOXM1 may also contribute metabolic disorders. FOXM1 is activated in highly proliferating normal cells and cancer cells; it promotes proliferation by progressing the cell cycle via directly activating the transcription of cyclin D1 and cyclin B1. An investigation by Hu et al (2014) reported that down-regulation of FOXM1 suppressed proliferation of hepatocellular carcinoma cells.
Other Ni and PM-dysregulated genes involved in metabolic syndrome
Besides the genes listed above, several other genes were identified from our gene list that play roles in diseases associated with MtS. One of these genes was PAX6. PAX6 (paired box 6) was down-regulated in both Ni and PM-treated mice and is crucial for β-cell function, insulin biosynthesis, and glucose-induced insulin secretion; decreased Pax6 diminishes processing of insulin (Gosmain et al, 2012b). Decreased Pax6 decreases release of glucagon (Gosmain et al, 2012a). Overexpression of PAX6 mRNA reduced synthesis of insulin (Wolf et al, 2010). CYP1A2 (cytochrome P450, family 1, subfamily A) mRNA was also lowered in livers of Ni or PM-treated mice. CYP1A2 is involved in the synthesis of lipids, steroids, and cholesterol. CYP1A2 protects against ROS production in mouse liver microsomes (Shertzer et al, 2004). C12orf4 (chromosome 12 open reading frame 4) was partially silenced by both Ni and PM and silencing of this gene is associated with carcinoma of the liver according to the Catalogue Of Somatic Mutations In Cancer (COSMIC).
Ni and PM induce similar alterations of MtS-associated genes
The toxicity of PM depends largely on its components. Lippmann et al (2007) noted that the cardiotoxicity effects of fine PM exposures are predominantly driven by Ni. Niu et al (2013) noted that metallic components of PM may be responsible for the cardiovascular disorders produced by PM exposures. Nickel has long been reported from epidemiological and molecular studies to promote cardiovascular disease (Bell et al, 2014; Ying et al, 2013), respiratory cancers (Grimsrud and Peto, 2006), insulin resistance (Xu et al, 2012) and mortality (Laden et al, 2000). Hsu and Lippmann (2007) previously found that the levels of Ni in concentrated ambient particulate (CAP) matter PM2.5 were strongly correlated to acute changes in heart rates and their variability. Xu et al (2012) noted that Ni exposure exerted effects on metabolism comparable to CAP matter PM2.5. Exposure to both Ni and PM significantly enhanced fasting glucose and worsened insulin resistance indices, when compared with exposure to CAP matter alone. The effects of the co-exposure were thought to be mediated by AMP-activated protein kinase. Another study that co-exposed mice to nickel and CAP also found synergistic effects between the two. Tumor necrosis factor-a and monocyte chemotactic protein 1 were both significantly up-regulated in co-exposed mice (Goebeler et al, 1999).
Given the numerous studies that pointed towards Ni as being a key player in the adverse health effects associated with PM exposures, it was not surprising to find that this metal affected the expression of MtS-associated genes in a similar fashion. The 3 exposure groups (Ni-50, Ni-100, and PM-100) shared 55 genes that displayed altered expression. The regulation of those genes between the two Ni groups was not similar; however, Ni-100 and PM-100 shared 52 out of 55 genes changed in the same direction. Ni-100 and PM-100 shared 107 of the same altered genes, and 104 out of 107 of those genes were regulated in the same direction. Data suggest that Ni and PM at the same dose (100 µg) induce similar alterations to genes involved in MtS.
The amount of Ni in the PM is 9.2 ng/m3. This is a high concentration compared to several other sites. Saudi Arabia burns a lot of oil and when oil is burned you produce Ni and vanadium. This may explain why Ni concentrations in Saudi Arabia PM are relatively high compared to other locations.
Conclusions
Particulate matter exposures are known to be associated with a number of adverse health outcomes, hospital admissions (Colucci et al, 2006; Vigotti et al, 2007), chronic obstructive pulmonary disease (Gan et al, 2013), lung cancer (Yanagi et al, 2012), asthma (Karakatsani et al, 2012) migraine headaches (Chiu and Yang, 2015), urticarial (Kousha and Valacchi, 2015) and cardiovascular diseases (Brook et al, 2010; Mazzoli-Rocha et al, 2010; Nishiwaki et al, 2012; Beckerman et al, 2012; Chang et al, 2013; Chiu et al, 2013). Both organic and inorganic components of PM hold potential to produce problems once inhaled (Ghio et al, 2012). It is difficult to pinpoint the exact PM component that is producing damage being evaluated. Since Ni was relatively high in the PM from Saudi Arabia, it was decided to compare gene expression changes occurring after a 4 week in vivo exposure to PM vs. gene alterations after in vivo exposure to the metal. Almost all of the changes in the 107 similar genes were altered in the same direction. Many of these genes were found to play roles in development of MtS risk factors or diseases associated with this syndrome.
Based upon our findings, it appears that exposure to Ni or PM at the same dose dysregulates the same MtS-associated pathways. Further understanding of the mechanisms by which exposure to Ni or PM produces these adverse effects may provide novel prevention and therapeutic strategies for better control and treatment of metabolic disorders such as obesity, type 2 diabetes, and insulin resistance.
It is likely that given the history of Ni with metabolic disorders, Ni in PM may be contributing to many of the differentially expressed genes induced by PM. In order to irrefutably conclude that Ni is the major culprit, an experiment needs to be conducted where all components of PM remain exactly the same, while Ni is removed completely. However, to acquire these two types of PM would be extremely difficult. Future experiments investigating PM components need to consider this limitation or try to find similar PM samples where one sample contains the component in question at low levels. Alternatively, an overexpression experiment similar to the investigation by Xu et al (2012) would also provide benefits.
Table 2.
Top ten up- and down-regulated genes for mice exposed to 100 µg of nickel chloride.
| Gene Name | Gene Symbol | Fold Change |
|---|---|---|
|
Cytokine inducible SH2- containing protein |
Cish | 3.54 |
| Myelocytomatosis oncogene | Myc | 2.85 |
|
Cytochrome P450, family 4, subfamily a, polypeptide 14 |
Cyp4a14 | 2.82 |
|
Pleckstrin homology-like domain, family A, member 1 |
Phlda1 | 2.70 |
|
Serine peptidase inhibitor, clade A, member 4 |
Serpina4 | 2.62 |
| Forkhead box Q1 | Foxq1 | 2.59 |
|
Solute carrier family 25, member 30 |
Slc25a30 | 2.29 |
|
Cytochrome P450, family 4, subfamily a, polypeptide 31 |
Cyp4a31 | 2.24 |
|
WD repeat and SOCS box- containing 1 |
Wsb1 | 1.85 |
|
Small nucleolar RNA, C/D box 14C |
Snord14c | 1.84 |
| Uridine phosphorylase 2 | Upp2 | −2.07 |
|
Thyroid hormone responsive |
Thrsp | −2.05 |
|
Arrestin domain containing 3 |
Arrdc3 | −1.92 |
|
Cytochrome P450, family 7, subfamily A, polypeptide 1 |
Cyp7a1 | −1.79 |
|
3-hydroxy-3- methylglutaryl-Coenzyme A reductase |
Hmgcr | −1.70 |
| MicroRNA 107 | Mir107 | −1.69 |
| Lanosterol synthase | Lss | −1.58 |
|
Serine/arginine-rich splicing factor 3 |
Srsf3 | −1.57 |
| Neuregulin 4 | Nrg4 | −1.55 |
|
Methylsterol monoxygenase 1 |
Sc4mol | −1.54 |
ACKNOWLEDGEMENTS
The authors thank NYU and KAU for technical and financial support. This work was supported by National Institute of Health (NIH) grants, ES010344, ES014454, ES000260, ES022935, ES023174 and ES005512 and by King Abdulaziz University (KAU), Jeddah, grant number 4/00/00/252.
Footnotes
Conflict of Interest Statement
None of the authors of the manuscript have a conflict of interest.
References
- Al-Malki JS, Al-Jaser MH, Warsy AS. Overweight and obesity in Saudi females of childbearing age. Int J Obes Relat Metab Disord. 2003;27:134–139. doi: 10.1038/sj.ijo.0802181. [DOI] [PubMed] [Google Scholar]
- Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, Al-Harthi SS, Arafah MR, Khalil MZ, Khan NB, Al-Khadra A, Al-Marzouki K, Nouh MS, Abdullah M, Attas O, Al-Shahid MS, Al-Mobeireek A. Diabetes mellitus in Saudi Arabia. Saudi Med J. 2004;25:1603–1610. [PubMed] [Google Scholar]
- Al-Othaimeen AI, Al-Nozha M, Osman AK. Obesity: an emerging problem in Saudi Arabia. Analysis of data from the National Nutrition Survey. East Mediterr Health J. 2007;13:441–448. [PubMed] [Google Scholar]
- Al-Rubeaan K, Al-Manaa H, Khoja T, Ahmad N, Al-Sharqawi A, Siddiqui K, AlNaqeb D, Aburisheh K, Youssef A, Al-Batil A, Al-Otaibi M, Ghamdi AA. The Saudi Abnormal Glucose Metabolism and Diabetes Impact Study (SAUDI-DM) Ann Saudi Med. 2014;34:465–475. doi: 10.5144/0256-4947.2014.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, Gouranton E, van Helden YG, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, Dembinska-Kiec A, Palou A, Keijer J, Landrier JF, Bonet ML, von Lintig J. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS One. 2011;6:e20644. doi: 10.1371/journal.pone.0020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Kim D, Dunayevich E. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21:935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman BS, Jerrett M, Finkelstein M, Kanaroglou P, Brook JR, Arain MA, Sears MR, Stieb D, Balmes J, Chapman K. The association between chronic exposure to traffic-related air pollution and ischemic heart disease. J Toxicol Environ Health A. 2012;75:402–411. doi: 10.1080/15287394.2012.670899. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Leaderer BP, Gent JF, Lee HJ, Koutrakis P, Wang Y, Dominici F, Peng RD. Associations of PM(2).(5) constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons >/= 65 years of age. Environ Health Persp. 2014;122:138–144. doi: 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocato J, Sun H, Shamy M, Kluz T, Alghamdi MA, Khoder MI, Chen LC, Costa M. Particulate matter from Saudi Arabia induces genes involved in inflammation, metabolic syndrome and atherosclerosis. J Toxicol Environ Health A. 2014;77:751–766. doi: 10.1080/15287394.2014.892446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Broude EV, Demidenko ZN, Vivo C, Swift ME, Davis BM, Blagosklonny MV, Roninson IB. p21 (CDKN1A) is a negative regulator of p53 stability. Cell Cycle. 2007;6:1468–1471. [PubMed] [Google Scholar]
- Chang CC, Kuo CC, Liou SH, Yang CY. Fine particulate air pollution and hospital admissions for myocardial infarction in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. 2013;76:440–448. doi: 10.1080/15287394.2013.771559. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Tsai SS, Weng HH, Yang CY. Short-term effects of fine particulate air pollution on emergency room visits for cardiac arrhythmias: A case-crossover study in Taipei. J Toxicol Environ Health A. 2013;76:614–623. doi: 10.1080/15287394.2013.801763. [DOI] [PubMed] [Google Scholar]
- Chiu HF, Yang CY. Air pollution and daily clinic visits for migraine in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. 2015;78:549–558. doi: 10.1080/15287394.2015.983218. [DOI] [PubMed] [Google Scholar]
- Chuang SS, Helvig C, Taimi M, Ramshaw HA, Collop AH, Amad M, White JA, Petkovich M, Jones G, Korczak B. CYP2U1, a novel human thymus- and brain-specific cytochrome P450, catalyzes omega- and (omega-1)-hydroxylation of fatty acids. J Biol Chem. 2004;279:6305–6314. doi: 10.1074/jbc.M311830200. [DOI] [PubMed] [Google Scholar]
- Colucci ME, Veronesi L, Roveda AM, Marangio E, Sansebastiano G. [Particulate matter (PM10) air pollution, daily mortality, and hospital admissions: recent findings] Ig Sanita Pubbl. 2006;62:289–304. [PubMed] [Google Scholar]
- Duverger N, Murry-Brelier A, Latta M, Reboul S, Castro G, Mayaux JF, Fruchart JC, Taylor JM, Steinmetz A, Denefle P. Functional characterization of human recombinant apolipoprotein AIV produced in Escherichia coli . Eur J Biochem. 1991;201:373–383. doi: 10.1111/j.1432-1033.1991.tb16294.x. [DOI] [PubMed] [Google Scholar]
- Fisher CD, Jackson JP, Lickteig AJ, Augustine LM, Cherrington NJ. Drug metabolizing enzyme induction pathways in experimental non-alcoholic steatohepatitis. Arch Toxicol. 2008;82:959–964. doi: 10.1007/s00204-008-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Oken E. Air pollution exposure and abnormal glucose tolerance during pregnancy: The project Viva cohort. Environ Health Persp. 2014;122:378–383. doi: 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa N, Araki E. [Type 2 diabetes and impaired glucose tolerance] Nihon Rinsho. 2013;71:270–274. [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WQ, Fitzgerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- Gnocchi D, Pedrelli M, Hurt-Camejo E, Parini P. Lipids around the clock: Focus on circadian rhythms and lipid metabolism. Biology (Basel) 2015;4:104–132. doi: 10.3390/biology4010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler M, Kilian K, Gillitzer R, Kunz M, Yoshimura T, Bröcker EB, Rapp UR, Ludwig S. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor-alpha-induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood. 1999;93:857–865. [PubMed] [Google Scholar]
- Goldberg IJ, Scheraldi CA, Yacoub LK, Saxena U, Bisgaier CL. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J Biol Chem. 1990;265:4266–4272. [PubMed] [Google Scholar]
- Gosmain Y, Cheyssac C, Masson MH, Guerardel A, Poisson C, Philippe J. Pax6 is a key component of regulated glucagon secretion. Endocrinology. 2012a;153:4204–4215. doi: 10.1210/en.2012-1425. [DOI] [PubMed] [Google Scholar]
- Gosmain Y, Katz LS, Masson MH, Cheyssac C, Poisson C, Philippe J. Pax6 is crucial for beta-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol Endocrinol. 2012b;26:696–709. doi: 10.1210/me.2011-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud TK, Peto J. Persisting risk of nickel related lung cancer and nasal cancer among Clydach refiners. Occup Environ Med. 2006;63:365–366. doi: 10.1136/oem.2005.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert SW. Regions of the retinoblastoma gene product required for its interaction with the E2F transcription factor are necessary for E2 promoter repression and pRb-mediated growth suppression. Mol Cell Biol. 1993;13:3384–3391. doi: 10.1128/mcb.13.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert SW, Chellappan SP, Horowitz JM, Nevins JR. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Liu D, Zhang Y, Lou G, Huang G, Chen B, Shen X, Gao M, Gong W, Zhou P, Dai S, Zeng Y, He F. LXRalpha-mediated downregulation of FOXM1 suppresses the proliferation of hepatocellular carcinoma cells. Oncogene. 2014;33:2888–2897. doi: 10.1038/onc.2013.250. [DOI] [PubMed] [Google Scholar]
- Huang YC. The role of in vitro gene expression profiling in particulate matter health research. J Toxicol Environ Health B. 2013;16:381–394. doi: 10.1080/10937404.2013.832649. [DOI] [PubMed] [Google Scholar]
- Kamel KS, Halperin ML. Acid-base problems in diabetic ketoacidosis. N Engl J Med. 2015;372:1969–1970. doi: 10.1056/NEJMc1502745. [DOI] [PubMed] [Google Scholar]
- Kamoun P, Aral B, Saudubray JM. [A new inherited metabolic disease: Delta1-pyrroline 5-carboxylate synthetase deficiency] Bull Acad Natl Med. 1998;182:131–137. discussion 138–139. [PubMed] [Google Scholar]
- Karakatsani A, Analitis A, Perifanou D, Ayres JG, Harrison RM, Kotronarou A, Kavouras IG, Pekkanen J, Hameri K, Kos GP, de Hartog JJ, Hoek G, Katsouyanni K. Particulate matter air pollution and respiratory symptoms in individuals having either asthma or chronic obstructive pulmonary disease: A European multicentre panel study. Environ Health. 2012;11:75. doi: 10.1186/1476-069X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodeir M, Shamy M, Alghamdi M, Zhong M, Sun H, Costa M, Chen LC, Maciejczyk P. Source apportionment and elemental composition of PM2.5 and PM10 in Jeddah City, Saudi Arabia. Atmos Pollut Res. 2012;3:331–340. doi: 10.5094/apr.2012.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- Kousha T, Valacchi G. The air quality health index and emergency department visits for urticaria in Windsor, Canada. J Toxicol Environ Health A. 2015;78:524–533. doi: 10.1080/15287394.2014.991053. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Persp. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Fang D, Xu D, Wang B, Zhao S, Yan S, Wang Y. Main air pollutants and diabetes-associated mortality: A systematic review and meta-analysis. Eur J Endocrinol. 2014;171:R183–R190. doi: 10.1530/EJE-14-0287. [DOI] [PubMed] [Google Scholar]
- Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioutas C, Hsiai T. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013;54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Persp. 2007;115:A294. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani KA, al-Amoudi NS, Kumosani TA. The state of nutrition in Saudi Arabia. Nutr Health. 2000;14:17–31. doi: 10.1177/026010600001400103. [DOI] [PubMed] [Google Scholar]
- Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, Zin WA. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol. 2010;26:481–498. doi: 10.1007/s10565-010-9158-2. [DOI] [PubMed] [Google Scholar]
- Nicholson J, Scherl A, Way L, Blackburn E A, Walkinshaw M D, Ball K L, Hupp T R. A systems wide mass spectrometric based linear motif screen to identify dominant in-vivo interacting proteins for the ubiquitin ligase MDM2. Cell Signal. 2014;26:1243–1257. doi: 10.1016/j.cellsig.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Nishiwaki Y, Michikawa T, Takebayashi T, Nitta H, Iso H, Inoue M, Tsugane S. Long-term exposure to particulate matter in relation to mortality and incidence of cardiovascular disease: The JPHC Study. J Atheroscler Thromb. 2012;20:296–309. doi: 10.5551/jat.15347. [DOI] [PubMed] [Google Scholar]
- Niu J, Liberda EN, Qu S, Guo X, Li X, Zhang J, Meng J, Yan B, Li N, Zhong M, Ito K, Wildman R, Liu H, Chen LC, Qu Q. The role of metal components in the cardiovascular effects of PM2.5. PLoS One. 2013;8:e83782. doi: 10.1371/journal.pone.0083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J. 2005;386:575–581. doi: 10.1042/BJ20041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onenli-Mungan N, Yuksel B, Elkay M, Topaloglu AK, Baykal T, Ozer G. Type II hyperprolinemia: a case report. Turk J Pediatr. 2004;46:167–169. [PubMed] [Google Scholar]
- Padwal R. Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity. Curr Opin Invest Drugs. 2009;10:1117–1125. [PubMed] [Google Scholar]
- Pang S, Lynn DA, Lo JY, Paek J, Curran SP. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat Commun. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–115. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Priestman DA, Mistry S, Halsall A. Mechanisms modifying glucose oxidation in diabetes mellitus. Diabetologia. 1994;37(Suppl 2):S155–S161. doi: 10.1007/BF00400839. [DOI] [PubMed] [Google Scholar]
- Rizzo AM, Corsetto PA, Farina F, Montorfano G, Pani G, Battaglia C, Sancini G, Palestini P. Repeated intratracheal instillation of PM10 induces lipid reshaping in lung parenchyma and in extra-pulmonary tissues. PLoS One. 2014;9:e106855. doi: 10.1371/journal.pone.0106855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JD, Voss JD, Knight B. The association of ambient air pollution and physical inactivity in the United States. PLoS One. 2014;9:e90143. doi: 10.1371/journal.pone.0090143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet J, Krewski D. Health effects associated with exposure to ambient air pollution. J Toxicol Environ Health A. 2007;70:227–242. doi: 10.1080/15287390600884644. [DOI] [PubMed] [Google Scholar]
- Satow R, Shitashige M, Kanai Y, Takeshita F, Ojima H, Jigami T, Honda K, Kosuge T, Ochiya T, Hirohashi S, Yamada T. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res. 2010;16:2518–28. doi: 10.1158/1078-0432.CCR-09-2214. [DOI] [PubMed] [Google Scholar]
- Schirra HJ, Anderson CG, Wilson WJ, Kerr L, Craik DJ, Waters MJ, Lichanska AM. Altered metabolism of growth hormone receptor mutant mice: a combined NMR metabonomics and microarray study. PLoS One. 2008;3:e2764. doi: 10.1371/journal.pone.0002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shertzer HG, Clay CD, Genter MB, Schneider SN, Nebert DW, Dalton TP. Cyp1a2 protects against reactive oxygen production in mouse liver microsomes. Free Radic Biol Med. 2004;36:605–617. doi: 10.1016/j.freeradbiomed.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Stefanska B, Huang J, Bhattacharyya B, Suderman M, Hallett M, Han ZG, Szyf M. Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res. 2011;71:5891–5903. doi: 10.1158/0008-5472.CAN-10-3823. [DOI] [PubMed] [Google Scholar]
- Sun H, Shamy M, Kluz T, Munoz AB, Zhong M, Laulicht F, Alghamdi MA, Khoder MI, Chen LC, Costa M. Gene expression profiling and pathway analysis of human bronchial epithelial cells exposed to airborne particulate matter collected from Saudi Arabia. Toxicol Appl Pharmacol. 2012;265:147–157. doi: 10.1016/j.taap.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Yoshie M, Miyajima E, Kano M, Tachikawa E. Stathmin regulates hypoxia-inducible factor-1alpha expression through the mammalian target of rapamycin pathway in ovarian clear cell adenocarcinoma. ISRN Pharmacol. 2013;2013:279593. doi: 10.1155/2013/279593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Tian L, Wang C, Hagen FK, Gormley M, Addya S, Soccio R, Casimiro MC, Zhou J, Powell MJ, Xu P, Deng H, Sauve AA, Pestell RG. Acetylation-defective mutant of Ppargamma is associated with decreased lipid synthesis in breast cancer cells. Oncotarget. 2014;5:7303–7315. doi: 10.18632/oncotarget.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigotti MA, Chiaverini F, Biagiola P, Rossi G. Urban air pollution and emergency visits for respiratory complaints in Pisa, Italy. J Toxicol Environ Health A. 2007;70:266–269. doi: 10.1080/15287390600884800. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: Target for metabolic syndrome. J Lipid Res. 2009;50(Suppl):S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171:R173–R182. doi: 10.1530/EJE-14-0365. [DOI] [PubMed] [Google Scholar]
- Wolf G, Hessabi B, Karkour A, Henrion U, Dahlhaus M, Ostmann A, Giese B, Fraunholz M, Grabarczyk P, Jack R, Walther R. The activation of the rat insulin gene II by BETA2 and PDX-1 in rat insulinoma cells is repressed by Pax6. Mol Endocrinol. 2010;24:2331–2342. doi: 10.1210/me.2009-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rao X, Wang TY, Jiang SY, Ying Z, Liu C, Wang A, Zhong M, Deiuliis JA, Maiseyeu A, Rajagopalan S, Lippmann M, Chen LC, Sun Q. Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part Fibre Toxicol. 2012;5:9. doi: 10.1186/1743-8977-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Assuncao JV, Barrozo LV. The impact of atmospheric particulate matter on cancer incidence and mortality in the city of Sao Paulo, Brazil. Cad Saude Publica. 2012;28:1737–1748. doi: 10.1590/s0102-311x2012000900012. [DOI] [PubMed] [Google Scholar]
- Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J, Kupper L, Williams R, Neas L, Cascio W, Devlin RB, Peden DB. Coarse particulate matter (PM2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Persp. 2007;115:709–714. doi: 10.1289/ehp.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xu X, Chen M, Liu D, Zhong M, Chen LC, Sun Q, Rajagopalan S. A synergistic vascular effect of airborne particulate matter and nickel in a mouse model. Toxicol Sci. 2013;135:72–80. doi: 10.1093/toxsci/kft136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KA, Nakamura Y, Arakawa H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Human Genet. 2004;49:134–140. doi: 10.1007/s10038-003-0122-3. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Huttemann M, Grossman LI, Chen LC, Rajagopalan S, Sun Q, Zhang K. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013;58:148–154. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]